The Impact of Whey Protein Supplementation on Sarcopenia Progression among the Elderly: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
- (1)
- low muscle strength (tested with handgrip strength and chair stand tests);
- (2)
- low muscle quantity or quality (confirmed with dual-energy X-ray absorptiometry (DEXA), computed tomography (CT), magnetic resonance imaging (MRI), etc.);
- (3)
- diminished physical performance (assessed with gait speed tests, 400-metre walking tests, etc.) [3].
2. Materials and Methods
2.1. Search Strategy and Inclusion Criteria
- (1)
- Human studies (studies in adults >60 years old);
- (2)
- Patients with diagnosed sarcopenia;
- (3)
- Languages: Polish, English, and German;
- (4)
- Randomised placebo-controlled clinical trial;
- (5)
- Intervention: whey protein supplementation compared to placebo/control group.
- (1)
- In animals;
- (2)
- Comprising non-sarcopenic patients;
- (3)
- Studies related to interventions other than whey protein (protein supplementation, general nutritional supplementation, and leucine supplementation).
2.2. Data Abstraction
2.3. Outcomes
2.4. Data Synthesis and Statistical Analysis
2.5. Risk of Bias
3. Results
3.1. Search Results
3.2. Characteristics of the Included Studies
3.3. Characteristics of Sarcopenia
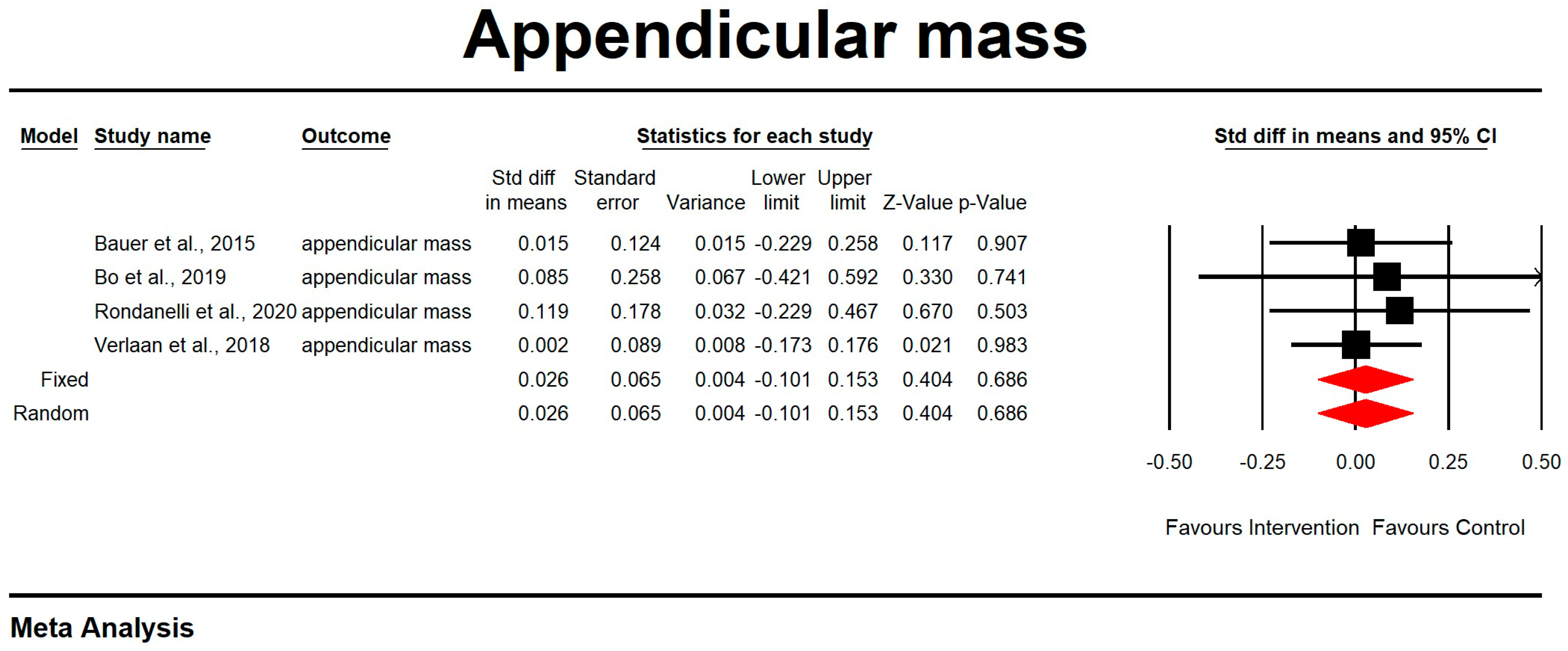
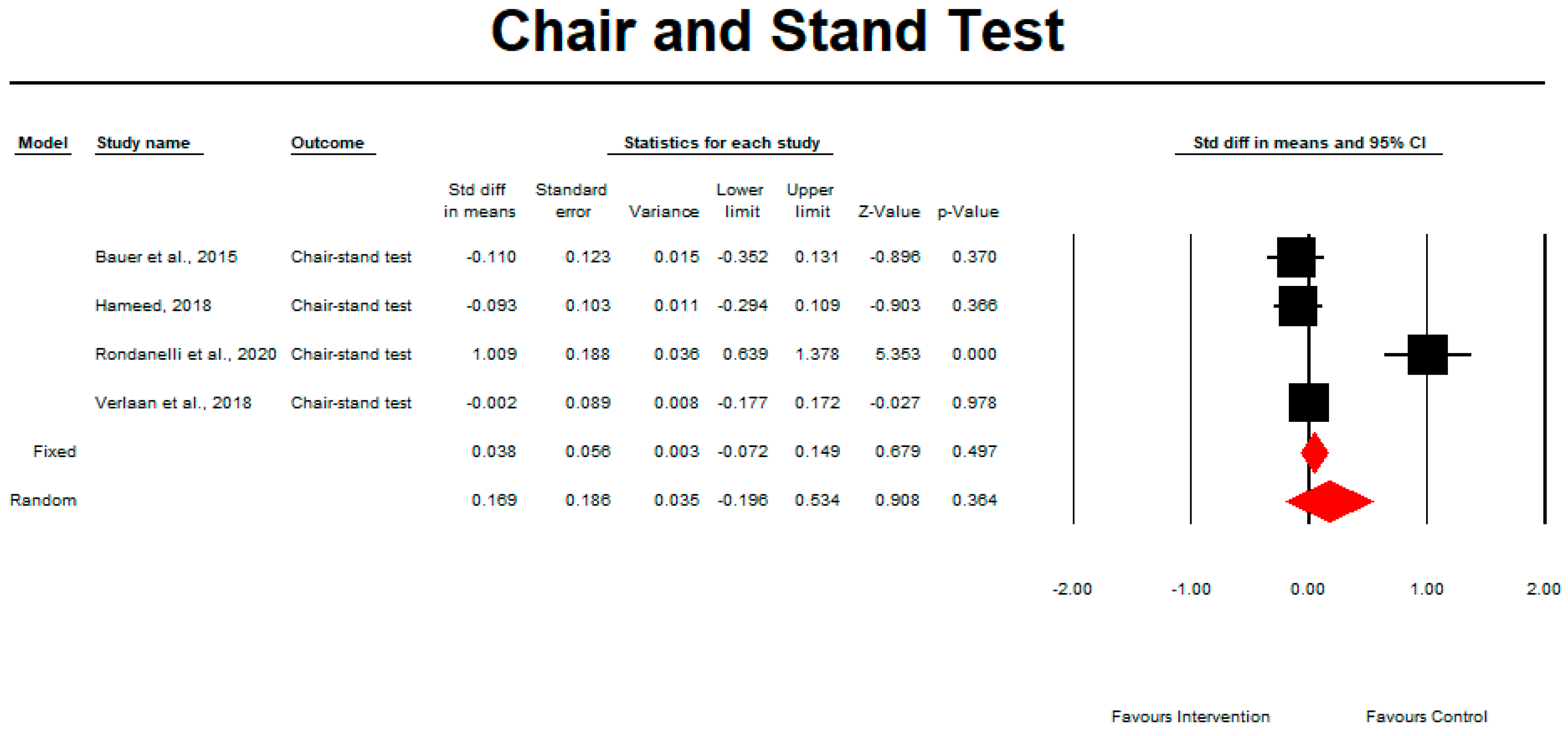
3.4. Muscle Mass, Muscle Strength, and Physical Performance
3.5. Characteristics of Interventions
3.6. Quality Assessment
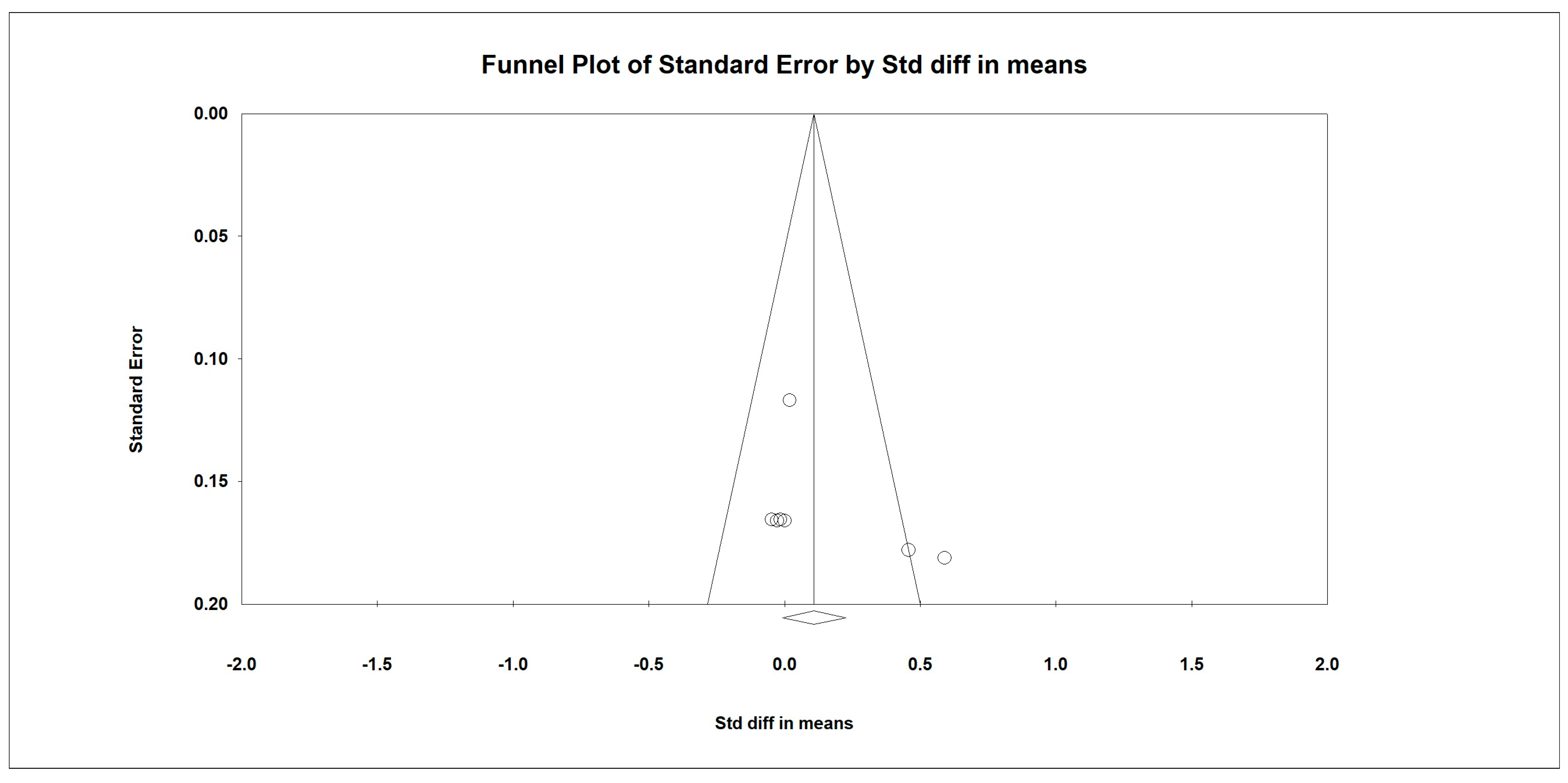
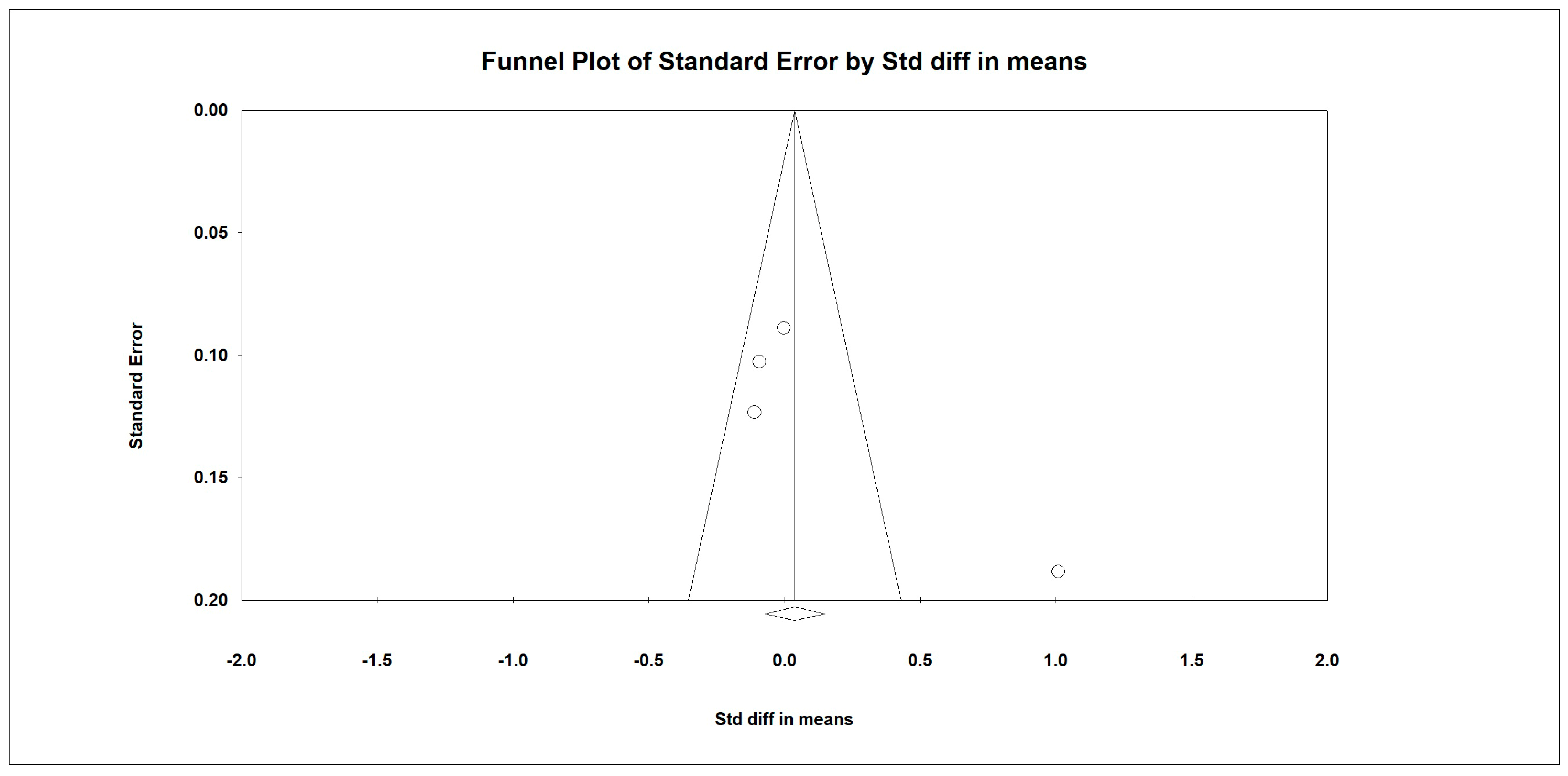
3.7. Meta-Analysis
Outcome Measures
3.8. The Effect of Protein Intake on Physical Performance
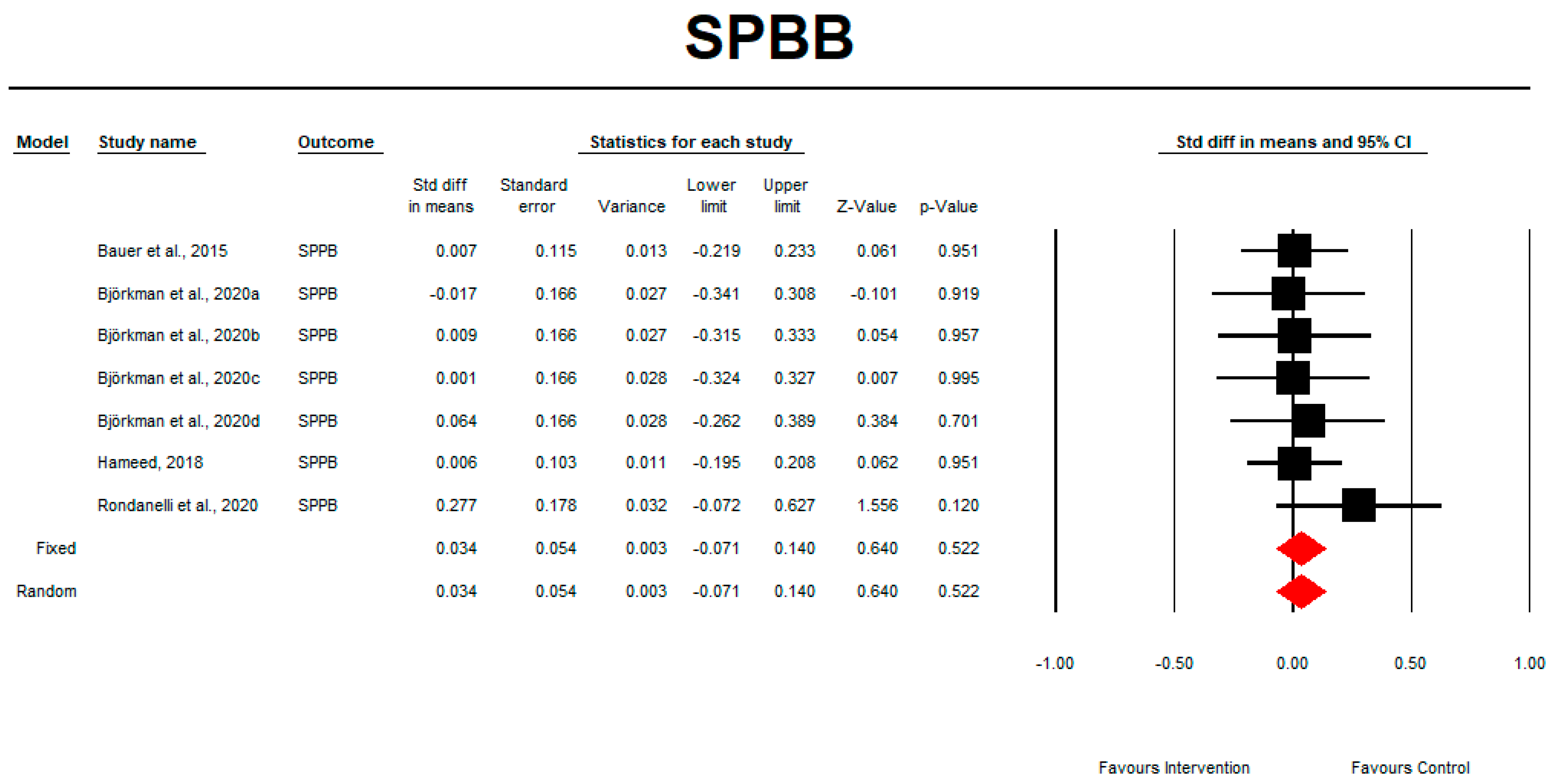
3.9. SPPB
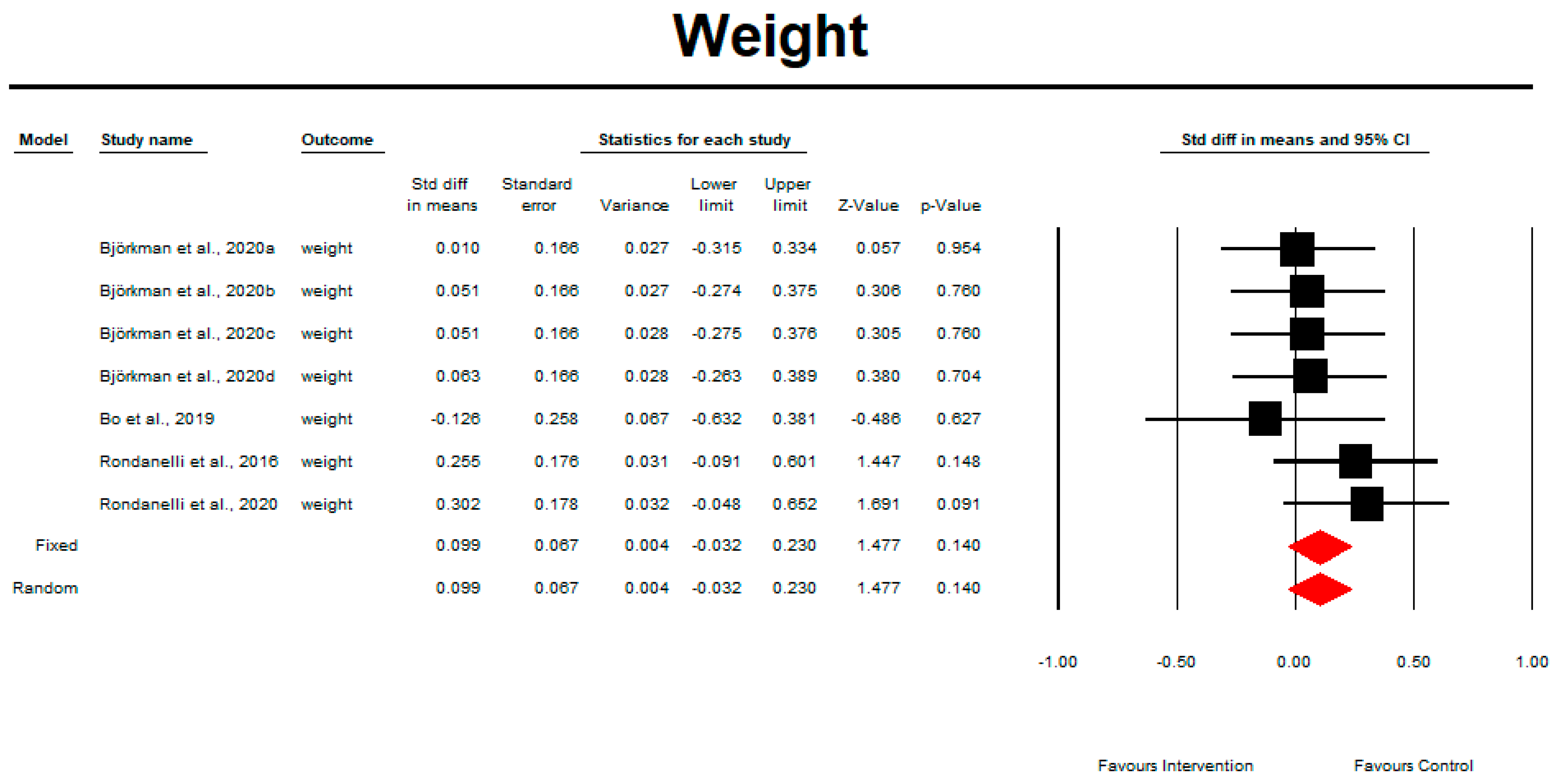
3.10. Weight
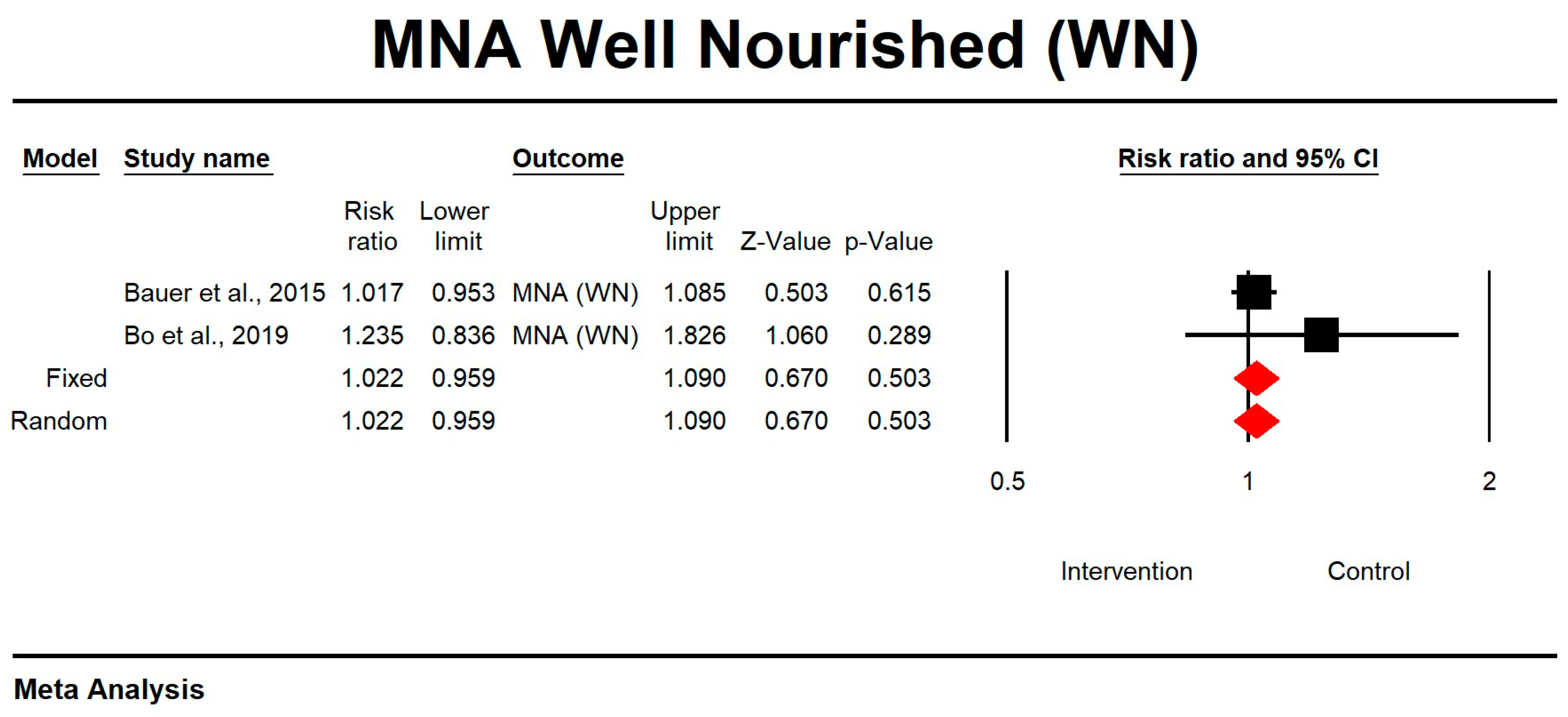
3.11. The Effect of Whey Protein Intake on MNA Results
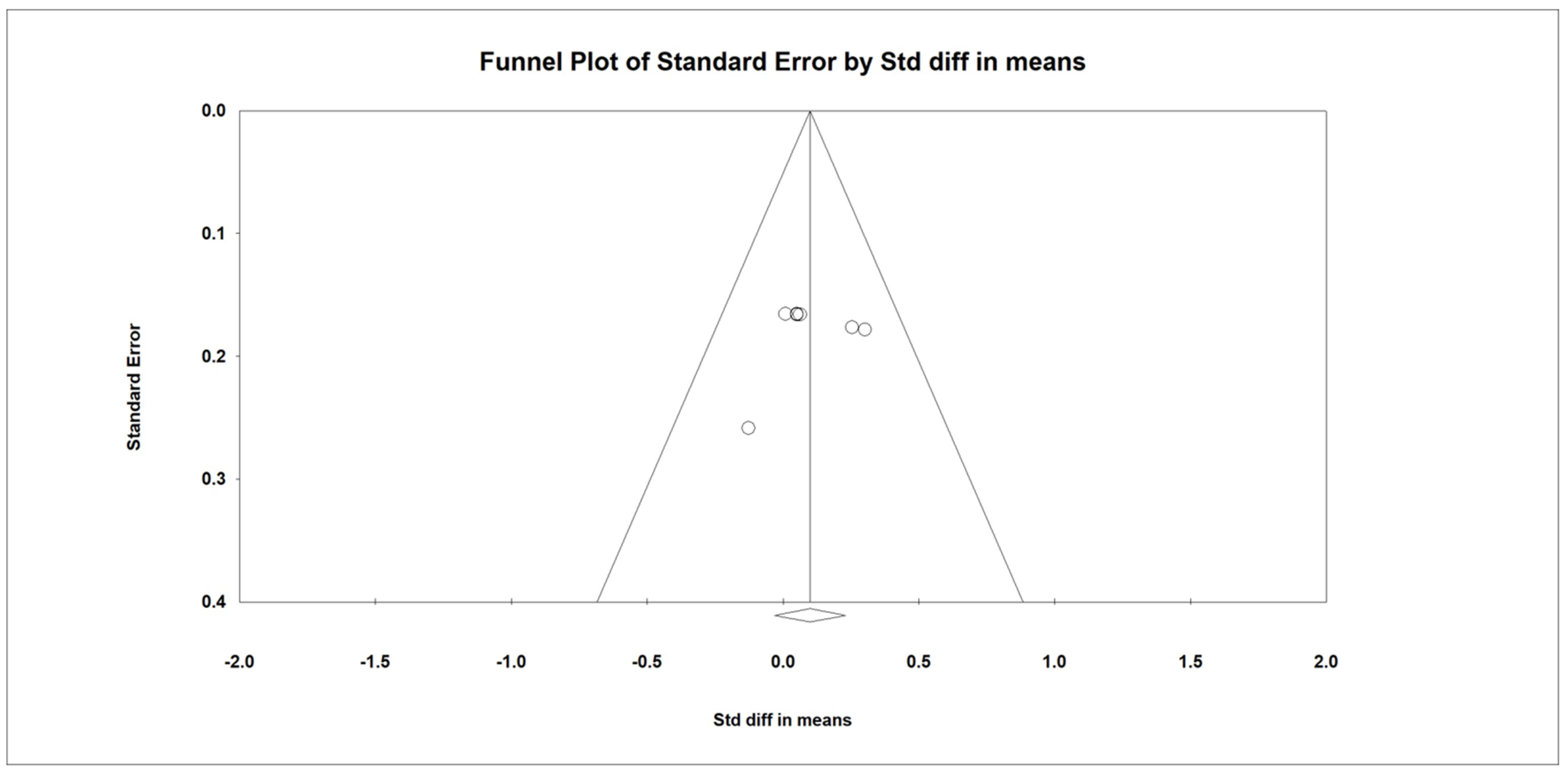
3.12. Meta-Regression Analysis
4. Discussion
4.1. Main Findings
4.2. Differences between Ours and Other Published Studies
