Pterostilbene Attenuates High-Intensity Swimming Exercise-Induced Glucose Absorption Dysfunction Associated with the Inhibition of NLRP3 Inflammasome-Induced IECs Pyroptosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Protocol
2.2. Oral Glucose Tolerance Test (OGTT) and d-Xylose Absorption Assay
2.3. Caspase-1 p20 Activity Assay
2.4. Immunofluorescence Staining
2.5. Mitochondrial Respiratory Complex Activities Assay
2.6. Analysis of Mitochondrial DNA (mtDNA) Content
2.7. Cell Culture and Treatment
2.8. Small Interference RNA (siRNA) Transfection
2.9. Cell Viability Assay
2.10. Glucose Uptake Assay
2.11. Intracellular ROS Measurement
2.12. ELISA
2.13. Seahorse XFp Cell Mito Stress Test
2.14. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.15. Western Blotting
2.16. Statistical Analysis
3. Results
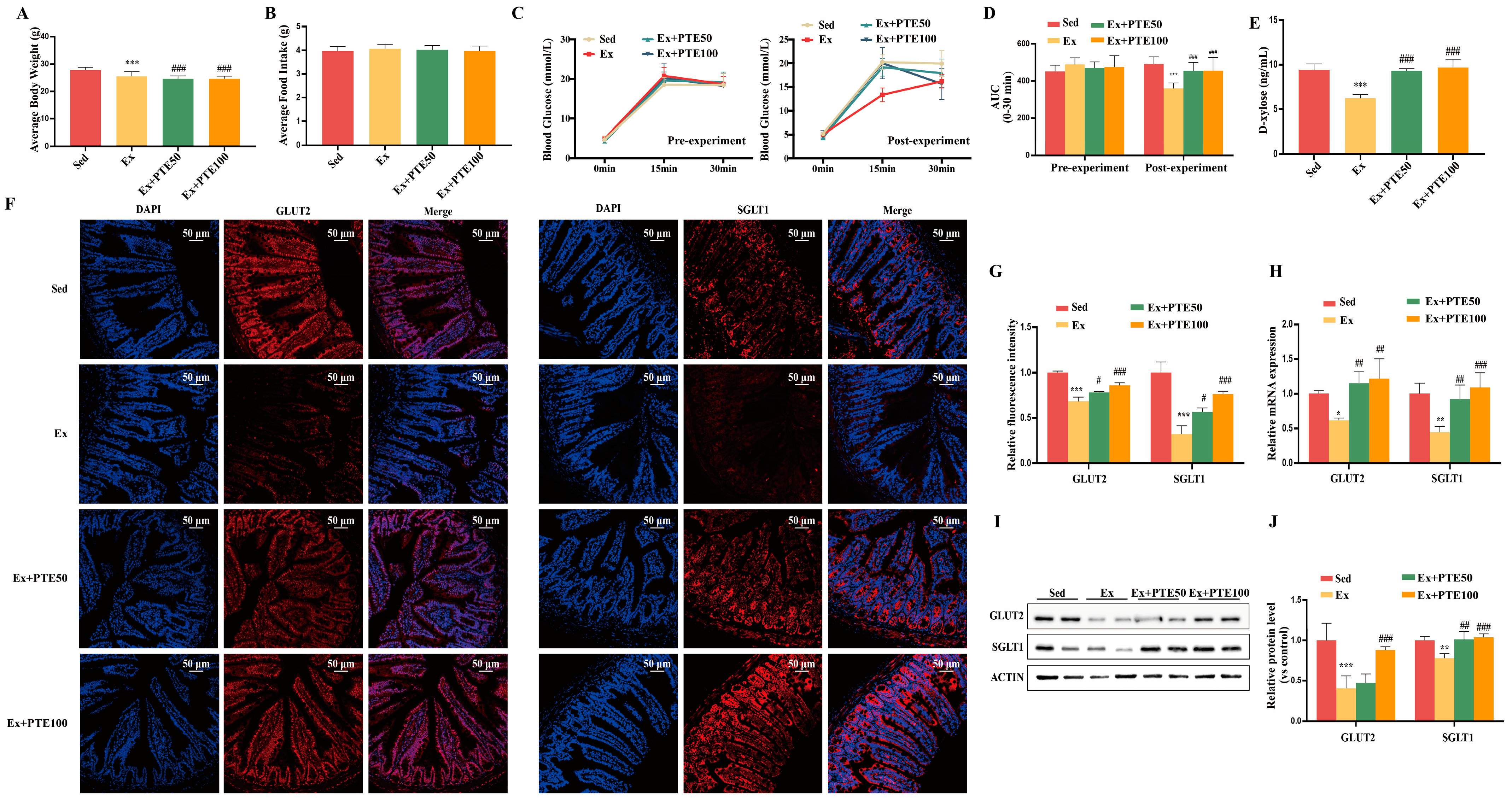
3.1. PTE Attenuated HISE-Induced Glucose Absorption Dysfunction in Mice
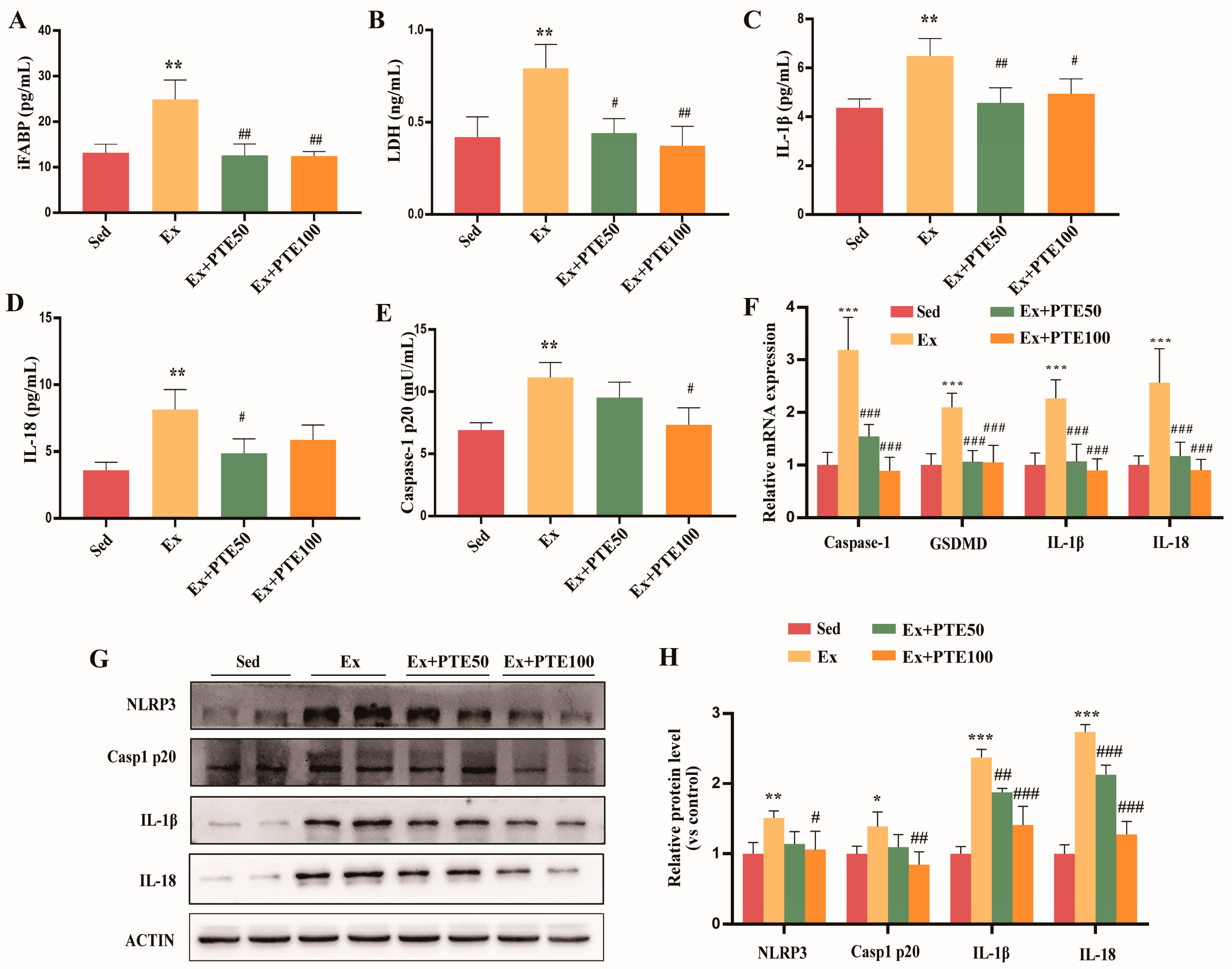
3.2. PTE Weakened Pyroptosis in the Small Intestine of Mice Accessing to HISE
3.3. PTE Eliminated Intestinal Mitochondrial Dysfunction in HISE Treated Mice
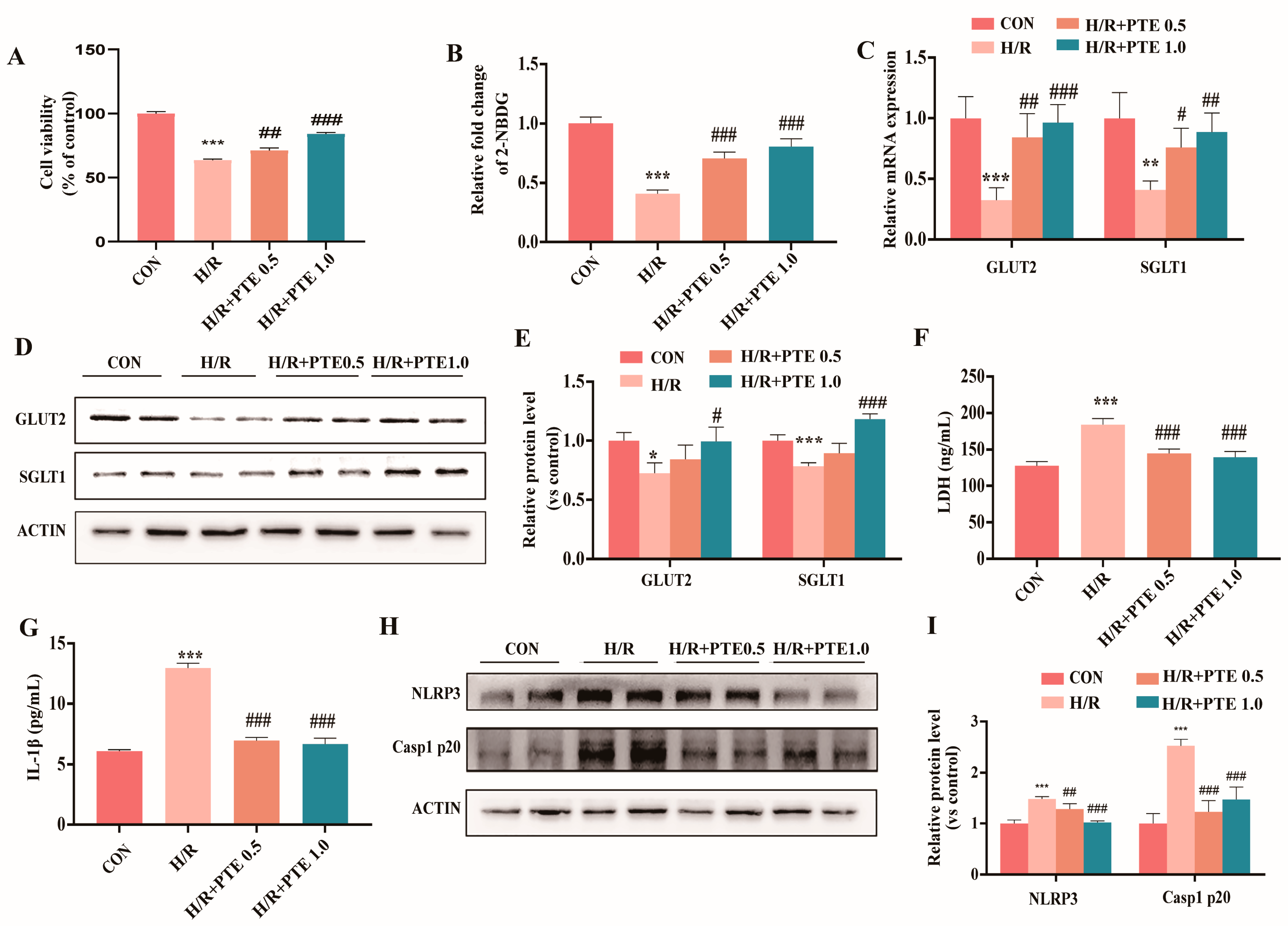
3.4. PTE Attenuated Glucose Absorption Dysfunction and Pyroptosis in H/R Treated IEC-6 Cells
3.5. PTE Improved H/R-Induced Mitochondrial Dysfunction in IEC-6 Cells
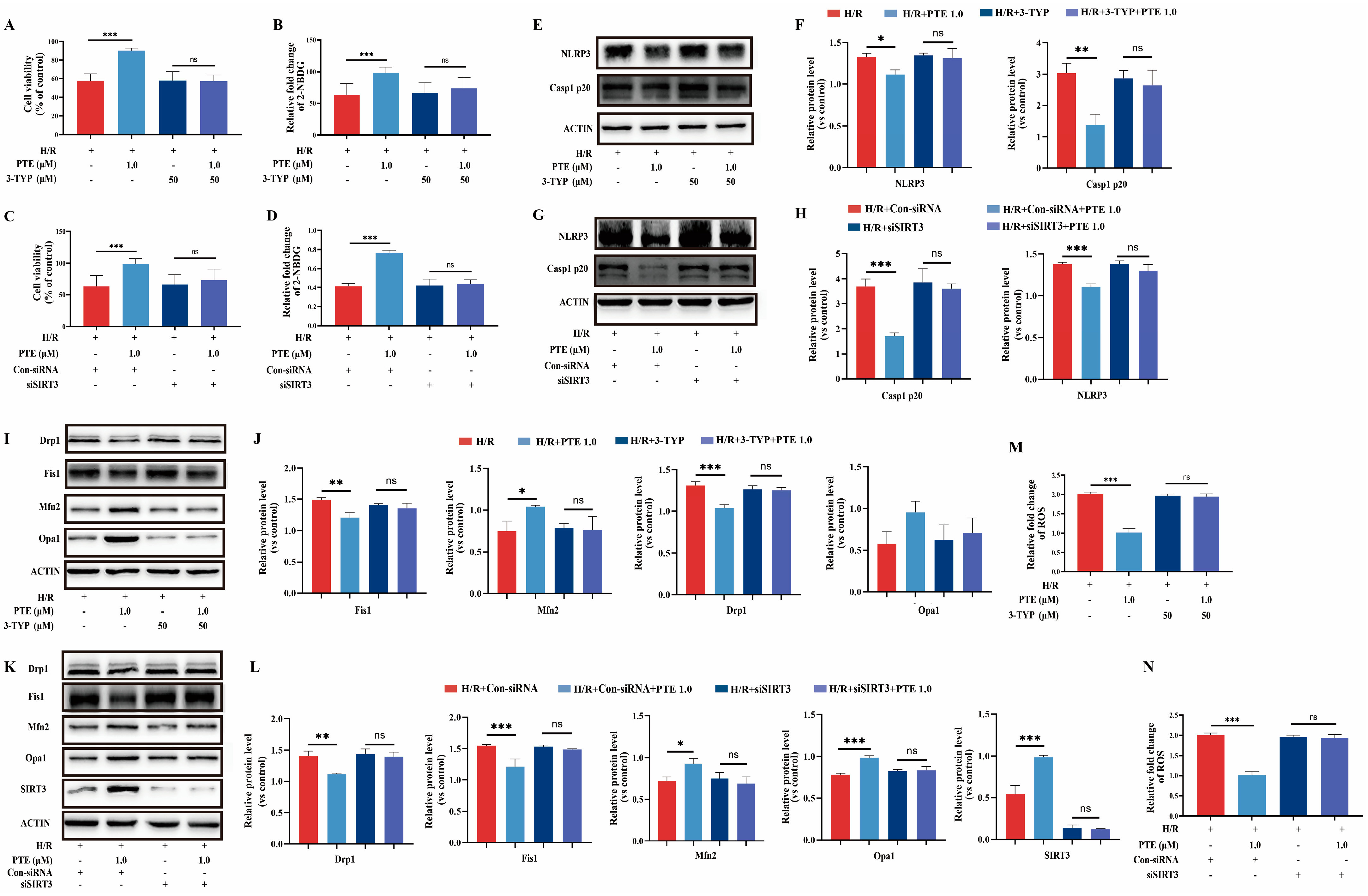
3.6. PTE Suppressed H/R-Induced Glucose Absorption Dysfunction and Pyroptosis as Well as Mitochondrial Dysfunction of IEC-6 Cells through a SIRT3-Dependent Mechanism In Vitro
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Guidelines Approved by the Guidelines Review Committee. In Global Recommendations on Physical Activity for Health; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Astrand, P.O.; Rodahl, K. Textbook of Work Physiology: Physiological Bases of Exercise; McGraw Hill: New York, NY, USA, 1986. [Google Scholar]
