Effects of Gut Microbiome Modulation on Reducing Adverse Health Outcomes among Elderly and Diabetes Patients during the COVID-19 Pandemic: A Randomised, Double-Blind, Placebo-Controlled Trial (IMPACT Study)
Abstract
1. Introduction
2. Materials and Methods
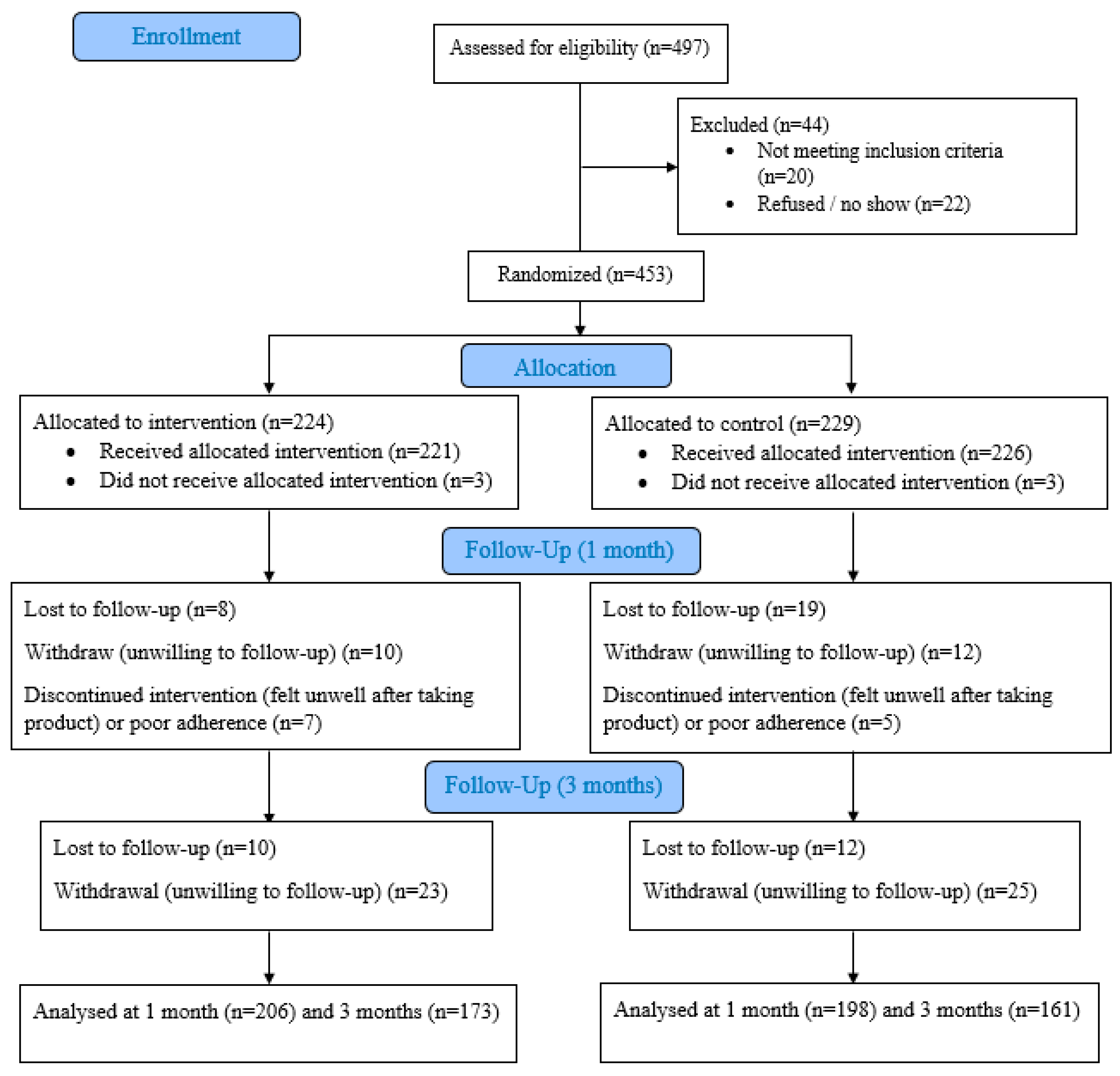
2.1. Study Design and Participants
