Consumption of Common Bean Suppresses the Obesogenic Increase in Adipose Depot Mass: Impact of Dose and Biological Sex
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Feeding Study
2.2. Experimental Diets
2.3. Histopathology
2.3.1. Fixation
2.3.2. Hematoxylin and Eosin (H&E) Staining
2.4. Western Blot-Based Nanocapillary Electrophoresis
2.5. RNA Isolation and RNA-Seq Analysis
2.6. Statistical Evaluation
3. Results
3.1. Body Weight Gain
3.2. Anthropometric Data at the End of the Study
3.3. Mechanisms
3.3.1. Hypothesis-Driven Analyses
3.3.2. Data-Driven Analyses
4. Discussion
4.1. Clinical Relevance and Physiological Considerations
4.2. One Size Does Not Fit All
4.3. Underlying Mechanisms
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martin, J.C.; Awoke, M.A.; Misso, M.L.; Moran, L.J.; Harrison, C.L. Preventing weight gain in adults: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2021, 22, e13280. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agriculture; U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025. Available online: https://www.dietaryguidelines.gov/sites/default/files/2020-12/Dietary_Guidelines_for_Americans_2020-2025.pdf (accessed on 21 December 2022).
- Ryan, D.; Barquera, S.; Barata Cavalcanti, O.; Ralston, J. The Global Pandemic of Overweight and Obesity. In Handbook of Global Health; Kickbusch, I., Ganten, D., Moeti, M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 739–773. [Google Scholar]
- Ajmera, R. A Dietitian’s Pick of the 15 Best Weight Loss Programs for 2023. Available online: https://www.healthline.com/nutrition/best-weight-loss-programs (accessed on 15 April 2023).
- Grand View Research. Weight Management Market Size, Share & Trends Analysis Report by Function (Diet, Fitness Equipment, Surgical Equipment, Services), by Region (APAC, North America) and Segment Forecasts, 2022–2030. Available online: https://www.grandviewresearch.com/industry-analysis/weight-management-market (accessed on 15 February 2023).
- Berciano, S.; Figueiredo, J.; Brisbois, T.D.; Alford, S.; Koecher, K.; Eckhouse, S.; Ciati, R.; Kussmann, M.; Ordovas, J.M.; Stebbins, K.; et al. Precision nutrition: Maintaining scientific integrity while realizing market potential. Front. Nutr. 2022, 9, 979665. [Google Scholar] [CrossRef] [PubMed]