- Roder, P.V.; Geillinger, K.E.; Zietek, T.S.; Thorens, B.; Koepsell, H.; Daniel, H. The role of SGLT1 and GLUT2 in intestinal glucose transport and sensing. PLoS ONE 2014, 9, e89977. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.J.S.; Snipe, R.M.J.; Kitic, C.M.; Gibson, P.R. Systematic review: Exercise-induced gastrointestinal syndrome-implications for health and intestinal disease. Aliment Pharmacol. Ther. 2017, 46, 246–265. [Google Scholar] [CrossRef] [PubMed]
- van Wijck, K.; Lenaerts, K.; Grootjans, J.; Wijnands, K.A.; Poeze, M.; van Loon, L.J.; Dejong, C.H.; Buurman, W.A. Physiology and pathophysiology of splanchnic hypoperfusion and intestinal injury during exercise: Strategies for evaluation and prevention. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G155–G168. [Google Scholar] [CrossRef]
- Rehrer, N.J.; Smets, A.; Reynaert, H.; Goes, E.; De Meirleir, K. Effect of exercise on portal vein blood flow in man. Med. Sci. Sport. Exerc. 2001, 33, 1533–1537. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.; Zhou, X.; Yu, L.; Yao, Y.; Zhang, Y.; Huang, Y.; Chen, M.; Yi, L.; Mi, M. Exhaustive Exercise Induces Gastrointestinal Syndrome through Reduced ILC3 and IL-22 in Mouse Model. Med. Sci. Sport. Exerc. 2020, 52, 1710–1718. [Google Scholar] [CrossRef]
- Hersh, D.; Monack, D.M.; Smith, M.R.; Ghori, N.; Falkow, S.; Zychlinsky, A. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc. Natl. Acad. Sci. USA 1999, 96, 2396–2401. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef]