2.2. Randomisation and Masking
2.3. Study Visits
2.4. Outcome Measures
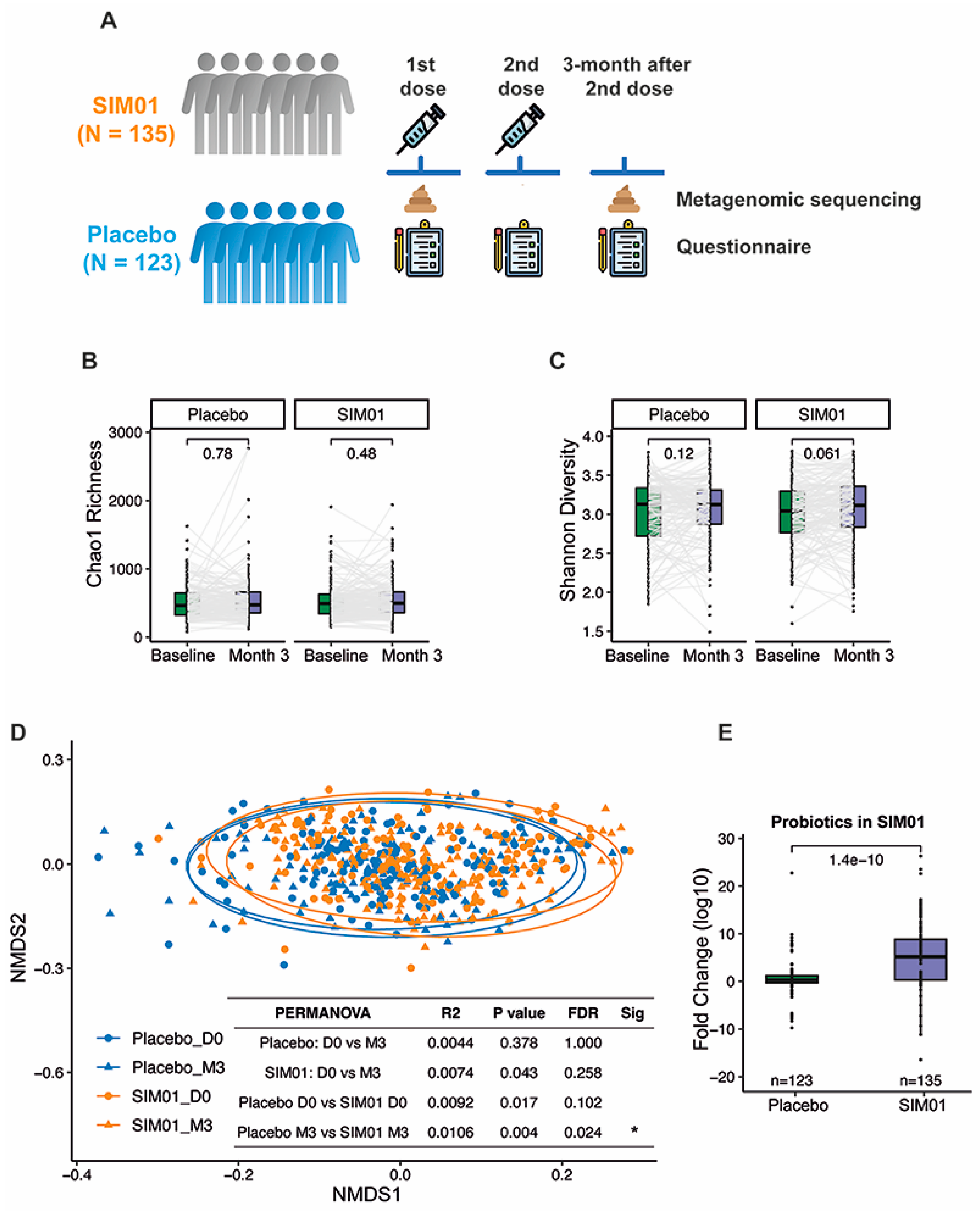
2.5. Stool Metagenomic Sequencing and Profiling
2.6. Statistical Analysis