- Weeramanthri, T.S.; Dawkins, H.J.S.; Baynam, G.; Bellgard, M.; Gudes, O.; Semmens, J.B. Editorial: Precision Public Health. Front. Public Health 2018, 6, 121. [Google Scholar] [CrossRef]
- Neuhouser, M.L. The importance of healthy dietary patterns in chronic disease prevention. Nutr. Res. 2019, 70, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Dicken, S.J.; Batterham, R.L. The Role of Diet Quality in Mediating the Association between Ultra-Processed Food Intake, Obesity and Health-Related Outcomes: A Review of Prospective Cohort Studies. Nutrients 2022, 14, 23. [Google Scholar] [CrossRef]
- Martini, D.; Godos, J.; Bonaccio, M.; Vitaglione, P.; Grosso, G. Ultra-Processed Foods and Nutritional Dietary Profile: A Meta-Analysis of Nationally Representative Samples. Nutrients 2021, 13, 3390. [Google Scholar] [CrossRef]
- Didinger, C.; Thompson, H.J. Defining nutritional and functional niches of legumes: A Call for clarity to distinguish a future role for pulses in the Dietary Guidelines for Americans. Nutrients 2021, 13, 1100. [Google Scholar] [CrossRef]
- Viguiliouk, E.; Blanco Mejia, S.; Kendall, C.W.C.; Sievenpiper, J.L. Can pulses play a role in improving cardiometabolic health? Evidence from systematic reviews and meta-analyses. Ann. N. Y. Acad. Sci. 2017, 1392, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, H.; Vasconcelos, M.; Gil, A.M.; Pinto, E. Benefits of pulse consumption on metabolism and health: A systematic review of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2021, 61, 85–96. [Google Scholar] [CrossRef]
- Thompson, H.J.; McGinley, J.N.; Neil, E.S.; Brick, M.A. Beneficial Effects of Common Bean on Adiposity and Lipid Metabolism. Nutrients 2017, 9, 998. [Google Scholar] [CrossRef] [PubMed]
- Neil, E.S.; McGinley, J.N.; Fitzgerald, V.K.; Lauck, C.A.; Tabke, J.A.; Streeter-McDonald, M.R.; Yao, L.; Broeckling, C.D.; Weir, T.L.; Foster, M.T.; et al. White Kidney Bean (Phaseolus vulgaris L.) Consumption Reduces Fat Accumulation in a Polygenic Mouse Model of Obesity. Nutrients 2019, 11, 2780. [Google Scholar] [CrossRef] [PubMed]
- Lutsiv, T.; McGinley, J.N.; Neil, E.S.; Foster, M.T.; Thompson, H.J. Thwarting Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD) with Common Bean: Dose- and Sex-Dependent Protection against Hepatic Steatosis. Nutrients 2023, 15, 526. [Google Scholar] [CrossRef]
