Admission Serum Magnesium Levels Is Associated with Short and Long-Term Clinical Outcomes in COVID-19 Patients
Abstract
1. Introduction
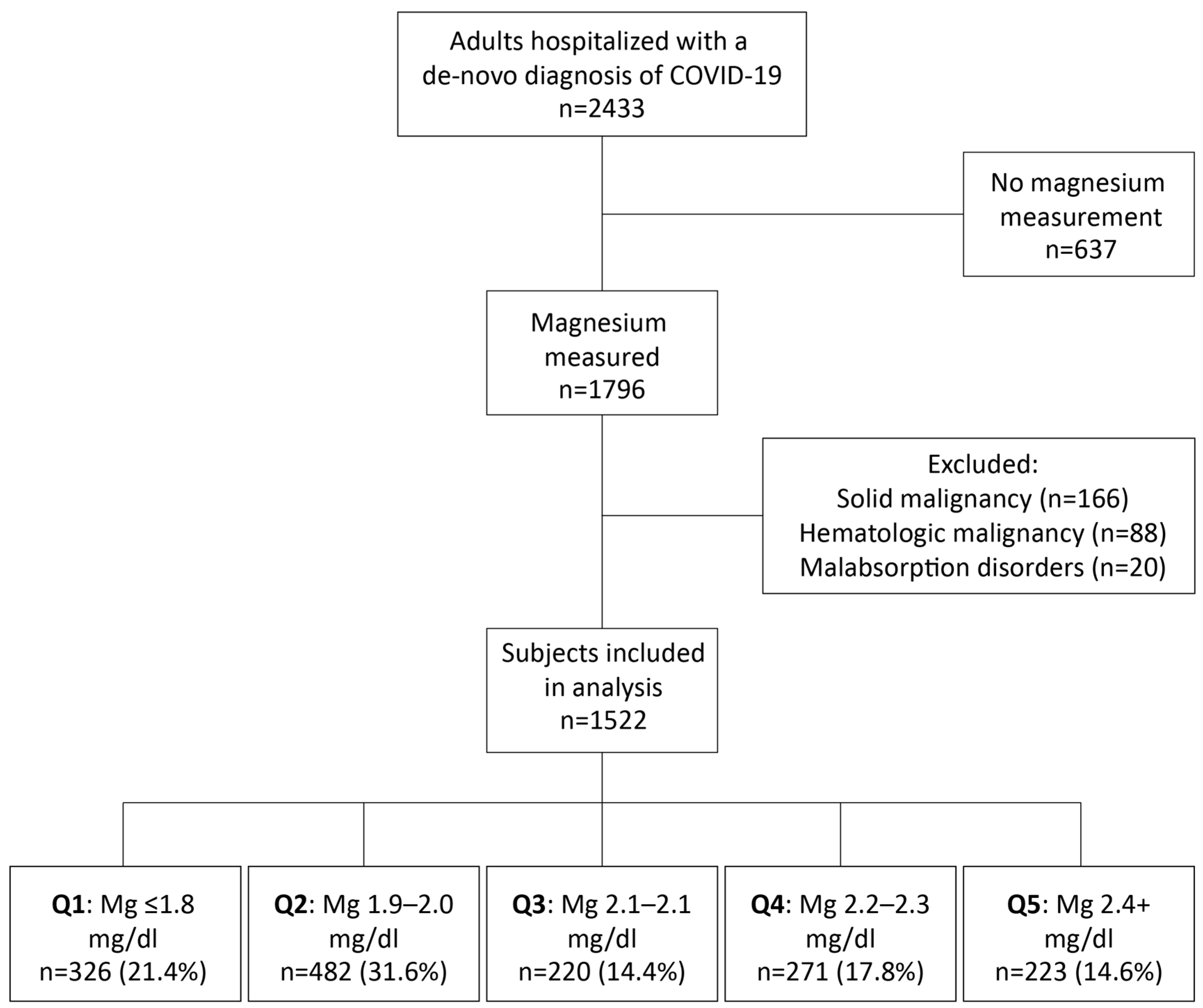