- Sutterwala, F.S.; Haasken, S.; Cassel, S.L. Mechanism of NLRP3 inflammasome activation. Ann. N. Y. Acad. Sci. 2014, 1319, 82–95. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef]
- Abderrazak, A.; Syrovets, T.; Couchie, D.; El Hadri, K.; Friguet, B.; Simmet, T.; Rouis, M. NLRP3 inflammasome: From a danger signal sensor to a regulatory node of oxidative stress and inflammatory diseases. Redox Biol. 2015, 4, 296–307. [Google Scholar] [CrossRef]
- Pernas, L.; Scorrano, L. Mito-Morphosis: Mitochondrial Fusion, Fission, and Cristae Remodeling as Key Mediators of Cellular Function. Annu. Rev. Physiol. 2016, 78, 505–531. [Google Scholar] [CrossRef]
- Seo, A.Y.; Joseph, A.M.; Dutta, D.; Hwang, J.C.; Aris, J.P.; Leeuwenburgh, C. New insights into the role of mitochondria in aging: Mitochondrial dynamics and more. J. Cell Sci. 2010, 123, 2533–2542. [Google Scholar] [CrossRef] [PubMed]
- Rath, E.; Moschetta, A.; Haller, D. Mitochondrial function—Gatekeeper of intestinal epithelial cell homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 497–516. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.Y.; Ho, C.T.; Chen, Y.K. Biological actions and molecular effects of resveratrol, pterostilbene, and 3′-hydroxypterostilbene. J. Food Drug Anal. 2017, 25, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Malik, S.; Acharya, J.D.; Mehendale, N.K.; Kamat, S.S.; Ghaskadbi, S.S. Pterostilbene reverses palmitic acid mediated insulin resistance in HepG2 cells by reducing oxidative stress and triglyceride accumulation. Free Radic. Res. 2019, 53, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lang, H.; Zhou, M.; Huang, L.; Hui, S.; Wang, X.; Chen, K.; Mi, M. The Preventive Effects of Pterostilbene on the Exercise Intolerance and Circadian Misalignment of Mice Subjected to Sleep Restriction. Mol. Nutr. Food Res. 2020, 64, e1900991. [Google Scholar] [CrossRef]