2.7. Patient and Public Involvement
3. Results
3.1. Participant Characteristics
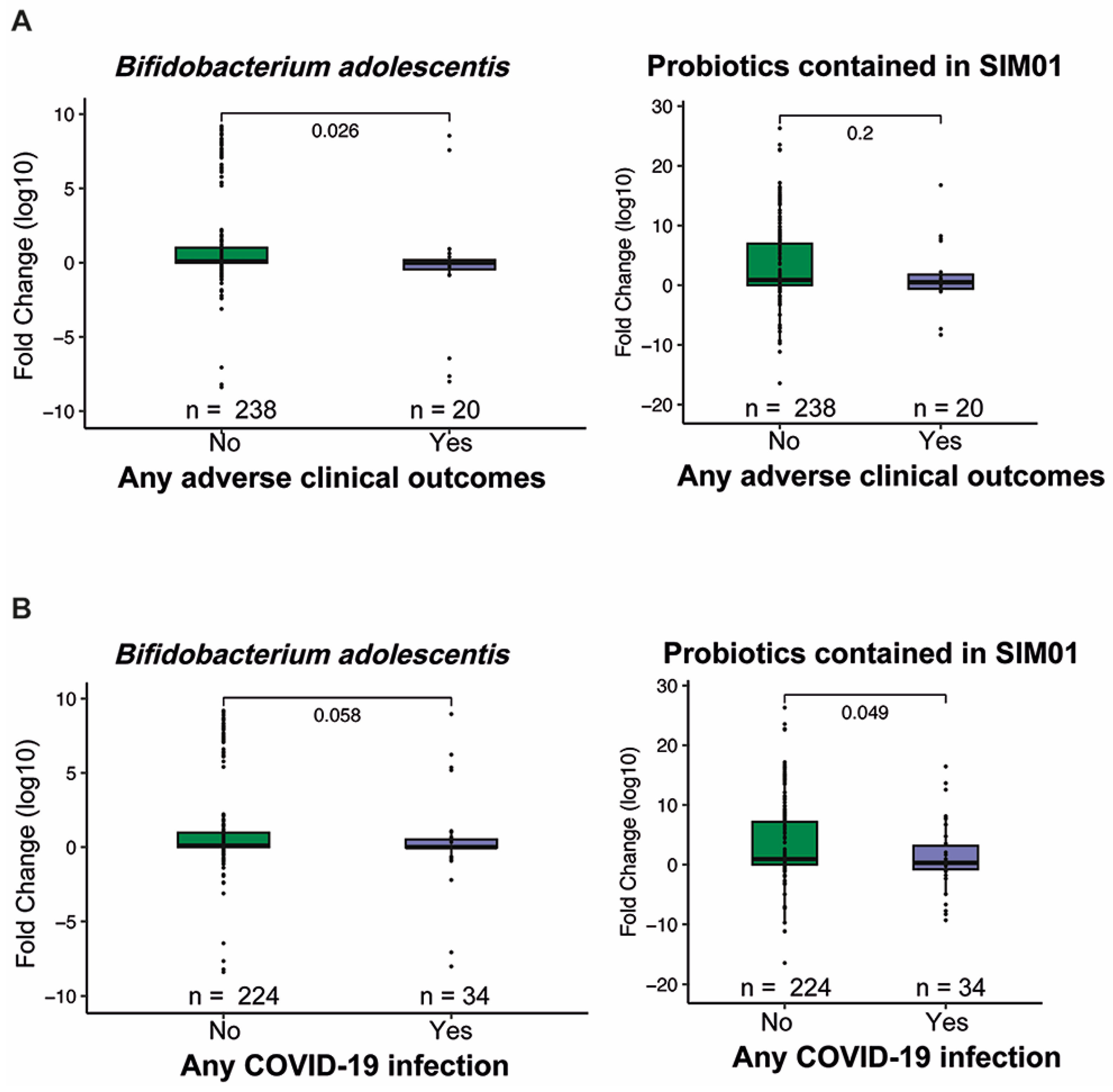
3.2. Adverse Health Outcomes