- Andrea Morán-Costoya, A.M.P.; Gianotti, M.; Lladó, I.; Valle, A. Sex Differences in Nonalcoholic Fatty Liver Disease: Estrogen Influence on the Liver–Adipose Tissue Crosstalk. Antioxid. Redox Signal. 2021, 35, 753–774. [Google Scholar] [CrossRef] [PubMed]
- Krämer, A.; Green, J.; Pollard, J., Jr.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2013, 30, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J.R. Use of high-fat diets to study rodent obesity as a model of human obesity. Int. J. Obes. 2019, 43, 1491–1492. [Google Scholar] [CrossRef]
- Bastías-Pérez, M.; Serra, D.; Herrero, L. Dietary Options for Rodents in the Study of Obesity. Nutrients 2020, 12, 3234. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S. Anatomy of the adipose organ. Eat Weight Disord. 2000, 5, 132–142. [Google Scholar] [CrossRef]
- Kumanyika, S.K. A Framework for Increasing Equity Impact in Obesity Prevention. Am. J. Public Health 2019, 109, 1350–1357. [Google Scholar] [CrossRef]
- Hagdorn, Q.A.J.; Bossers, G.P.L.; Koop, A.-M.C.; Piek, A.; Eijgenraam, T.R.; Feen, D.E.v.d.; Silljé, H.H.W.; Boer, R.A.d.; Berger, R.M.F. A novel method optimizing the normalization of cardiac parameters in small animal models: The importance of dimensional indexing. Am. J. Physiol.-Heart Circ. Physiol. 2019, 316, H1552–H1557. [Google Scholar] [CrossRef]
- Novelli, E.L.; Diniz, Y.S.; Galhardi, C.M.; Ebaid, G.M.; Rodrigues, H.G.; Mani, F.; Fernandes, A.A.; Cicogna, A.C.; Novelli Filho, J.L. Anthropometrical parameters and markers of obesity in rats. Lab Anim. 2007, 41, 111–119. [Google Scholar] [CrossRef]
- Siersbæk, M.S.; Ditzel, N.; Hejbøl, E.K.; Præstholm, S.M.; Markussen, L.K.; Avolio, F.; Li, L.; Lehtonen, L.; Hansen, A.K.; Schrøder, H.D.; et al. C57BL/6J substrain differences in response to high-fat diet intervention. Sci. Rep. 2020, 10, 14052. [Google Scholar] [CrossRef]
- Lutsiv, T.; McGinley, J.N.; Neil-McDonald, E.S.; Weir, T.L.; Foster, M.T.; Thompson, H.J. Relandscaping the Gut Microbiota with a Whole Food: Dose-Response Effects to Common Bean. Foods 2022, 11, 1153. [Google Scholar] [CrossRef]