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Patient Characteristics
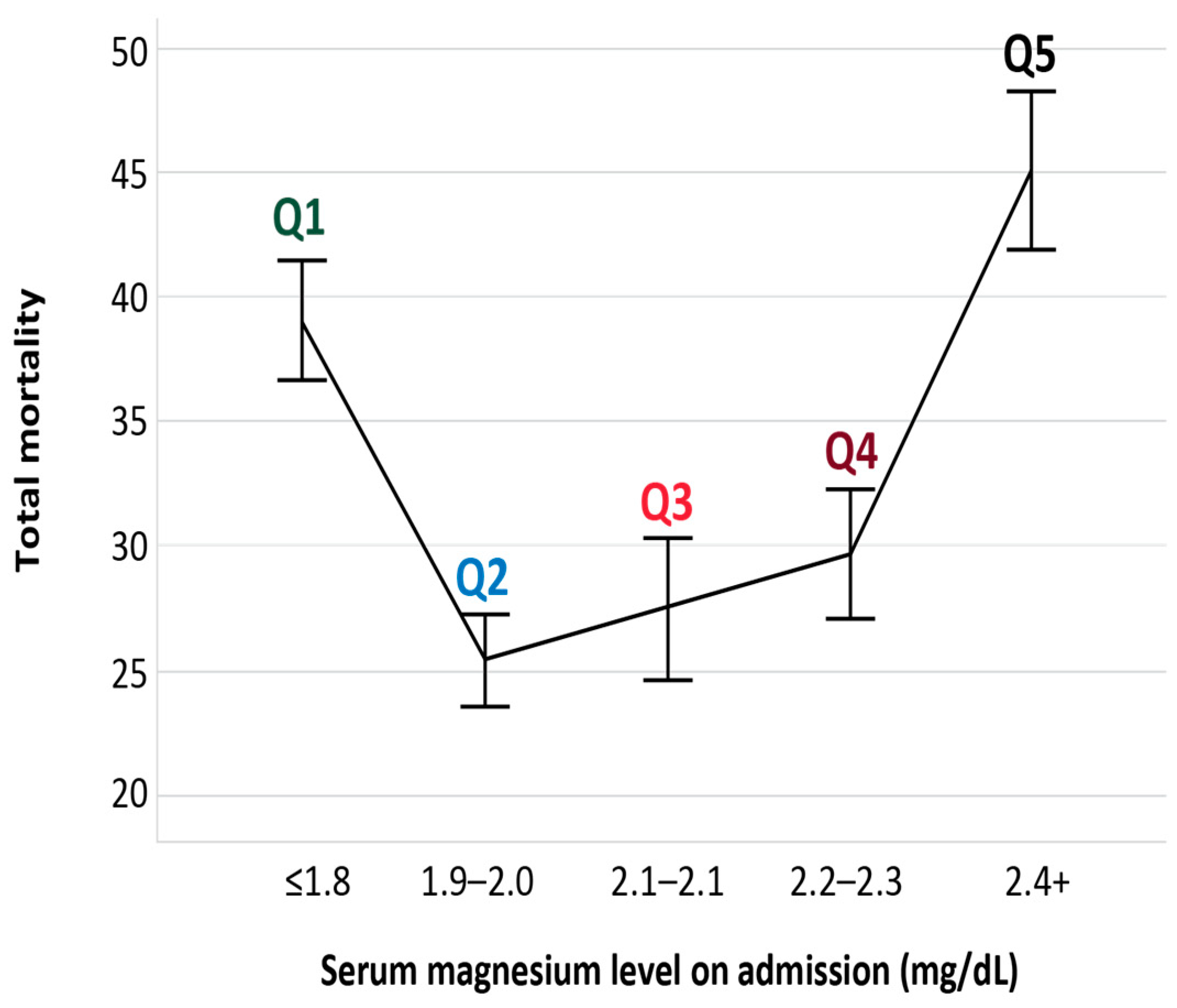
3.2. Outcomes
3.3. Multivariate Analysis
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 12 March 2023).