4.3. Strengths and Limitations
4.4. Implications for Current Practise and Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Decade of Healthy Ageing (2020–2030). Available online: https://www.who.int/ageing/decade-of-healthy-ageing (accessed on 15 May 2022).
- Peel, N.; Bartlett, H.; McClure, R. Healthy ageing: How is it defined and measured? Australas. J. Ageing 2004, 23, 115–119. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), the Extended Group for EWGSOP2. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997, 127, 990S–991S. [Google Scholar] [CrossRef] [PubMed]
- Falcon, L.J.; Harris-Love, M.O. Sarcopenia and the new ICD-10-CM code: Screening, staging, and diagnosis considerations. Fed. Pract. 2017, 34, 24–32. [Google Scholar]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef] [PubMed]
- Von Haehling, S.; Morley, J.E.; Anker, S.D. An overview of sarcopenia: Facts and numbers on prevalence and clinical impact. J. Cachexia Sarcopenia Muscle 2010, 1, 129–133. [Google Scholar] [CrossRef]
- Shafiee, G.; Keshtkar, A.; Soltani, A.; Ahadi, Z.; La rijani, B.; Heshmat, R. Prevalence of sarcopenia in the world: A systematic review and meta- analysis of general population studies. J. Diabetes Metab. Disord. 2017, 16, 21. [Google Scholar] [CrossRef]
- Zanini, B.; Simonetto, A.; Zubani, M.; Castellano, M.; Gilioli, G. The effects of cow-milk protein supplementation in elderly population: Systematic review and narrative synthesis. Nutrients 2020, 12, 2548. [Google Scholar] [CrossRef]
- Walrand, S.; Boline, Y. Optimizing protein intake in aging. Curr. Opin. Clin. Nutr. Metab. Care 2005, 8, 89–94. [Google Scholar] [CrossRef]
- Beasley, J.M.; Shikany, J.M.; Thomson, C.A. The role of dietary protein intake in the prevention of sarcopenia of aging. Nutr. Clin. Pract. 2013, 28, 684–690. [Google Scholar] [CrossRef]
- Wolfe, R.R. Update on protein intake: Importance of milk proteins for health status of the elderly. Nutr. Rev. 2015, 73, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-JentoftA, J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-Based Recommendations for Optimal Dietary Protein Intake in Older People: A Position Paper From the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef] [PubMed]
- Boulos, C.; Salameh, P.; Barberger-Gateau, P. Social isolation and risk for malnutrition among older people. Geriatr. Gerontol. Int. 2017, 17, 286–294. [Google Scholar] [CrossRef] [PubMed]
- WHO. Protein and Amino Acid Requirements in Human Nutrition; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Joint FAO/WHO/UNU Expert Consultation on Protein and Amino Acid Requirements in Human Nutrition (2002: Geneva, Switzerland); Food and Agriculture Organization of the United Nations; World Health Organization; United Nations University. Protein and amino acid requirements in human nutrition: Report of a joint FAO/WHO/UNU expert consultation. World Health Organization. 2007. Available online: https://apps.who.int/iris/handle/10665/43411 (accessed on 20 April 2023).