- McGinley, J.N.; Fitzgerald, V.K.; Neil, E.S.; Omerigic, H.M.; Heuberger, A.L.; Weir, T.L.; McGee, R.; Vandemark, G.; Thompson, H.J. Pulse Crop Effects on Gut Microbial Populations, Intestinal Function, and Adiposity in a Mouse Model of Diet-Induced Obesity. Nutrients 2020, 12, 593. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.T.; Malinowska, E.; Jura, M.; Kozak, L.P. C57BL/6J mice as a polygenic developmental model of diet-induced obesity. Physiol. Rep. 2017, 5, e13093. [Google Scholar] [CrossRef] [PubMed]
- Koza, R.A.; Nikonova, L.; Hogan, J.; Rim, J.S.; Mendoza, T.; Faulk, C.; Skaf, J.; Kozak, L.P. Changes in gene expression foreshadow diet-induced obesity in genetically identical mice. PLoS Genet. 2006, 2, e81. [Google Scholar] [CrossRef]
- Bondarenko, V.; Løkke, C.R.; Dobrowolski, P.; Mentzel, C.J.; Castro-Mejía, J.L.; Hansen, C.H.F.; Sørensen, D.B.; Nielsen, D.S.; Krych, L.; Hansen, A.K. Controlling the uncontrolled variation in the diet induced obese mouse by microbiomic characterization. Sci. Rep. 2022, 12, 13767. [Google Scholar] [CrossRef] [PubMed]
- Ricci, M.R.; Levin, B.E. Ontogeny of diet-induced obesity in selectively bred Sprague-Dawley rats. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2003, 285, R610–R618. [Google Scholar] [CrossRef]
- Koch, L.G.; Britton, S.L. Divergent Selection for Aerobic Capacity in Rats as a Model for Complex Disease. Integr. Comp. Biol. 2005, 45, 405–415. [Google Scholar] [CrossRef]
- Loos, R.J.F.; Yeo, G.S.H. The genetics of obesity: From discovery to biology. Nat. Rev. Genet. 2022, 23, 120–133. [Google Scholar] [CrossRef]
- Turner, N.; Cooney, G.J.; Kraegen, E.W.; Bruce, C.R. Fatty acid metabolism, energy expenditure and insulin resistance in muscle. J. Endocrinol. 2014, 220, T61–T79. [Google Scholar] [CrossRef]
- Balasescu, E.; Pandia, L.D.; Nedelcu, R.I.; Brinzea, A.; Ion, D.A. Obesity—A closer look to cell mechanisms disfunction. Rom. J. Med. Pract. 2021, 16, 77. [Google Scholar] [CrossRef]
- Ji, A.; Trumbauer, A.C.; Noffsinger, V.P.; Jeon, H.; Patrick, A.C.; De Beer, F.C.; Webb, N.R.; Tannock, L.R.; Shridas, P. Serum Amyloid A is not obligatory for high-fat, high-sucrose, cholesterol-fed diet-induced obesity and its metabolic and inflammatory complications. PLoS ONE 2022, 17, e0266688. [Google Scholar] [CrossRef]
- Chakarov, S.; Blériot, C.; Ginhoux, F. Role of adipose tissue macrophages in obesity-related disorders. J. Exp. Med. 2022, 219, 1948. [Google Scholar] [CrossRef] [PubMed]