- Li, P.; Chen, L.; Liu, Z.; Pan, J.; Zhou, D.; Wang, H.; Gong, H.; Fu, Z.; Song, Q.; Min, Q.; et al. Clinical features and short-term outcomes of elderly patients with COVID-19. Int. J. Infect. Dis. 2020, 97, 245–250. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Cai, D.; Zhao, S.; Li, D.; Chang, F.; Tian, X.; Huang, G.; Zhu, Z.; Liu, D.; Dou, X.; Li, S.; et al. Nutrient intake is associated with longevity characterization by metabolites and element profiles of healthy centenarians. Nutrients 2016, 8, 564. [Google Scholar] [CrossRef]
- Saris, N.-E.L.; Mervaala, E.; Karppanen, H.; Khawaja, J.A.; Lewenstam, A. Magnesium. An update on physiological, clinical and analytical aspects. Clin. Chim. Acta 2000, 294, 1–26. [Google Scholar] [CrossRef]
- Seelig, M.S. Consequences of magnesium deficiency on the enhancement of stress reactions; preventive and therapeutic implications (a review). J. Am. Coll. Nutr. 1994, 13, 429–446. [Google Scholar] [CrossRef]
- Rosanoff, A.; West, C.; Elin, R.J.; Micke, O.; Baniasadi, S.; Barbagallo, M.; Campbell, E.; Cheng, F.-C.; Costello, R.B.; Gamboa-Gomez, C.; et al. Recommendation on an updated standardization of serum magnesium reference ranges. Eur. J. Nutr. 2022, 61, 3697–3706. [Google Scholar] [CrossRef] [PubMed]
- Shechter, M. Magnesium and cardiovascular system. Magnes. Res. 2010, 23, 60–72. [Google Scholar]
- Rosanoff, A. Changing crop magnesium concentrations: Impact on human health. Plant Soil 2013, 368, 139–153. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Lüscher, T. COVID-19 is, in the end, an endothelial disease. Eur. Heart J. 2020, 41, 3038–3044. [Google Scholar] [CrossRef] [PubMed]
- Marini, J.J.; Gattinoni, L. Management of COVID-19 Respiratory Distress. JAMA 2020, 323, 2329. [Google Scholar] [CrossRef] [PubMed]
- Middeldorp, S.; Coppens, M.; Haaps, T.F.; Foppen, M.; Vlaar, A.P.; Müller, M.C.; Bouman, C.C.; Beenen, L.F.; Kootte, R.S.; Heijmans, J.; et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 2020, 18, 1995–2002. [Google Scholar] [CrossRef]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Elalamy, I.; Kastritis, E.; Sergentanis, T.N.; Politou, M.; Psaltopoulou, T.; Gerotziafas, G.; Dimopoulos, M.A. Hematological findings and complications of COVID-19. Am. J. Hematol. 2020, 95, 834–847. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.M.; Malpuech-Brugère, C.; Zimowska, W.; Rayssiguier, Y.; Mazur, A. Low magnesium promotes endothelial cell dysfunction: Implications for atherosclerosis, inflammation and thrombosis. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2004, 1689, 13–21. [Google Scholar] [CrossRef]
- Darooghegi Mofrad, M.; Djafarian, K.; Mozaffari, H.; Shab-Bidar, S. Effect of magnesium supplementation on endothelial function: A systematic review and meta-analysis of randomized controlled trials. Atherosclerosis 2018, 273, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Shechter, M.; Sharir, M.; Paul Labrador, M.J.; Forrester, J.; Silver, B.; Noel Bairey Merz, C. Oral Magnesium Therapy Improves Endothelial Function in Patients with Coronary Artery Disease. 2000. Available online: http://www.circulationaha.org (accessed on 21 November 2022).