- McDonnell, E.; Peterson, B.S.; Bomze, H.M.; Hirschey, M.D. SIRT3 regulates progression and development of diseases of aging. Trends Endocrinol. Metab. 2015, 26, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, M.; Zeng, X.; Yang, J.; Deng, H.; Yi, L.; Mi, M.T. Resveratrol regulates mitochondrial reactive oxygen species homeostasis through Sirt3 signaling pathway in human vascular endothelial cells. Cell Death Dis. 2014, 5, e1576. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, W.; Zhu, X.; Ran, L.; Lang, H.; Yi, L.; Mi, M.; Zhu, J. Pterostilbene Enhances Endurance Capacity via Promoting Skeletal Muscle Adaptations to Exercise Training in Rats. Molecules 2020, 25, 186. [Google Scholar] [CrossRef]
- Yuan, X.; Xu, S.; Huang, H.; Liang, J.; Wu, Y.; Li, C.; Yuan, H.; Zhao, X.; Lai, X.; Hou, S. Influence of excessive exercise on immunity, metabolism, and gut microbial diversity in an overtraining mice model. Scand. J. Med. Sci. Sport. 2018, 28, 1541–1551. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, E.K.; Choi, E.J. High-intensity swimming exercise increases dust mite extract and 1-chloro-2,4-dinitrobenzene-derived atopic dermatitis in BALB/c mice. Inflammation 2014, 37, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, A.; Shimomura, Y.; Murakami, T.; Ichikawa, M.; Nakai, N.; Fujitsuka, C.; Kanematsu, M.; Fujitsuka, N. Glycogen depletion of the intrafusal fibers in a mouse muscle spindle during prolonged swimming. Am. J. Physiol. 1996, 271, R398–R408. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Xu, B.; Sun, Y.; Lian, M.; Li, Y.; Lin, Y.; Chen, D.; Diao, Y.; Almoiliqy, M.; Wang, L. Paeoniflorin protects against intestinal ischemia/reperfusion by activating LKB1/AMPK and promoting autophagy. Pharmacol. Res. 2019, 146, 104308. [Google Scholar] [CrossRef]