- Coral, D.E.; Fernandez-Tajes, J.; Tsereteli, N.; Pomares-Millan, H.; Fitipaldi, H.; Mutie, P.M.; Atabaki-Pasdar, N.; Kalamajski, S.; Poveda, A.; Miller-Fleming, T.W.; et al. A phenome-wide comparative analysis of genetic discordance between obesity and type 2 diabetes. Nat. Metab. 2023, 5, 237–247. [Google Scholar] [CrossRef]
- Ruiz-Ojeda, F.J.; Plaza-Díaz, J.; Anguita-Ruiz, A.; Méndez-Gutiérrez, A.; Aguilera, C.M. Adipose Extracellular Matrix Remodeling in Obesity and Insulin Resistance. In Cellular and Biochemical Mechanisms of Obesity; Tappia, P.S., Ramjiawan, B., Dhalla, N.S., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 215–229. [Google Scholar]
- Wu, Y.; Lee, M.-J.; Ido, Y.; Fried, S.K. High-fat diet-induced obesity regulates MMP3 to modulate depot- and sex-dependent adipose expansion in C57BL/6J mice. Am. J. Physiol.-Endocrinol. Metab. 2017, 312, E58–E71. [Google Scholar] [CrossRef]
- Maquoi, E.; Munaut, C.; Colige, A.; Collen, D.; Lijnen, H.R. Modulation of adipose tissue expression of murine matrix metalloproteinases and their tissue inhibitors with obesity. Diabetes 2002, 51, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Dali-Youcef, N.; Vix, M.; Costantino, F.; El-Saghire, H.; Lhermitte, B.; Callari, C.; D’Agostino, J.; Perretta, S.; Paveliu, S.; Gualtierotti, M.; et al. Interleukin-32 Contributes to Human Nonalcoholic Fatty Liver Disease and Insulin Resistance. Hepatol. Commun. 2019, 3, 1205–1220. [Google Scholar] [CrossRef]
- Prashanth, G.; Vastrad, B.; Tengli, A.; Vastrad, C.; Kotturshetti, I. Investigation of candidate genes and mechanisms underlying obesity associated type 2 diabetes mellitus using bioinformatics analysis and screening of small drug molecules. BMC Endocr. Disord. 2021, 21, 80. [Google Scholar] [CrossRef]
- Zhao, C.; Yao, X.; Chen, X.; Wu, W.; Xi, F.; Yang, G.; Yu, T. Knockdown of ubiquitin D inhibits adipogenesis during the differentiation of porcine intramuscular and subcutaneous preadipocytes. Cell Prolif. 2018, 51, e12401. [Google Scholar] [CrossRef] [PubMed]
- West-Eberhard, M.J. Nutrition, the visceral immune system, and the evolutionary origins of pathogenic obesity. Proc. Natl. Acad. Sci. USA 2019, 116, 723–731. [Google Scholar] [CrossRef]