- Maier, J. Low magnesium and atherosclerosis: An evidence-based link. Mol. Asp. Med. 2003, 24, 137–146. [Google Scholar] [CrossRef]
- Sugimoto, J.; Romani, A.M.; Valentin-Torres, A.M.; Luciano, A.A.; Kitchen, C.M.R.; Funderburg, N.; Mesiano, S.; Bernstein, H.B. Magnesium Decreases Inflammatory Cytokine Production: A Novel Innate Immunomodulatory Mechanism. J. Immunol. 2012, 188, 6338–6346. [Google Scholar] [CrossRef] [PubMed]
- Rude, R.K.; Adams, J.S.; Ryzen, E.; Endres, D.B.; Niimi, H.; Horst, R.L.; Haddad, J.G.; Singer, F.R. Low Serum Concentrations of 1,25-Dihydroxyvitamin D in Human Magnesium Deficiency. J. Clin. Endocrinol. Metab. 1985, 61, 933–940. [Google Scholar] [CrossRef]
- Castiglioni, S.; Cazzaniga, A.; Locatelli, L.; Maier, J.A. Burning magnesium, a sparkle in acute inflammation: Gleams from experimental models. Magnes. Res. 2017, 30, 8–15. [Google Scholar] [CrossRef]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagall, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574. [Google Scholar] [CrossRef]
- Bikdeli, B.; Madhavan, M.; Jimenez, D.; Chuich, T.; Dreyfus, I.; Driggin, E.; Nigoghossian, C.D.; Ageno, W.; Madjid, M.; Guo, Y.; et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 2950–2973. [Google Scholar] [CrossRef] [PubMed]
- Alter, H.J.; Koepsell, T.D.; Hilty, W.M. Intravenous Magnesium as an Adjuvant in Acute Bronchospasm: A Meta-Analysis. Ann. Emerg. Med. 2000, 36, 191–197. [Google Scholar] [CrossRef]
- Zhu, L.; Bao, X.; Bi, J.; Lin, Y.; Shan, C.; Fan, X.; Bian, J.; Wang, X. Serum magnesium in patients with severe acute respiratory syndrome coronavirus 2 from Wuhan, China. Magnes. Res. 2021, 34, 103–113. [Google Scholar]
- Quilliot, D.; Bonsack, O.; Jaussaud, R.; Mazur, A. Dysmagnesemia in COVID-19 cohort patients: Prevalence and associated factors. Magnes. Res. 2020, 33, 114–122. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Mercado, M.; Rodriguez-Moran, M.; Ramírez-Renteria, C.; Martínez-Aguilar, G.; Marrero-Rodríguez, D.; Ferreira-Hermosillo, A.; Simental-Mendía, L.E.; Remba-Shapiro, I.; Gamboa-Gómez, G.I.; et al. Magnesium-to-Calcium Ratio and Mortality from COVID-19. Nutrients 2022, 14, 1686. [Google Scholar] [CrossRef]
- Tan, C.W.; Ho, L.P.; Kalimuddin, S.; Cherng, B.P.; The, Y.E.; Thien, S.Y.; Wong, H.M.; Tern, P.J.W.; Chandran, M.; Chay, J.W.M.; et al. Cohort study to evaluate effect of vitamin D, magnesium, and vitamin B12 in combination on severe outcome progression in older patients with coronavirus (COVID-19). Nutrition 2020, 79–80, 111017. [Google Scholar] [CrossRef]
- Israeli Ministry of Health. A Uniform Definition of Disease Severity in Hospitalized COVID-19 Patients. 2020. Available online: https://www.gov.il/he/departments/publications/reports/mr-294754420 (accessed on 5 December 2022).
- Costello, R.B.; Elin, R.J.; Rosanoff, A.; Wallace, T.C.; Guerrero-Romero, F.; Hruby, A.; Lutsey, P.L.; Nielsen, F.H.; Rodriguez-Moran, M.L.; Song, Y.; et al. Perspective: The Case for an Evidence-Based Reference Interval for Serum Magnesium: The Time Has Come. Adv. Nutr. 2016, 7, 977–993. [Google Scholar] [CrossRef]