- Stellingwerff, T.; Cox, G.R. Systematic review: Carbohydrate supplementation on exercise performance or capacity of varying durations. Appl. Physiol. Nutr. Metab. 2014, 39, 998–1011. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.J.; Snipe, R.; Camões-Costa, V.; Scheer, V.; Murray, A. The Impact of Gastrointestinal Symptoms and Dermatological Injuries on Nutritional Intake and Hydration Status During Ultramarathon Events. Sport. Med. Open 2016, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- Stuempfle, K.J.; Hoffman, M.D. Gastrointestinal distress is common during a 161-km ultramarathon. J. Sport. Sci. 2015, 33, 1814–1821. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Gao, W.; Shao, F. Pyroptosis: Gasdermin—Mediated Programmed Necrotic Cell Death. Trends Biochem. Sci. 2017, 42, 245–254. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Dixit, V.M. Mechanisms and functions of inflammasomes. Cell 2014, 157, 1013–1022. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Z.; Ruan, J.; Pan, Y.; Magupalli, V.G.; Wu, H.; Lieberman, J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 2016, 535, 153–158. [Google Scholar] [CrossRef]
- Latz, E. The inflammasomes: Mechanisms of activation and function. Curr. Opin. Immunol. 2010, 22, 28–33. [Google Scholar] [CrossRef]
- Franchi, L.; Eigenbrod, T.; Muñoz-Planillo, R.; Nuñez, G. The inflammasome: A caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat. Immunol. 