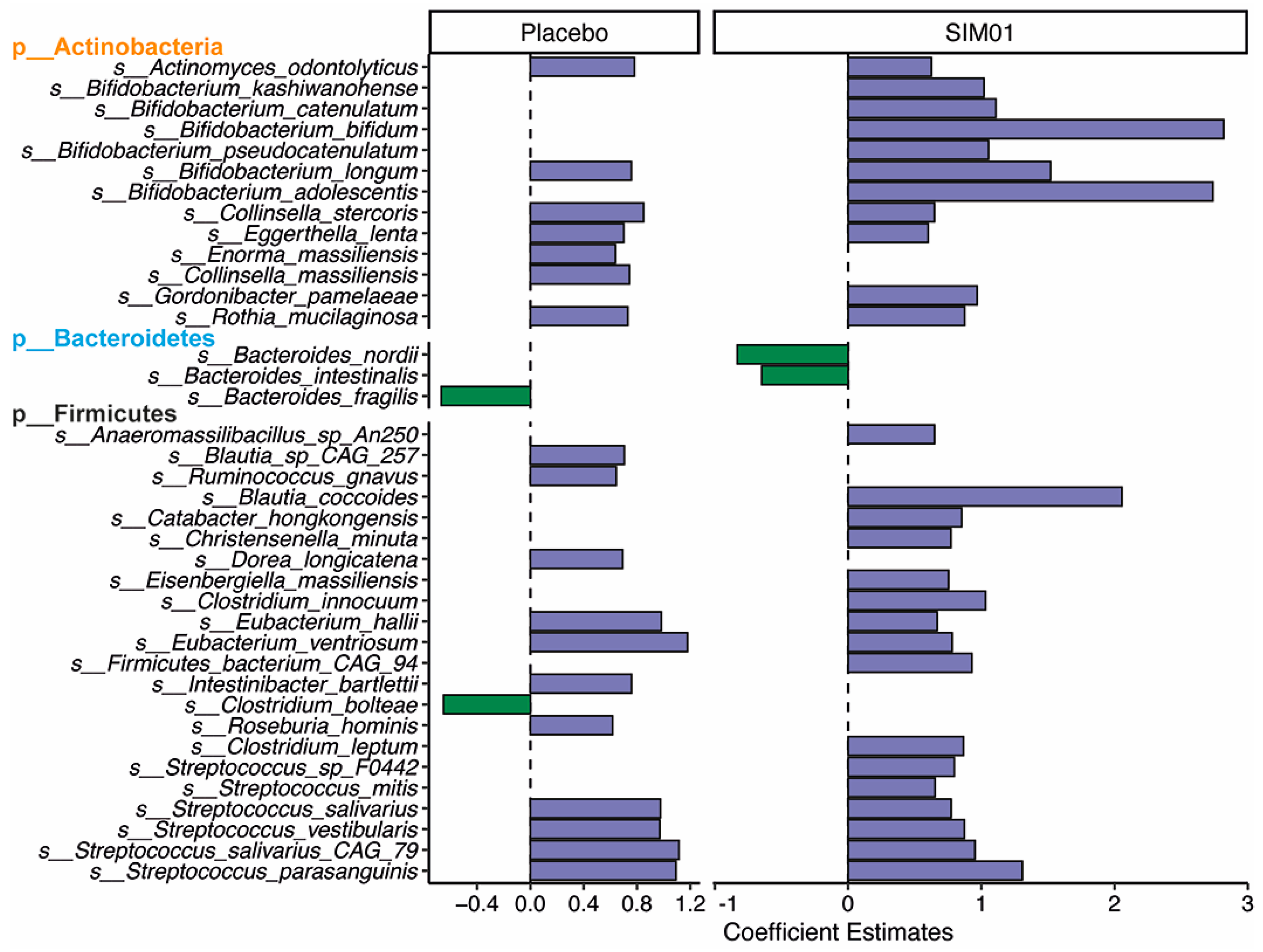
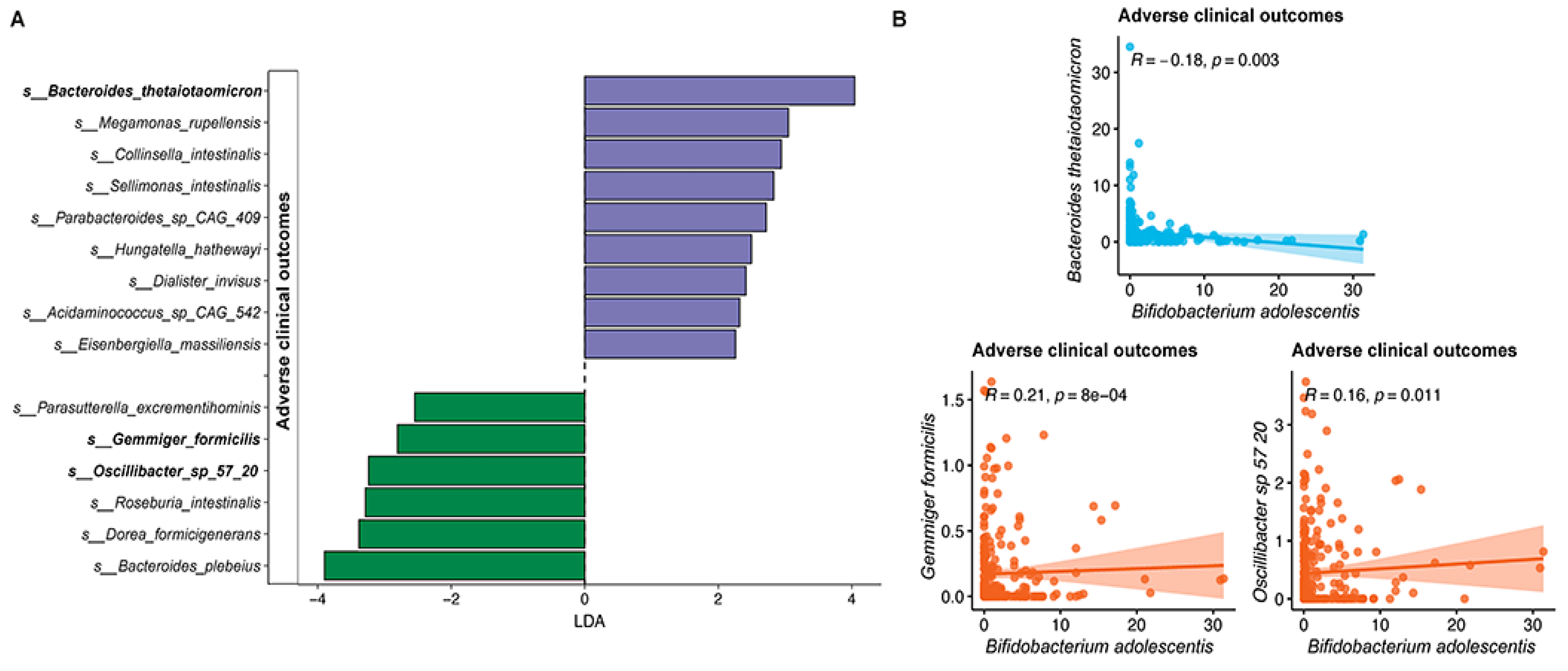
3.3. Faecal Microbiota Composition
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 30 December 2022).