- Micke, O.; Vormann, J.; Kraus, A.; Kisters, K. Serum magnesium: Time for a standardized and evidence-based reference range. Magnes. Res. 2021, 34, 84–89. [Google Scholar]
- Pham, P.-C.T.; Pham, P.-M.T.; Pham S v Miller, J.M.; Pham, P.-T.T. Hypomagnesemia in Patients with Type 2 Diabetes. Clinical J. Am. Soc. Nephrol. 2007, 2, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Danziger, J.; William, J.H.; Scott, D.J.; Lee, J.; Lehman, L.-W.; Mark, R.G.; Howell, M.D.; Celi, L.A.; Mukamal, K.J. Proton-pump inhibitor use is associated with low serum magnesium concentrations. Kidney Int. 2013, 83, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Gebhard, C.; Regitz-Zagrosek, V.; Neuhauser, H.K.; Morgan, R.; Klein, S.L. Impact of sex and gender on COVID-19 outcomes in Europe. Biol. Sex Differ. 2020, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.S.; Moses, A.A.; Nickolas, T.L.; Husain, S.A.; Mohan, S. Increased Mortality Associated with Hypermagnesemia in Severe COVID-19 Illness. Kidney360 2021, 2, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Sepandi, M.; Taghdir, M.; Alimohamadi, Y.; Afrashteh, S.; Hosamirudsari, H. Factors Associated with Mortality in COVID-19 Patients: A Systematic Review and Meta-Analysis. Iran. J. Public Health 2020, 49, 1211–1221. [Google Scholar] [CrossRef]
- Henry, B.M.; Lippi, G. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Int. Urol. Nephrol. 2020, 52, 1193–1194. [Google Scholar] [CrossRef]
- Coburn, J.W. The Physicochemical State and Renal Handling of Divalent Ions in Chronic Renal Failure. Arch. Intern. Med. 1969, 124, 302. [Google Scholar] [CrossRef] [PubMed]
- van Laecke, S.; Nagler E v Verbeke, F.; van Biesen, W.; Vanholder, R. Hypomagnesemia and the risk of death and gfr decline in chronic kidney disease. Am. J. Med. 2013, 126, 825–831. [Google Scholar] [CrossRef]
- Sakaguchi, Y.; Fujii, N.; Shoji, T.; Hayashi, T.; Rakugi, H.; Isaka, Y. Hypomagnesemia is a significant predictor of cardiovascular and non-cardiovascular mortality in patients undergoing hemodialysis. Kidney Int. 2014, 85, 174–181. [Google Scholar] [CrossRef]
- Henry, B.M.; de Oliveira, M.H.S.; Benoit, S.; Plebani, M.; Lippi, G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): A meta-analysis. Clin. Chem. Lab. Med. (CCLM) 2020, 58, 1021–1028. [Google Scholar] [CrossRef]
- Kermali, M.; Khalsa, R.K.; Pillai, K.; Ismail, Z.; Harky, A. The role of biomarkers in diagnosis of COVID-19—A systematic review. Life Sci. 2020, 254, 117788. [Google Scholar] [CrossRef]
- Ichkawa, Y.; Wada, H.; Ezaki, M.; Tanaka, M.; Hiromori, S.; Shiraki, K.; Moritani, I.; Yamamoto, A.; Tashiro, H.; Shimpo, H.; et al. Elevated D-Dimer Levels Predict a Poor Outcome in Critically Ill Patients. Clin. Appl. Thromb./Hemost. 2020, 26, 1076029620973084. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H.; Levi, M.; Thachil, J. Coagulopathy in COVID-19. J. Thromb. Haemost. 2020, 18, 2103–2109. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, G.; Gudur, A.; Vela-Ortiz, M.; Jodelka, J.; Livert, D.; Krishnamurthy, M. Where there is sodium there may be sepsis. J. Community Hosp. Intern. Med. Perspect. 2019, 9, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Sarvazad, H.; Cahngaripour, S.H.; Eskandari Roozbahani, N.; Izadi, B. Evaluation of electrolyte status of sodium, potassium and magnesium, and fasting blood sugar at the initial admission of individuals with COVID-19 without underlying disease in Golestan Hospital, Kermanshah. New Microbes New Infect. 2020, 38, 100807. [Google Scholar] [CrossRef] [PubMed]