- Khan, S.; Chan, Y.T.; Revelo, X.S.; Winer, D.A. The Immune Landscape of Visceral Adipose Tissue During Obesity and Aging. Front. Endocrinol. 2020, 11, 267. [Google Scholar] [CrossRef] [PubMed]
- Blaszczak, A.M.; Jalilvand, A.; Hsueh, W.A. Adipocytes, Innate Immunity and Obesity: A Mini-Review. Front. Immunol. 2021, 12, 650768. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef] [PubMed]
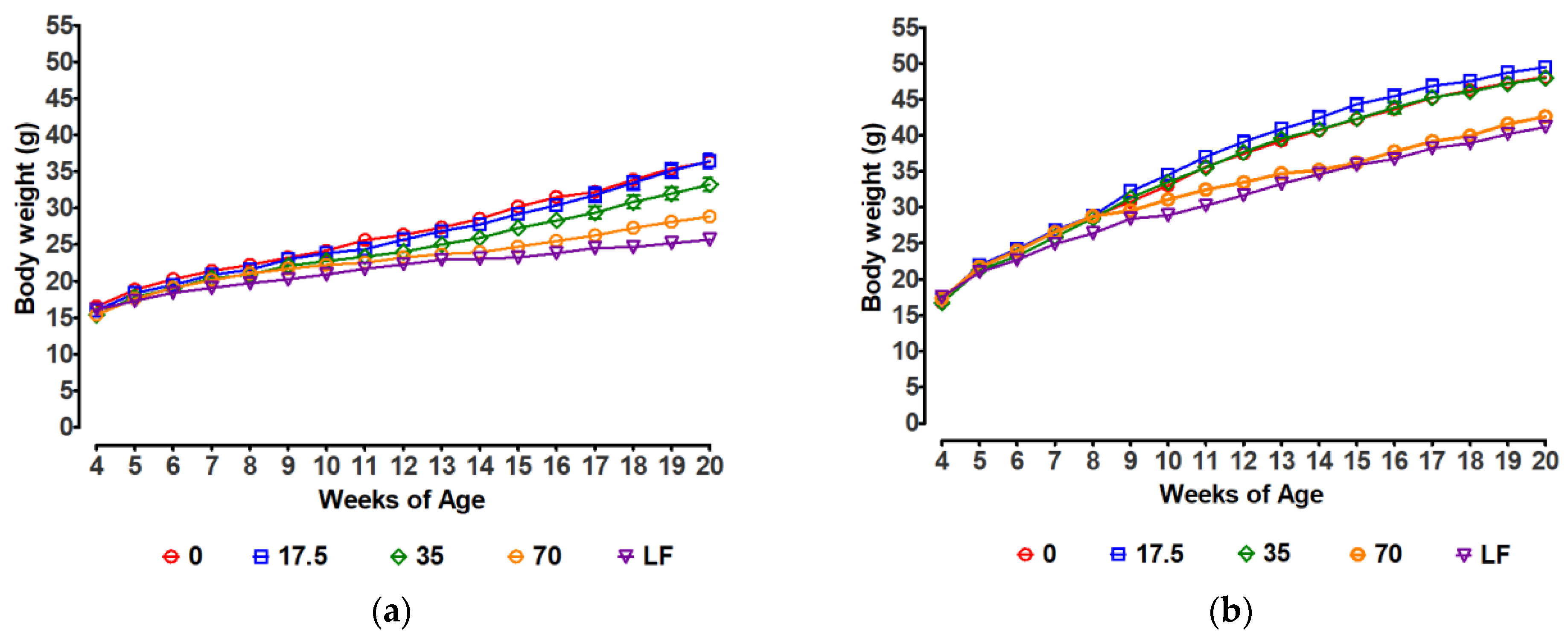
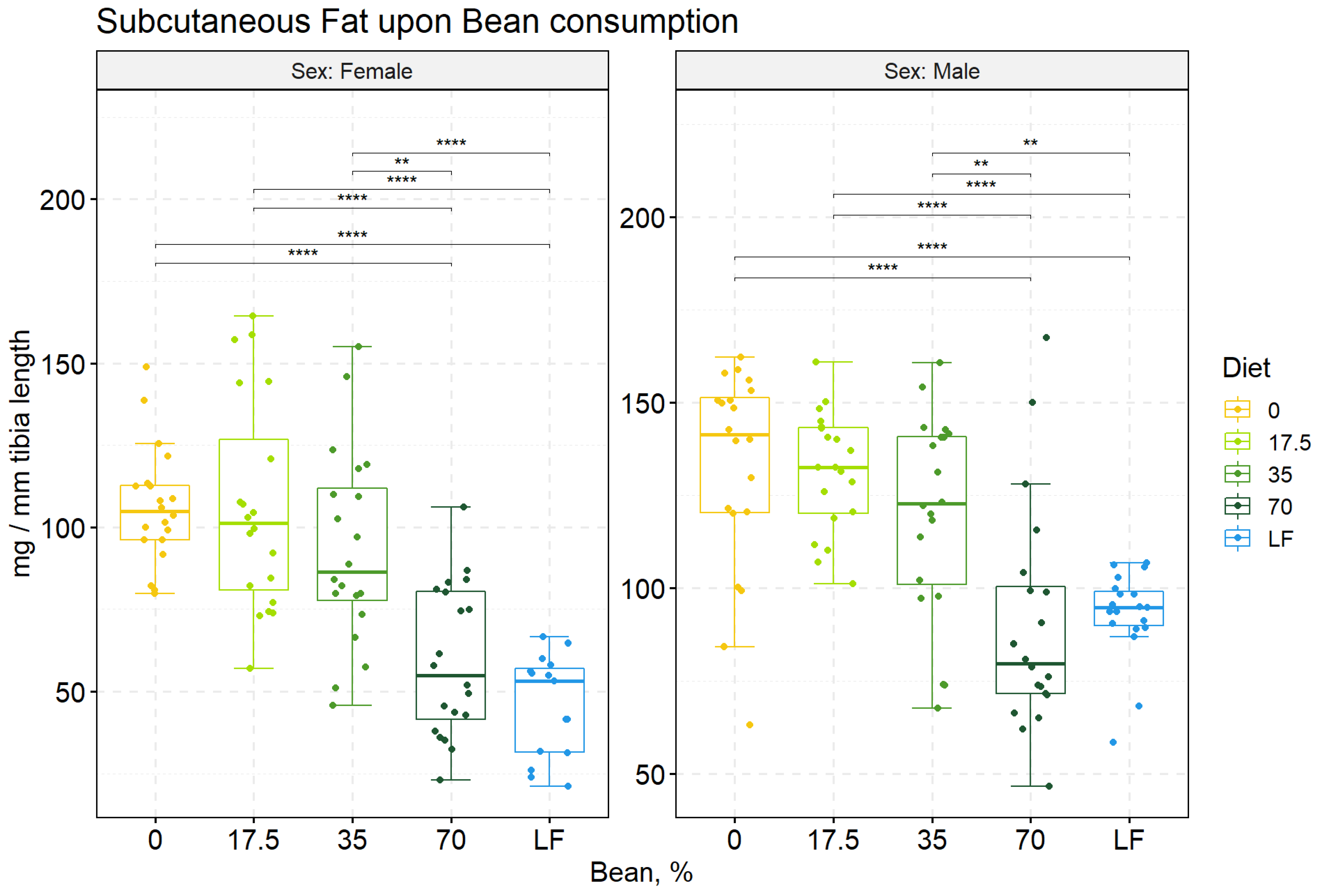




| Females | |||||
|---|---|---|---|---|---|
| Percent of the Total Dietary Protein from Beans | Body Weight, g | Body Mass Index, g/mm | Percent Body Fat, % | Subcutaneous Fat Mass, g/mm | Visceral Fat Mass, g/mm |
| 0 | 36.3 ± 0.6 a | 2.1 ± 0.03 a | 3.8 ± 0.1 a | 106 ± 4 a | 205 ± 7 a |
| 17.5 | 36.4 ± 1.0 a | 2.1 ± 0.05 a | 4.0 ± 0.1 a | 106 ± 7 a | 208 ± 11 a |
| 35 | 33.2 ± 0.9 b | 1.9 ± 0.05 a | 3.6 ± 0.1 a | 93 ± 7 a | 175 ± 12 a |
| 70 | 28.8 ± 0.7 c | 1.7 ± 0.04 b | 2.6 ± 0.1 b | 59 ± 5 b | 119 ± 10 b |
| Low-fat Control | 25.6 ± 0.6 c | 1.5 ± 0.04 b | 2.2 ± 0.1 c | 46 ± 4 b | 76 ± 8 b |
| Males | |||||
| Percent of the Total Dietary Protein from Beans | Body Weight, g | Body Mass Index, g/mm | Percent Body Fat, % | Subcutaneous Fat Mass,g/mm | Visceral Fat Mass, g/mm |
| 0 | 49.2 ± 0.6 a | 2.8 ± 0.05 a | 4.1 ± 0.1 a | 132 ± 6 a | 196 ± 6 a |
| 17.5 | 50.7 ± 0.6 a | 2.9 ± 0.03 a | 4.3 ± 0.1 a | 131 ± 4 a | 198 ± 5 a |
| 35 | 49.1 ± 0.7 a | 2.7 ± 0.04 a | 4.2 ± 0.1 a | 120 ± 6 a | 196 ± 7 a |
| 70 | 44.3 ± 0.7 b | 2.5 ± 0.05 b | 3.5 ± 0.1 b | 90 ± 7 b | 198 ± 7 a |
| Low-fat Control | 42.4 ± 0.7 b | 2.4 ± 0.04 b | 3.7 ± 0.1 b | 93 ± 3 b | 188 ± 4 a |
| Percent of the Total Dietary Protein from Beans | Mesenteric Fat Mass, mg/mm | Perigonadal Fat Mass, mg/mm | Retroperitoneal Fat Mass, mg/mm | |||
|---|---|---|---|---|---|---|
| Females | Males | Females | Males | Females | Males | |
| 0 | 35.2 ± 3.1 a | 65.8 ± 3.1 a | 147.0 ± 6.1 a | 102.9 ± 6.1 a | 22.9 ± 0.9 a | 27.9 ± 0.9 a |
| 17.5 | 39.0 ± 3.1 a | 69.5 ± 3.1 a | 147.0 ± 6.1 a | 100.7 ± 6.1 a | 22.0 ± 0.9 a | 28.2 ± 0.9 a |
| 35 | 32.9 ± 3.1 a | 61.9 ± 3.1 a | 123.4 ± 6.1 a | 106.9 ± 6.1 a | 18.6 ± 0.9 a,b | 27.4 ± 0.9 a |
| 70 | 23.5 ± 3.1 b | 44.6 ± 3.1 b | 82.1 ± 6.1 b | 124.8 ± 6.1 b | 13.2 ± 0.9 c | 29.1 ± 0.9 a |