- Gupta, S.; Koirala, J.; Khardori, R.; Khardori, N. Infections in diabetes mellitus and hyperglycemia. Infect Dis. Clin. N. Am. 2007, 21, 617–638. [Google Scholar] [CrossRef] [PubMed]
- Van Crevel, R.; Van de Vijver, S.; Moore, D.A.J. The global diabetes epidemic: What does it mean for infectious diseases in tropical countries? Lancet Diabetes Endocrinol. 2017, 5, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected with SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Casqueiro, J.; Casqueiro, J.; Alves, C. Infections in patients with diabetes mellitus: A review of pathogenesis. Indian J. Endocrinol. Metab. 2012, 16, S27–S36. [Google Scholar] [PubMed]
- Summers, K.L.; Marleau, A.M.; Mahon, J.L.; McManus, R.; Hramiak, I.; Singh, B. Reduced IFN-alpha secretion by blood dendritic cells in human diabetes. Clin. Immunol. 2006, 121, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Xia, C.Q.; Butfiloski, E.; Clare-Salzler, M. Effect of high glucose on cytokine production by human peripheral blood immune cells and type I interferon signaling in monocytes: Implications for the role of hyperglycemia in the diabetes inflammatory process and host defense against infection. Clin. Immunol. 2018, 195, 139–148. [Google Scholar] [CrossRef]
- Bansod, S.; Ahirwar, A.K.; Sakarde, A.; Asia, P.; Gopal, N.; Alam, S.; Kaim, K.; Ahirwar, P.; Sorte, S.R. COVID-19 and geriatric population: From pathophysiology to clinical perspectives. Horm. Mol. Biol. Clin. Investig. 2021, 42, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Mueller, A.L.; McNamara, M.S.; Sinclair, D.A. Why does COVID-19 disproportionately affect older people? Aging 2020, 12, 9959–9981. [Google Scholar] [CrossRef] [PubMed]
- Marquez, E.J.; Chung, C.H.; Marches, R.; Rossi, R.J.; Nehar-Belaid, D.; Eroglu, A.; Mellert, D.J.; Kuchel, G.A.; Banchereau, J.; Ucar, D. Sexual-dimorphism in human immune system aging. Nat. Commun. 2020, 11, 751. [Google Scholar] [CrossRef]
- Jaillon, S.; Berthenet, K.; Garlanda, C. Sexual Dimorphism in Innate Immunity. Clin. Rev. Allergy Immunol. 2019, 56, 308–321. [Google Scholar] [CrossRef]
- El-Sayed, A.; Aleya, L.; Kamel, M. Microbiota’s role in health and diseases. Environ. Sci. Pollut. Res. Int. 2021, 28, 36967–36983. [Google Scholar] [CrossRef] [PubMed]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Bahuguna, A.; Ramalingam, S.; Kim, M. Developing a sustainable bioprocess for the cleaner production of xylooligosaccharides: An approach towards lignocellulosic waste management. J. Clean. Prod. 2021, 316, 128332. [Google Scholar] [CrossRef]
- Yan, F.; Polk, D.B. Probiotics and immune health. Curr. Opin. Gastroenterol. 2011, 27, 496–501. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Z.; Mak, J.W.Y.; Chow, K.M.; Lui, G.; Li, T.C.; Wong, C.K.; Chan, P.K.; Ching, J.Y.; Fujiwara, Y.; et al. Gut microbiota-derived synbiotic formula (SIM01) as a novel adjuvant therapy for COVID-19: An open-label pilot study. J. Gastroenterol. Hepatol. 2022, 37, 823–831. [Google Scholar] [CrossRef]
- Ng, S.C.; Peng, Y.; Zhang, L.; Mok, C.K.P.; Zhao, S.; Li, A.; Ching, J.Y.L.; Liu, Y.; Yan, S.; Chan, D.L.S.; et al. Gut microbiota composition is associated with SARS-CoV-2 vaccine immunogenicity and adverse events. Gut 2022, 71, 1106–1116. [Google Scholar] [CrossRef]
- Institute of Digestive Disease. Available online: https://www.idd.cuhk.edu.hk/40-of-hong-kong-people-show-gut-dysbiosis-comparable-to-that-of-covid-19-patients-cuhk-microbiome-immunity-formula-hastens-recovery-of-covid-19-patients-and-offers-hope-to-boost-immunity/ (accessed on 3 December 2022).