| Q1: ≤1.8 n = 326 | Q2: 1.9–2.0 n = 482 | Q3: 2.1–2.1 n = 220 | Q4: 2.2–2.3 n = 271 | Q5 ≥ 2.4 n = 223 | Total n = 1522 | p | |
|---|---|---|---|---|---|---|---|
| Age | 69 ± 17 | 67 ± 17 | 68 ± 16 | 66 ± 17 | 70 ± 16 | 68 ± 17 | 0.039 |
| Male gender | 166 (50.9) | 254 (52.7) | 133 (60.5) | 181 (66.8) | 153 (68.6) | 887 (58) | <0.001 |
| BMI | 28 ± 6 | 28 ± 5 | 28 ± 6 | 28 ± 6 | 29 ± 6 | 28.5 ± 6 | 0.533 |
| CHF | 19 (5.8) | 23 (4.8) | 11 (5.0) | 16 (5.9) | 9 (4.0) | 78 (5.1) | 0.854 |
| IHD | 47 (14.4) | 65 (13.5) | 25 (11.4) | 35 (12.9) | 27 (12.1) | 199 (13.1) | 0.854 |
| Hypertension | 102 (31.3) | 150 (31.1) | 69 (31.4) | 96 (35.4) | 73 (32.7) | 490 (32.2) | 0.778 |
| Diabetes | 78 (23.9) | 79 (16.4) | 28 (12.7) | 32 (11.8) | 39 (17.5) | 256 (16.8) | <0.001 |
| Dyslipidemia | 37 (11.3) | 62 (12.9) | 20 (9.1) | 26 (9.6) | 36 (16.1) | 181 (11.9) | 0.117 |
| Stroke/TIA | 39 (12.0) | 59 (12.2) | 22 (10) | 22 (8.1) | 24 (10.8) | 166 (11.0) | 0.460 |
| VTE | 13 (4.0) | 25 (5.2) | 10 (4.5) | 13 (4.8) | 7 (3.1) | 68 (4.5) | 0.781 |
| CKD | 20 (6.1) | 19 (3.9) | 11 (5.0) | 9 (3.3) | 21 (9.4) | 80 (5.3) | 0.017 |
| COPD | 13 (4.0) | 21 (4.4) | 10 (4.5) | 8 (3.0) | 10 (4.5) | 62 (4.1) | 0.878 |
| WBC | 7.4 ± 4.5 | 8 ± 16.8 | 7.6 ± 4.3 | 8.3 ± 8.1 | 9.9 ± 5.3 | 8.2 ± 10.6 | <0.001 |
| Lymphocytes | 1.13 ± 2.07 | 1.9 ± 15.6 | 1.16 ± 1.27 | 1.1 ± 1.42 | 0.95 ± 0.67 | 1.36 ± 8.88 | <0.001 |
| Hemoglobin | 12.13 ± 2.18 | 12.46 ± 2.17 | 12.8 ± 2 | 12.6 ± 2 | 12.6 ± 2 | 12.5 ± 2 | <0.001 |
| Platelets | 207 ± 109 | 207 ± 85 | 222 ± 103 | 209 ± 86 | 246 ± 118 | 215 ± 100 | <0.001 |
| Creatinine | 1.24 ± 0.97 | 1.08 ± 0.86 | 1.11 ± 0.87 | 1.2 ± 1.14 | 1.88 ± 2.02 | 1.26 ± 1.2 | <0.001 |
| CRCL | 71.5 ± 31.7 | 78.2 ± 28.4 | 77.6 ± 27.6 | 76.8 ± 80.2 | 59.5 ± 34.1 | 73.8 ± 30.9 | <0.001 |
| Glucose | 150 ± 89 | 139 ± 67 | 130 ± 62 | 139 ± 73 | 167 ± 86 | 145 ± 77 | <0.001 |
| Sodium | 136 ± 6.4 | 137 ± 5.2 | 137 ± 4.9 | 137 ±5.6 | 139 ± 10 | 137 ± 6.5 | <0.001 |
| Pottasium | 4.2 ± 0.68 | 4.2 ± 0.61 | 4.45 ± 0.73 | 4.39 ±0.68 | 4.48 ± 0.75 | 4.35 ± 0.69 | <0.001 |
| Total calcium | 8.7 ± 0.61 | 8.7 ± 0.58 | 8.82 ± 0.65 | 8.69 ± 0.58 | 8.74 ± 0.67 | 8.76 ± 0.61 | 0.053 |
| ALT | 28.1 ± 24.8 | 33.9 ± 33.5 | 32.7 ± 26 | 48 ± 127.1 | 43.2 ± 41.6 | 36.4 ± 61.3 | <0.001 |
| Troponin | 149 ± 1057 | 122 ± 836 | 183 ± 1534 | 164 ± 898 | 96 ± 246 | 141 ± 975 | 0.904 |
| CRP | 91 ± 86 | 86 ± 80 | 93 ± 85 | 121 ± 91 | 149 ± 104 | 104 ± 91 | <0.001 |
| LDH | 344 ± 160 | 340 ± 155 | 399 ± 230 | 462 ± 481 | 517 ± 322 | 397 ± 286 | <0.001 |
| Ferritin | 544 ± 830 | 540 ± 698 | 579 ± 625 | 844 ±1488 | 1102 ± 1676 | 687 ± 1106 | <0.001 |