| Low-fat Control | 15.3 ± 3.6 b | 41.5 ± 3.1 b | 52.1 ± 7.1 b | 120.1 ± 6.1 b | 9.0 ± 1.1 c | 26.1 ± 0.9 a |
| Factorial ANOVA | ||||||
| Diet, p < 0.001 Sex, p < 0.001 | Diet, p < 0.001 Sex, p = 0.8 | Diet, p < 0.001 Sex, p < 0.001 | ||||
| Protein | Fat Mass Tissue | Female, Normalized AU | Male, Normalized AU | Factorial ANOVA | ||
|---|---|---|---|---|---|---|
| 0% | 70% | 0% | 70% | |||
| PPARγ | Mesenteric | 82.6 ± 26.4 | 59.0 ± 26.4 | 153.1 ± 26.4 | 96.9 ± 26.4 | Diet, p = 0.08; Sex, p = 0.058; Tissue, p < 0.001 |
| p = 0.12 | p = 0.08 | |||||
| Subcutaneous | 211.6 ± 26.4 | 195.5 ± 26.4 | 241.9 ± 26.4 | 205.4 ± 26.4 | ||
| p = 0.6 | p = 0.5 | |||||
| SCD | Mesenteric | 41.9 ± 32.6 | 69.4 ± 32.6 | 16.6 ± 2.0 ± 2.0 | 12.0 ± 2.0 | Diet, p = 0.65; Sex, p = 0.099; Tissue, p = 0.047 |
| p = 0.6 | p = 0.13 | |||||
| Subcutaneous | 9.8 ± 1.1 | 10.6 ± 1.1 | 14.1 ± 2.0 | 10.3 ± 2.0 | ||
| p = 0.62 | p = 0.19 | |||||
| FASN | Mesenteric | 31.5 ± 11.3 | 37.3 ± 11.3 | 14.4 ± 5.7 | 7.8 ± 5.7 | Diet, p = 0.51; Sex, p = 0.20; Tissue, p < 0.001 |
| p = 0.7 | p = 0.4 | |||||
| Subcutaneous | 1900 ± 337 | 1676 ± 337 | 1489 ± 313 | 1282 ± 313 | ||
| p = 0.7 | p = 0.6 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thompson, H.J.; Lutsiv, T.; McGinley, J.N.; Fitzgerald, V.K.; Neil, E.S. Consumption of Common Bean Suppresses the Obesogenic Increase in Adipose Depot Mass: Impact of Dose and Biological Sex. Nutrients 2023, 15, 2015. https://doi.org/10.3390/nu15092015
Thompson HJ, Lutsiv T, McGinley JN, Fitzgerald VK, Neil ES. Consumption of Common Bean Suppresses the Obesogenic Increase in Adipose Depot Mass: Impact of Dose and Biological Sex. Nutrients. 2023; 15(9):2015. https://doi.org/10.3390/nu15092015
Chicago/Turabian StyleThompson, Henry J., Tymofiy Lutsiv, John N. McGinley, Vanessa K. Fitzgerald, and Elizabeth S. Neil. 2023. "Consumption of Common Bean Suppresses the Obesogenic Increase in Adipose Depot Mass: Impact of Dose and Biological Sex" Nutrients 15, no. 9: 2015. https://doi.org/10.3390/nu15092015
APA StyleThompson, H. J., Lutsiv, T., McGinley, J. N., Fitzgerald, V. K., & Neil, E. S. (2023). Consumption of Common Bean Suppresses the Obesogenic Increase in Adipose Depot Mass: Impact of Dose and Biological Sex. Nutrients, 15(9), 2015. https://doi.org/10.3390/nu15092015