- Zuo, T.; Zhang, F.; Lui, G.C.Y.; Yeoh, Y.K.; Li, A.Y.L.; Zhan, H.; Wan, Y.; Chung, A.C.K.; Cheung, C.P.; Chen, N.; et al. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology 2020, 159, e8. [Google Scholar] [CrossRef]
- Yeoh, Y.K.; Zuo, T.; Lui, G.C.; Zhang, F.; Liu, Q.; Li, A.Y.; Chung, A.C.; Cheung, C.P.; Tso, E.Y.; Fung, K.S.; et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 2021, 70, 698–706. [Google Scholar] [CrossRef]
- Abdelhafiz, A.H.; Emmerton, D.; Sinclair, A.J. Diabetes in COVID-19 pandemic-prevalence, patient characteristics and adverse outcomes. Int. J. Clin. Pract. 2021, 75, e14112. [Google Scholar] [CrossRef] [PubMed]
- Bethel, M.A.; Sloan, F.A.; Belsky, D.; Feinglos, M.N. Longitudinal incidence and prevalence of adverse outcomes of diabetes mellitus in elderly patients. Arch. Intern. Med. 2007, 167, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Parker, C.N.; Parker, T.J.; Kinnear, E.M.; Derhy, P.H.; Alvarado, A.M.; Huygens, F.; Lazzarini, P.A. Incidence and risk factors for developing infection in patients presenting with uninfected diabetic foot ulcers. PLoS ONE 2017, 12, e0177916. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, L.; Qi, Y.; Xu, J.; Ge, Q.; Fan, Y.; Chen, D.; Zhang, Y.; Wang, L.; Hou, T.; et al. Bifidobacterium adolescentis regulates catalase activity and host metabolism and improves healthspan and lifespan in multiple species. Nat. Aging 2021, 1, 991–1001. [Google Scholar] [CrossRef]
- Zhang, F.; Lau, R.I.; Liu, Q.; Su, Q.; Chan, F.K.L.; Ng, S.C. Gut microbiota in COVID-19: Key microbial changes, potential mechanisms and clinical applications. Nat. Rev. Gastroenterol. Hepatol. 2022, 1–15. [Google Scholar] [CrossRef]
- Wischmeyer, P.E.; Tang, H.; Ren, Y.; Bohannon, L.; Ramirez, Z.E.; Andermann, T.M.; Messina, J.A.; Sung, J.A.; Jensen, D.; Jung, S.-H.; et al. Efficacy of Probiotic Treatment as Post-Exposure Prophylaxis for COVID-19: A Double-Blind, Placebo Controlled Randomized Trial. Res. Sq. 2022; preprint. [Google Scholar] [CrossRef]
- Su, Q.; Lau, R.I.; Liu, Q.; Chan, F.K.L.; Ng, S.C. Post-acute COVID-19 syndrome and gut dysbiosis linger beyond 1 year after SARS-CoV-2 clearance. Gut, 2022; Epub ahead of print. [Google Scholar] [CrossRef]

| Microbiome Immunity Formula SIM01 (n = 224) | Control (n = 229) | All Subjects (n = 453) | |
|---|---|---|---|
| Age (mean, SD), years | 67.4 (8.3) | 67.6 (8.0) | 67.5 (8.1) |
| Sex (n, %) | |||
| Female | 107 (47.8) | 121 (52.8) | 228 (50.3) |
| Male | 117 (52.2) | 108 (47.2) | 225 (49.7) |
| Body weight (mean; SD), kg | 64.0 (13.6) | 62.9 (13.2) | 63.7 (13.5) |
| Smoking status (n, %) | |||
| Current | 13 (5.8) | 15 (6.6) | 28 (6.2) |
| Ex-smoker | 26 (11.6) | 15 (6.6) | 41 (9.1) |
| Nonsmoker | 185 (82.6) | 199 (86.8) | 384 (84.7) |
| Alcohol drinking (n, %) | |||
| Current drinker | 31 (13.84) | 17 (7.42) | 48 (10.60) |
| Exdrinker | 7 (3.13) | 11 (4.81) | 18 (3.97) |
| Nondrinker | 186 (83.0) | 201 (87.8) | 387 (85.4) |
| Chronic disease (n, %) | |||
| Hypertension | 115 (51.3) | 108 (47.1) | 223 (49.2) |
| Diabetes Mellitus | 111 (49.5) | 104 (45.4) | 215 (47.5) |