| D-dimer | 3074 ± 8001 | 3160 ± 9094 | 2042 ± 3964 | 2861 ± 8578 | 3682 ± 7913 | 3004 ± 8027 | <0.001 |
| INR | 1.14 ± 0.26 | 1.14 ± 0.35 | 1.12 ± 0.18 | 1.18 ± 0.26 | 1.22 ± 0.48 | 1.16 ± 0.32 | <0.001 |
| Antiplatelet | 103 (31.6) | 146 (30.3) | 67 (30.5) | 69 (25.5) | 63 (28.3) | 448 (29.4) | 0.521 |
| Steroids | 23 (7.1) | 22 (4.6) | 5 (2.3) | 10 (3.7) | 4 (1.8) | 64 (4.2) | 0.016 |
| Diuretics | 65 (19.9) | 93 (19.3) | 43 (19.5) | 52 (19.2) | 51 (22.9) | 304(20) | 0.837 |
| CCB | 79 (24.2) | 88 (18.3) | 39 (17.7) | 45 (16.6) | 54 (24.2) | 305 (20) | 0.046 |
| PPI | 92 (28.2) | 109 (22.6) | 52 (23.6) | 48 (17.7) | 49 (22.0) | 350 (23) | 0.049 |
| Q1: ≤1.8 n = 326 | Q2: 1.9–2.0 n = 482 | Q3: 2.1–2.1 n = 220 | Q4: 2.2–2.3 n = 271 | Q5 ≥ 2.4 n = 223 | Total n = 1522 | p | |
|---|---|---|---|---|---|---|---|
| O2 Saturation on admission—% ± SD | 93 ± 6.1 | 93 ± 6.4 | 92 ± 8 | 91 ± 7.7 | 89 ± 10. | 92 ± 7.6 | <0.001 |
| O2 Saturation ≤93% during hospitalization | 266 (84) | 367 (80) | 169 (80) | 222 (85) | 200 (92) | 1224 (83) | <0.001 |
| Hospitalization length of stay—days ± SD | 10.4 ± 17.1 | 10.6 ± 21.3 | 12.9 ± 22.8 | 10.2 ± 16.6 | 13.4 ± 23.4 | 11.2 ± 20.3 | 0.274 |
| Intensive care unit admission | 29 (8.9) | 39 (8.1) | 22 (10) | 30 (11.1) | 34 (15) | 154 (10) | 0.007 |
| In hospital mortality | 53 (19) | 40 (9.5) | 21 (10.7) | 44 (17.8) | 60 (30) | 218 (16) | <0.001 |
| Total mortality | 115 (35) | 113 (23) | 59 (26.8) | 74 (27.3) | 101 (45.3) | 462 (30) | <0.001 |
| HR | 95% CI | p | |
|---|---|---|---|
| Age | 1.050 | 1.042–1.058 | <0.001 |
| Creatinine | 1.134 | 1.072–1.199 | <0.001 |
| ALT | 0.997 | 0.995–0.999 | 0.003 |
| ALK-P | 1.001 | 1.00–1.002 | 0.051 |
| CRP | 1.004 | 1.003–1.005 | <0.001 |
| LDH | 1.001 | 1.001–1.001 | <0.001 |
| Desaturation under 93% | 0.408 | 0.261–0.638 | <0.001 |
| Sodium | 1.033 | 1.020–1.045 | <0.001 |
| Diabetes | 1.372 | 1.087–1.731 | 0.008 |
| sMg Q2 | 1 | 0.009 | |
| sMg Q1 | 1.573 | 1.204–2.055 | <0.001 |
| sMg Q3 | 1.102 | 0.793–1.532 | 0.563 |
| sMg Q4 | 1.051 | 0.772–1.431 | 0.750 |
| sMg sQ5 | 1.288 | 0.090–1.727 | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segev, A.; Sagir, A.; Matetzky, S.; Segev, A.; Atar, S.; Shechter, M. Admission Serum Magnesium Levels Is Associated with Short and Long-Term Clinical Outcomes in COVID-19 Patients. Nutrients 2023, 15, 2016. https://doi.org/10.3390/nu15092016
Segev A, Sagir A, Matetzky S, Segev A, Atar S, Shechter M. Admission Serum Magnesium Levels Is Associated with Short and Long-Term Clinical Outcomes in COVID-19 Patients. Nutrients. 2023; 15(9):2016. https://doi.org/10.3390/nu15092016
Chicago/Turabian StyleSegev, Amitai, Adam Sagir, Shlomi Matetzky, Amit Segev, Shaul Atar, and Michael Shechter. 2023. "Admission Serum Magnesium Levels Is Associated with Short and Long-Term Clinical Outcomes in COVID-19 Patients" Nutrients 15, no. 9: 2016. https://doi.org/10.3390/nu15092016
APA StyleSegev, A., Sagir, A., Matetzky, S., Segev, A., Atar, S., & Shechter, M. (2023). Admission Serum Magnesium Levels Is Associated with Short and Long-Term Clinical Outcomes in COVID-19 Patients. Nutrients, 15(9), 2016. https://doi.org/10.3390/nu15092016