- Akhavan, T.; Luhovyy, B.L.; Panahi, S.; Kubant, R.; Brown, P.H.; Anderson, G.H. Mechanism of action of pre-meal consumption of whey protein on glycemic control in young adults. J. Nutr. Biochem. 2014, 25, 36–43. [Google Scholar] [CrossRef]
- Dawson, B.; Taylor, J.; Favaloro, E.J. Potential benefits of improved protein intake in older people. Nutr. Diet. 2008, 65, 151–156. [Google Scholar] [CrossRef]
- Gryson, C.; Walrand, S.; Giraudet, C.; Rousset, P.; Migne, C.; Bonhomme, C.; Le Ruyet, P.; Boirie, Y. “Fast proteins” with a unique essential amino acid content as an optimal nutrition in the elderly: Growing evidence. Clin. Nutr. 2014, 33, 642–648. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J. Am. Stat. Assoc. 2000, 95, 89–98. [Google Scholar]
- Bauer, J.M.; Verlaan, S.; Bautmans, I.; Brandt, K.; Donini, L.M.; Maggio, M.; McMurdo, M.E.; Mets, T.; Seal, C.; Wijers, S.L.; et al. Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: A randomised, double-blind, placebo-controlled trial. J. Am. Med. Dir. Assoc. 2015, 16, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.M.; Mikušová, L.; Verlaan, S.; Bautmans, I.; Brandt, K.; Donini, L.M.; Maggio, M.; Mets, T.; Wijers, S.L.J.; Garthoff, J.A.; et al. PROVIDE Consortium. Safety and tolerability of 6-month supplementation with a vitamin D, calcium and leucine-enriched whey protein medical nutrition drink in sarcopenic older adults. Aging Clin. Exp. Res. 2020, 32, 1501–1514. [Google Scholar] [CrossRef]
- Björkman, M.P.; Suominen, M.H.; Kautiainen, H.; Jyväkorpi, S.K.; Finne-Soveri, H.U.; Strandberg, T.E.; Pitkälä, K.H.; Tilvis, R.S. Effect of protein supplementation on physical performance in older people with sarcopenia-a randomised controlled trial. J. Am. Med. Dir. Assoc. 2020, 21, 226–232.e1. [Google Scholar] [CrossRef]
- Bo, Y.; Liu, C.; Ji, Z.; Yang, R.; An, Q.; Zhang, X.; You, J.; Duan, D.; Sun, Y.; Zhu, Y.; et al. A high whey protein, vitamin D and E supplement preserves muscle mass, strength, and quality of life in sarcopenic older adults: A double-blind randomised controlled trial. Clin. Nutr. 2019, 38, 159–164. [Google Scholar] [CrossRef]
- Hameed, R.H. The effect of vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults. Indian J. Public Health Res. Dev. 2018, 9, 1074–1079. [Google Scholar] [CrossRef]
- Hill, T.R.; Verlaan, S.; Biesheuvel, E.; Eastell, R.; Bauer, J.M.; Bautmans, I.; Brandt, K.; Donini, L.M.; Maggio, M.; Mets, T.; et al. A vitamin D, calcium and leucine-enriched whey protein nutritional supplement improves measures of bone health in sarcopenic non-malnourished older adults: The PROVIDE Study. Calcif. Tissue Int. 2019, 105, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Liberman, K.; Njemini, R.; Luiking, Y.; Forti, L.N.; Verlaan, S.; Bauer, J.M.; Memelink, R.; Brandt, K.; Donini, L.M.; Maggio, M.; et al. Thirteen weeks of supplementation of vitamin D and leucine-enriched whey protein nutritional supplement attenuates chronic low-grade inflammation in sarcopenic older adults: The PROVIDE study. Aging Clin. Exp. Res. 2019, 31, 845–854. [Google Scholar] [CrossRef]
- Rondanelli, M.; Klersy, C.; Terracol, G.; Talluri, J.; Maugeri, R.; Guido, D.; Faliva, M.A.; Solerte, B.S.; Fioravanti, M.; Lukaski, H.; et al. Whey protein, amino acids, and vitamin D supplementation with physical activity increases fat-free mass and strength, functionality, and quality of life and decreases inflammation in sarcopenic elderly. Am. J. Clin. Nutr. 2016, 103, 830–840. [Google Scholar] [CrossRef]
- Rondanelli, M.; Cereda, E.; Klersy, C.; Faliva, M.A.; Peroni, G.; Nichetti, M.; Gasparri, C.; Iannello, G.; Spadaccini, D.; Infantino, V.; et al. Improving rehabilitation in sarcopenia: A randomised-controlled trial utilizing a muscle-targeted food for special medical purposes. J. Cachexia Sarcopenia Muscle 2020, 11, 1535–1547. [Google Scholar] [CrossRef] [PubMed]
- Verlaan, S.; Maier, A.B.; Bauer, J.M.; Bautmans, I.; Brandt, K.; Donini, L.M.; Maggio, M.; McMurdo, M.E.T.; Mets, T.; Seal, C.; et al. Sufficient levels of 25-hydroxyvitamin D and protein intake required to increase muscle mass in sarcopenic older adults—The PROVIDE study. Clin. Nutr. 2018, 37, 551–557. [Google Scholar] [CrossRef]
- Cochrane Handbook for Systematic Review of Interventions. Available online: https://handbook-5-1.cochrane.org/chapter_8/table_8_5_a_the_cochrane_collaborations_tool_for_assessing.htm (accessed on 15 May 2022).
- Makanae, Y.; Fujita, S. Role of Exercise and Nutrition in the Prevention of Sarcopenia. J. Nutr. Sci. Vitaminol. 2015, 61, S125–S127. [Google Scholar] [CrossRef]
- Kirk, B.; Mooney, K.; Amirabdollahian, F.; Khaiyat, O. Exercise and dietary-protein as a countermeasure to skeletal muscle weakness: Liverpool Hope University—Sarcopenia Aging Trial (LHU-SAT). Front. Physiol. 2019, 10, 445. [Google Scholar] [CrossRef] [PubMed]
- Esmarck, B.; Andersen, J.L.; Olsen, S.; Richter, E.A.; Mizuno, M.; Kjaer, M. Timing of postexercise protein intake is important for muscle hypertrophy with resistance training in elderly humans. J. Physiol. 2001, 535 Pt 1, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Park, H.Y.; Kim, J.; Hwang, H.; Jung, Y.; Kreider, R.; Lim, K. Effects of whey protein supplementation prior to, and following, resistance exercise on body composition and training responses: A randomized double-blind placebo-controlled study. J. Exerc. Nutrition. Biochem. 2019, 23, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Tu, D.Y.; Kao, F.M.; Tsai, S.T.; Tung, T.H. Sarcopenia among the elderly population: A systematic review and meta-analysis of randomised controlled trials. Healthcare 2021, 9, 650. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.X.M.; Yao, J.; Zirek, Y.; Reijnierse, E.M.; Maier, A.B. Muscle mass, strength, and physical performance predicting activities of daily living: A meta-analysis. J. Cachexia Sarcopenia Muscle 2020, 11, 3–25. [Google Scholar] [CrossRef]