| Hyperlipidaemia | 108 (48.2) | 105 (45.8) | 213 (47) |
| Fatty Liver | 9 (4) | 15 (6.6) | 24 (5.3) |
| Gout | 8 (3.5) | 9 (3.9) | 17 (3.8) |
| Cardiovascular Disease | 17 (7.6) | 21 (9.2) | 38 (8.4) |
| OSAS | 6 (2.7) | 10 (4.4) | 16 (3.5) |
| Asthma | 9 (4) | 5 (2.2) | 14 (3.1) |
| CVA | 2 (0.9) | 4 (1.7) | 6 (1.3) |
| Use of chronic medications (n, %) | |||
| Antihypertensive agents | 103 (46.0) | 104 (45.4) | 207 (45.7) |
| Oral hypoglycemic agents/insulin | 76 (33.9) | 66 (28.8) | 142 (31.3) |
| Lipid-lowering agents | 97 (43.3) | 94 (41.0) | 191 (42.2) |
| Antiplatelets/anticoagulants | 39 (17.4) | 39 (17.0) | 78 (17.2) |
| H2 receptor antagomist/PPI | 58 (25.9) | 59 (25.8) | 117 (25.8) |
| Others * | 97 (43.3) | 93 (40.6) | 190 (41.9) |
| Month 1 | Month 3 | |
|---|---|---|
| Microbiome immunity formula SIM01 | 6/206 (2.9%) | 0/173 (0%) |
| Gastrointestinal: Bloating (1) Constipation (3) Diarrhoea (1) Immunological: Dermatitis (1) | ||
| Vitamin C | 25/198 (12.6%) Gastrointestinal: Bloating (3) Constipation (5) Diarrhoea (3) Abdominal discomfort (2) Loss of appetite (1) Immunological: Eczematous rash (2) Urticarial rash (1) Allergic skin reaction (2) Infection: Infected liver cyst (1) Infected wound (4) Septic shock (1) | 5/161 (3.1%) Gastrointestinal: Constipation (2) Infection: Wound infection (2) COVID-19 (1) |
| p value | ||
| Overall | 0.0002 * | 0.0252 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, M.C.S.; Zhang, L.; Ching, J.Y.L.; Mak, J.W.Y.; Huang, J.; Wang, S.; Mok, C.K.P.; Wong, A.; Chiu, O.-L.; Fung, Y.-T.; et al. Effects of Gut Microbiome Modulation on Reducing Adverse Health Outcomes among Elderly and Diabetes Patients during the COVID-19 Pandemic: A Randomised, Double-Blind, Placebo-Controlled Trial (IMPACT Study). Nutrients 2023, 15, 1982. https://doi.org/10.3390/nu15081982
Wong MCS, Zhang L, Ching JYL, Mak JWY, Huang J, Wang S, Mok CKP, Wong A, Chiu O-L, Fung Y-T, et al. Effects of Gut Microbiome Modulation on Reducing Adverse Health Outcomes among Elderly and Diabetes Patients during the COVID-19 Pandemic: A Randomised, Double-Blind, Placebo-Controlled Trial (IMPACT Study). Nutrients. 2023; 15(8):1982. https://doi.org/10.3390/nu15081982
Chicago/Turabian StyleWong, Martin C. S., Lin Zhang, Jessica Y. L. Ching, Joyce W. Y. Mak, Junjie Huang, Shilan Wang, Chris K. P. Mok, Angie Wong, Oi-Lee Chiu, Yee-Ting Fung, and et al. 2023. "Effects of Gut Microbiome Modulation on Reducing Adverse Health Outcomes among Elderly and Diabetes Patients during the COVID-19 Pandemic: A Randomised, Double-Blind, Placebo-Controlled Trial (IMPACT Study)" Nutrients 15, no. 8: 1982. https://doi.org/10.3390/nu15081982
APA StyleWong, M. C. S., Zhang, L., Ching, J. Y. L., Mak, J. W. Y., Huang, J., Wang, S., Mok, C. K. P., Wong, A., Chiu, O.-L., Fung, Y.-T., Cheong, P.-K., Tun, H.-M., Ng, S. C., & Chan, F. K. L. (2023). Effects of Gut Microbiome Modulation on Reducing Adverse Health Outcomes among Elderly and Diabetes Patients during the COVID-19 Pandemic: A Randomised, Double-Blind, Placebo-Controlled Trial (IMPACT Study). Nutrients, 15(8), 1982. https://doi.org/10.3390/nu15081982