2009, 10, 241–247. [Google Scholar] [CrossRef]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Shirasuna, K.; Usui, F.; Karasawa, T.; Kimura, H.; Kawashima, A.; Mizukami, H.; Ohkuchi, A.; Nishimura, S.; Sagara, J.; Noda, T.; et al. Nanosilica-induced placental inflammation and pregnancy complications: Different roles of the inflammasome components NLRP3 and ASC. Nanotoxicology 2015, 9, 554–567. [Google Scholar] [CrossRef]
- Archer, S.L. Mitochondrial dynamics--mitochondrial fission and fusion in human diseases. N. Engl. J. Med. 2013, 369, 2236–2251. [Google Scholar] [CrossRef]
- Muñoz, J.P.; Ivanova, S.; Sánchez-Wandelmer, J.; Martínez-Cristóbal, P.; Noguera, E.; Sancho, A.; Díaz-Ramos, A.; Hernández-Alvarez, M.I.; Sebastián, D.; Mauvezin, C.; et al. Mfn2 modulates the UPR and mitochondrial function via repression of PERK. EMBO J. 2013, 32, 2348–2361. [Google Scholar] [CrossRef] [PubMed]
- Nie, Q.; Wang, C.; Song, G.; Ma, H.; Kong, D.; Zhang, X.; Gan, K.; Tang, Y. Mitofusin 2 deficiency leads to oxidative stress that contributes to insulin resistance in rat skeletal muscle cells. Mol. Biol. Rep. 2014, 41, 6975–6983. [Google Scholar] [CrossRef]
- Kai, J.; Yang, X.; Wang, Z.; Wang, F.; Jia, Y.; Wang, S.; Tan, S.; Chen, A.; Shao, J.; Zhang, F.; et al. Oroxylin a promotes PGC-1alpha/Mfn2 signaling to attenuate hepatocyte pyroptosis via blocking mitochondrial ROS in alcoholic liver disease. Free Radic. Biol. Med. 2020, 153, 89–102. [Google Scholar] [CrossRef]
- de Oliveira, E.P.; Burini, R.C.; Jeukendrup, A. Gastrointestinal complaints during exercise: Prevalence, etiology, and nutritional recommendations. Sport. Med. 2014, 44 (Suppl. 1), S79–S85. [Google Scholar] [CrossRef] [PubMed]