- Liao, C.D.; Tsauo, J.Y.; Wu, Y.T.; Cheng, C.P.; Chen, H.C.; Huang, Y.C.; Chen, H.C.; Liou, T.H. Effects of protein supplementation combined with resistance exercise on body composition and physical function in older adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2017, 106, 1078–1091. [Google Scholar] [CrossRef]
- Luo, D.; Lin, Z.; Li, S.; Liu, S.J. Effect of nutritional supplement combined with exercise intervention on sarcopenia in the elderly: A meta-analysis. Int. J. Nurs. Sci. 2017, 4, 389–401. [Google Scholar] [CrossRef]
- Wu, P.Y.; Huang, K.S.; Chen, K.M.; Chou, C.P.; Tu, Y.K. Exercise, nutrition, and combined exercise and nutrition in older adults with sarcopenia: A systematic review and network meta-analysis. Maturitas 2021, 145, 38–48. [Google Scholar] [CrossRef]
- Ligthart-Melis, G.C.; Luiking, Y.C.; Kakourou, A.; Cederholm, T.; Maier, A.B.; de van der Schueren, M.A.E. Frailty, sarcopenia, and malnutrition frequently (co-)occur in hospitalized older adults: A systematic review and meta-analysis. J. Am. Med. Dir. Assoc. 2020, 21, 1216–1228. [Google Scholar] [CrossRef]






| No. | Reference (Localisation) | Age [Years] (Mean ± SD) | Subjects/ Males (n) | Health Care Setting | Funding | Intervention | Daily Dose (g) | Ingredients (Names) | Comparator | Duration (Week) | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Bauer et al., 2015 [24] (multicentre) | 77.71 ± 6.87 | 380/131 | Out patient/ In patient | Industry | Leucine enriched whey protein and vitamin D | 2 × 40 | WP-MND (FortiFit, Nutricia N.V., The Netherlands) | PBO | 13 | Whey protein intervention improves muscle mass and lower-extremity function in sarcopenic older adults |
| 2. | Bauer et al., 2020 [25] (multicentre) | 77.71 ± 6.87 | 233/131 | Out patient/ In patient | Industry | Leucine enriched whey protein, calcium, and vitamin D | 2 × 21 | WP-MND (FortiFit, Nutricia N.V., The Netherlands) | PBO | 13 | No impact of whey protein intervention on kidney function deterioration or symptoms of vitamin D or calcium toxicity |
| 3. | Björkman et al., 2020 [26] (Finland) | 83.64 ± 4.9 (P vs. C) 83.8 ± 4.32 (P vs. I) | 218/92 | Out patients | Academia/ Government |
| 2 × 20 | nd | PBO | 52 | Whey protein intervention and low-intensity home-based physical exercise did not attenuate the deterioration of muscle and physical performance in sarcopenic older adults |
| 4. | Bo et al., 2019 [27] (China) | 74.03 ± 6.29 | 60/27 | nd | Industry/ Academia | Whey protein, vitamin D, and vitamin E | 2 × 23 | nd | PBO | 26 | “Whey protein intervention significantly improves RSMI, muscle strength, and anabolic markers such as IGF-I and IL-2 in older adults with sarcopenia” |
| 5. | Hameed, 2018 [28] (Iraq) | 77.71 ± 6.87 | 380/131 | nd | nd | Leucine enriched whey protein and vitamin D | 2 × 20 | nd | PBO | 13 | Whey protein intervention improves muscle mass and lower-extremity function in sarcopenic older adults |
| 6. | Hill et al., 2019 [29] (multicentre) | 77.71 ± 6.87 | 302/131 | Out patient/ In patient | Industry | Leucine enriched whey protein, calcium, and vitamin D | 1 × 20 | WP-MND (FortiFit, Nutricia N.V., The Netherlands) | PBO | 13 | “Whey protein intervention improved 25(OH)D, suppressed PTH and had small but positive effects on BMD, indicative of improved bone health, in sarcopenic non-malnourished older adults” |
| 7. | Liberman et al., 2019 [30] (multicentre) | 77.72 ± 6.82 | 288/128 | Out patient/In patient | Industry | Leucine enriched whey protein and vitamin D | 2 × 20 | WP-MND (FortiFit, Nutricia N.V., The Netherlands) | PBO | 13 | “Whey protein intervention may attenuate the progression of CLIP in older sarcopenic persons with mobility limitations” |
| 8. | Rondanelli et al., 2016 [31] (Italy) | 80.51 ± 7.44 | 130/53 | In patients | Industry | Whey protein, amino acids with leucine, and vitamin D Regular controlled physical activity | 1 × 22 | nd | PBO | 12 | Whey protein intervention and age-appropriate exercise improve fat-free mass and strength in sarcopenic older adults |
| 9. | Rondanelli et al., 2020 [32] (Italy) | 81.5 ± 6.11 | 127/43 | In patients | Industry/ Academia | Leucine enriched whey protein and vitamin D; Rehabilitation | 2 × 20 | Fortifit®, Nutricia | PBO | From 4 to 8 | Whey protein intervention improves physical performance, function, and muscle mass in sarcopenic older adults |
| 10. | Verlaan et al., 2018 [33] (multicentre) | 77.73 ± 6.88 | 380/258 | Out patient/ In patient | Industry | Leucine enriched whey protein and vitamin D | 2 × 20 | WP-MND (FortiFit, Nutricia N.V., The Netherlands) | PBO | 13 | “Whey protein intervention increases muscle mass and improves lower-extremity function in sarcopenic older adults” |
| No. | Reference (Localisation) | (EWGSOP) Criteria (Yes/No) | Definition of Sarcopenia | Body Composition Assessment (DXA/BIA) and Nutritional Status (BMI, MNA) | Muscle Strength and Physical Performance Assessment | ||||
|---|---|---|---|---|---|---|---|---|---|
| Handgrip Strength | SPPB | Chair-Stand Time | Balance Test | Gait Speed | |||||
| 1. | Bauer et al., 2015 [24] (multicentre) | YES (EWGSOP) | Sarcopenia was defined as “the age-related loss of muscle mass, strength, and function makes up a large component of physical frailty” | DXA/ BMI by BIA, MNA | Handgrip dynamometry | SPPB score | Chair-stand test score | Balance tests | 4-m walk |
| 2. | Bauer et al., 2020 [25] (multicentre) | YES (EWGSOP) | Sarcopenia was defined as “low skeletal muscle mass index (SMI) combined with mild to moderate limitations in physical performance” | BMI, MNA-SF | nd | nd | nd | nd | nd |
| 3. | Björkman et al., 2020 [26] (Finland) | YES (EWGSOP) | Sarcopenia was defined as “low muscle strength, low muscle quantity or quality, and low physical performance” | BIA, tetrapolar BIS device | Handgrip dynamometry | nd | Chair-stand test score | nd | 4-m walk |
| 4. | Bo et al., 2019 [27] (China) | YES (EWGSOP) |
| BIA, MNA | Handgrip dynamometry | nd | Chair stand test score | nd | 6-m walk |
| 5. | Hameed, 2018 [28] (Iraq) | YES (EWGSOP) | “Sarcopenia was measured using hydraulic hand dynamometer, SPPB (balance, chair stand test, and gait speed)” | MNA | Handgrip dynamometry | SPPB score | Chair-stand test score | Balance tests | nd |
| 6. | Hill et al., 2019 [29] (multicentre) | nd | “Sarcopenia was determined by Short Physical Performance Battery (SPPB; 0–12) scores between 4 and 9, and a low skeletal muscle mass index (SMI; skeletal muscle mass/BW × 100) ≤ 37% in men and ≤ 28% in women using bioelectric impedance analysis” | DXA, BIA/ BMI, MNA | nd | SPPB score | nd | nd | nd |
| 7. | Liberman et al., 2019 [30] (multicentre) | YES (EWGSOP) | Sarcopenia was defined as “a muscle failure disease that is caused by adverse muscle changes that accumulate over life” | DXA, BMI | nd | SPPB score | nd | nd | nd |
| 8. | Rondanelli et al., 2016 [31] (Italy) | YES (EWGSOP) | Sarcopenia was defined as “the age-related depletion of skeletal muscle mass and loss of strength” | DXA, BIA | Handgrip dynamometry | nd | Chair-stand test score | nd | nd |
| 9. | Rondanelli et al., 2020 [32] (Italy) | YES (EWGSOP) | Sarcopenia was defined “according to European Working Group on Sarcopenia in Older People (EWGSOP) 2010 criteria in terms of the outcome of body composition by bioimpedance analysis [(skeletal muscle mass/body weight × 100) ≤ 37% in men and ≤28% in women], handgrip strength, and gait speed” | DXA, BIA | Handgrip dynamometry | SPPB score | Chair-stand test score | Balance tests | 4-m walk |
| 10. | Verlaan et al., 2018 [33] (multicentre) | YES (EWGSOP) | Sarcopenia was defined as “the geriatric syndrome characterized by low muscle mass, strength, and function” | DXA/ BMI by BIA, MNA-SF | Handgrip dynamometry | SPPB score | Chair-stand test score | nd | 4-m walk |
| Reference (Localisation) | Random Sequence Generation (Selection Bias) | Allocation Concealment (Selection Bias) | Blinding of Participants and Personnel (Performance Bias) | Blinding of Outcome Assessment (Detection Bias) | Incomplete Outcome Data | Selective Reporting (Reporting Bias) | Other Sources of Bias | Number of Low Risk of Bias Assessments | Final Assessment of Study Quality |
|---|---|---|---|---|---|---|---|---|---|
| Bauer et al., 2015 [24] | ? | L | L | L | L | L | ? | 5 | HIGH |
| Bauer et al., 2020 [25] | ? | L | L | L | L | L | ? | 5 | HIGH |
| Björkman et al., 2020 [26] | ? | L | L | L | L | L | ? | 5 | HIGH |
| Bo et al., 2019 [27] | L | L | L | L | L | L | ? | 6 | HIGH |
| Hameed et al., 2018 [28] | H | H | ? | ? | H | H | ? | 0 | LOW |
| Hill et al., 2019 [29] | L | ? | L | L | L | L | ? | 5 | HIGH |
| Liberman et al., 2019 [30] | L | L | ? | ? | L | L | ? | 4 | HIGH |
| Rondanelli et al., 2016 [31] | L | L | L | L | L | L | ? | 5 | HIGH |
| Rondanelli et al., 2020 [32] | L | L | L | L | L | L | ? | 6 | HIGH |
| Verlaan et al., 2018 [33] | ? | ? | ? | ? | L | L | ? | 2 | ? |
| Covariates | Appendicular Mass | |||||
|---|---|---|---|---|---|---|
| Q | df | Coefficient | SE | Z | p | |
| Study duration (weeks) | 0.02 | 1 | −0.0022 | 0.0157 | −0.14 | 0.8868 |
| % male | 0.02 | 1 | 0.0039 | 0.0255 | 0.15 | 0.8773 |
| Age | 0.10 | 1 | 0.0123 | 0.0396 | 0.31 | 0.7566 |
| Dose | 0.02 | 1 | −0.0005 | 0.0034 | −0.15 | 0.8769 |
| % analysed | 0.37 | 1 | −0.0004 | 0.0006 | −0.61 | 0.5443 |
| Handgrip strength | ||||||
| Q | df | Coefficient | SE | Z | p | |
| Study duration (weeks) | 5.01 | 1 | −0.0083 | 0.0037 | −2.24 | 0.0252 |
| % male | 2.76 | 1 | 0.0407 | 0.0245 | 1.66 | 0.0968 |
| Age | 0.47 | 1 | −0.0311 | 0.0455 | −0.68 | 0.4934 |
| Dose | 0.84 | 1 | −0.0051 | 0.0056 | −0.92 | 0.3589 |
| % analysed | 3.14 | 1 | −0.0018 | 0.0010 | −1.77 | 0.0765 |
| Chair and stand test | ||||||
| Study duration (weeks) | 29.12 | 1 | −0.1522 | 0.0282 | −5.40 | 0.0000 |
| % male | 2.72 | 1 | −0.8611 | 0.5221 | −1.65 | 0.0991 |
| Age | 29.20 | 1 | 0.2822 | 0.0522 | 5.40 | 0.0000 |
| Dose | 0.09 | 1 | −0.0031 | 0.0104 | −0.30 | 0.7625 |
| % analysed | 29.12 | 1 | −0.0042 | 0.0008 | −5.40 | 0.0000 |
| SPPB | ||||||
| Study duration (weeks) | 0.21 | 1 | −0.0012 | 0.0027 | −0.45 | 0.6503 |
| % male | 0.00 | 1 | −0.0010 | 0.0291 | −0.03 | 0.9739 |
| Age | 0.04 | 1 | 0.0039 | 0.0187 | 0.21 | 0.8353 |
| Dose | 0.01 | 1 | −0.0002 | 0.0025 | −0.07 | 0.9415 |
| % analysed | 0.72 | 1 | −0.0005 | 0.0006 | −0.85 | 0.3971 |
| Weight | ||||||
| Study duration (weeks) | 1.96 | 1 | −0.0048 | 0.0034 | −1.40 | 0.1615 |
| % male | 0.03 | 1 | 0.0027 | 0.0145 | 0.18 | 0.8537 |
| Age | 0.00 | 1 | 0.0015 | 0.0263 | 0.06 | 0.9557 |
| Dose | 0.10 | 1 | −0.0029 | 0.0093 | −0.31 | 0.7571 |
| % analysed | 0.41 | 1 | −0.0008 | 0.0013 | −0.64 | 0.5232 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamińska, M.S.; Rachubińska, K.; Grochans, S.; Skonieczna-Żydecka, K.; Cybulska, A.M.; Grochans, E.; Karakiewicz, B. The Impact of Whey Protein Supplementation on Sarcopenia Progression among the Elderly: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 2039. https://doi.org/10.3390/nu15092039
Kamińska MS, Rachubińska K, Grochans S, Skonieczna-Żydecka K, Cybulska AM, Grochans E, Karakiewicz B. The Impact of Whey Protein Supplementation on Sarcopenia Progression among the Elderly: A Systematic Review and Meta-Analysis. Nutrients. 2023; 15(9):2039. https://doi.org/10.3390/nu15092039
Chicago/Turabian StyleKamińska, Magdalena Sylwia, Kamila Rachubińska, Szymon Grochans, Karolina Skonieczna-Żydecka, Anna Maria Cybulska, Elżbieta Grochans, and Beata Karakiewicz. 2023. "The Impact of Whey Protein Supplementation on Sarcopenia Progression among the Elderly: A Systematic Review and Meta-Analysis" Nutrients 15, no. 9: 2039. https://doi.org/10.3390/nu15092039
APA StyleKamińska, M. S., Rachubińska, K., Grochans, S., Skonieczna-Żydecka, K., Cybulska, A. M., Grochans, E., & Karakiewicz, B. (2023). The Impact of Whey Protein Supplementation on Sarcopenia Progression among the Elderly: A Systematic Review and Meta-Analysis. Nutrients, 15(9), 2039. https://doi.org/10.3390/nu15092039