- Tseng, A.H.; Shieh, S.S.; Wang, D.L. SIRT3 deacetylates FOXO3 to protect mitochondria against oxidative damage. Free Radic. Biol. Med. 2013, 63, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, C.; Kumar, C.; Gnad, F.; Nielsen, M.L.; Rehman, M.; Walther, T.C.; Olsen, J.V.; Mann, M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 2009, 325, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.P.; Ka, S.M.; Hsu, W.H.; Chen, A.; Chao, L.K.; Lin, C.C.; Hsieh, C.C.; Chen, M.C.; Chiu, H.W.; Ho, C.L.; et al. Resveratrol inhibits NLRP3 inflammasome activation by preserving mitochondrial integrity and augmenting autophagy. J. Cell Physiol. 2015, 230, 1567–1579. [Google Scholar] [CrossRef] [PubMed]


| Genes | Species | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|---|
| Glut2 | mouse | CTCAGCTTTATTCTGGGCAATC | TTTCTGGACAGAAGAGCAGTAG |
| Sglt1 | mouse | GCCATGTTTTCCACTAATCGTG | CACTTCCAATGTTACTGGCAAA |
| Glut2 | rat | CACCAGCACATACGACACCAGAC | TGGACACAGACAGAGACCAGAGC |
| Sglt1 | rat | CTATCAGCGTCGTCACCGTCTTG | GGCTCCTCCTCTCCTGCATCC |
| Gsdmd | mouse | CTAGCTAAGGCTCTGGAGACAA | GATTCTTTTCATCCCAGCAGTC |
| Casp1 | mouse | AGAGGATTTCTTAACGGATGCA | TCACAAGACCAGGCATATTCTT |
| IL-1β | mouse | GCAGCAGCACATCAACAAGAGC | AGGTCCACGGGAAAGACACAGG |
| IL-18 | mouse | AGACCTGGAATCAGACAACTTT | TCAGTCATATCCTCGAACACAG |
| Sirt3 | mouse | TCTATACACAGAACATCGACGG | GCATGTAGCTGTTACAAAGGTC |
| Drp1 | mouse | ACTGATTCAATCCGTGATGAGT | GTAACCTATTCAGGGTCCTAGC |
| Fis1 | mouse | CCTGGTTCGAAGCAAATACAAT | CTTTTCATATTCCTTGAGCCGG |
| Mfn2 | mouse | TCTCCCTCTGACACCTGCCAAC | ACACCACTCCTCCGACCACAAG |
| Opa1 | mouse | CTTACATGCAGAATCCTAACGC | CCAAGTCTGTAACAATACTGCG |
| Nd1 | mouse | TCCCCTACCAATACCACACCC | ATTGTTTGGGCTACGGCTCG |
| β-actin | mouse | CTACCTCATGAAGATCCTGACC | CACAGCTTCTCTTTGATGTCAC |
| β-actin | rat | CTGAGAGGGAAATCGTGCGTGAC | AGGAAGAGGATGCGGCAGTGG |
| Antibody | Company | Dilution |
|---|---|---|
| GLUT2 | Abcam | 1:1000 |
| SGLT1 | Abcam | 1:1000 |
| NLRP3 | Abcam | 1:1000 |
| CASPASE-1 P20 | Proteintech | 1:1000 |
| IL-1β | Abcam | 1:1000 |
| IL-18 | Abcam | 1:1000 |
| DRP1 | BD biosciences | 1:1000 |
| FIS1 | BD biosciences | 1:1000 |
| MFN2 | Abcam | 1:1000 |
| OPA1 | BD biosciences | 1:1000 |
| SIRT3 | Cell signaling | 1:1000 |
| β-ACTIN | Cell signaling | 1:1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, L.; Hou, P.; Jing, J.; Zhou, M.; Wang, L.; Wu, L.; Zhu, J.; Yi, L.; Mi, M. Pterostilbene Attenuates High-Intensity Swimming Exercise-Induced Glucose Absorption Dysfunction Associated with the Inhibition of NLRP3 Inflammasome-Induced IECs Pyroptosis. Nutrients 2023, 15, 2036. https://doi.org/10.3390/nu15092036
Zheng L, Hou P, Jing J, Zhou M, Wang L, Wu L, Zhu J, Yi L, Mi M. Pterostilbene Attenuates High-Intensity Swimming Exercise-Induced Glucose Absorption Dysfunction Associated with the Inhibition of NLRP3 Inflammasome-Induced IECs Pyroptosis. Nutrients. 2023; 15(9):2036. https://doi.org/10.3390/nu15092036
Chicago/Turabian StyleZheng, Lin, Pengfei Hou, Jinjin Jing, Min Zhou, Le Wang, Luting Wu, Jundong Zhu, Long Yi, and Mantian Mi. 2023. "Pterostilbene Attenuates High-Intensity Swimming Exercise-Induced Glucose Absorption Dysfunction Associated with the Inhibition of NLRP3 Inflammasome-Induced IECs Pyroptosis" Nutrients 15, no. 9: 2036. https://doi.org/10.3390/nu15092036
APA StyleZheng, L., Hou, P., Jing, J., Zhou, M., Wang, L., Wu, L., Zhu, J., Yi, L., & Mi, M. (2023). Pterostilbene Attenuates High-Intensity Swimming Exercise-Induced Glucose Absorption Dysfunction Associated with the Inhibition of NLRP3 Inflammasome-Induced IECs Pyroptosis. Nutrients, 15(9), 2036. https://doi.org/10.3390/nu15092036






