Biophysical Parameters of Plasma-Derived Extracellular Vesicles as Potential Biomarkers of Bone Disturbances in Breast Cancer Patients Receiving an Individualized Nutrition Intervention
Abstract
1. Introduction
2. Materials and Methods
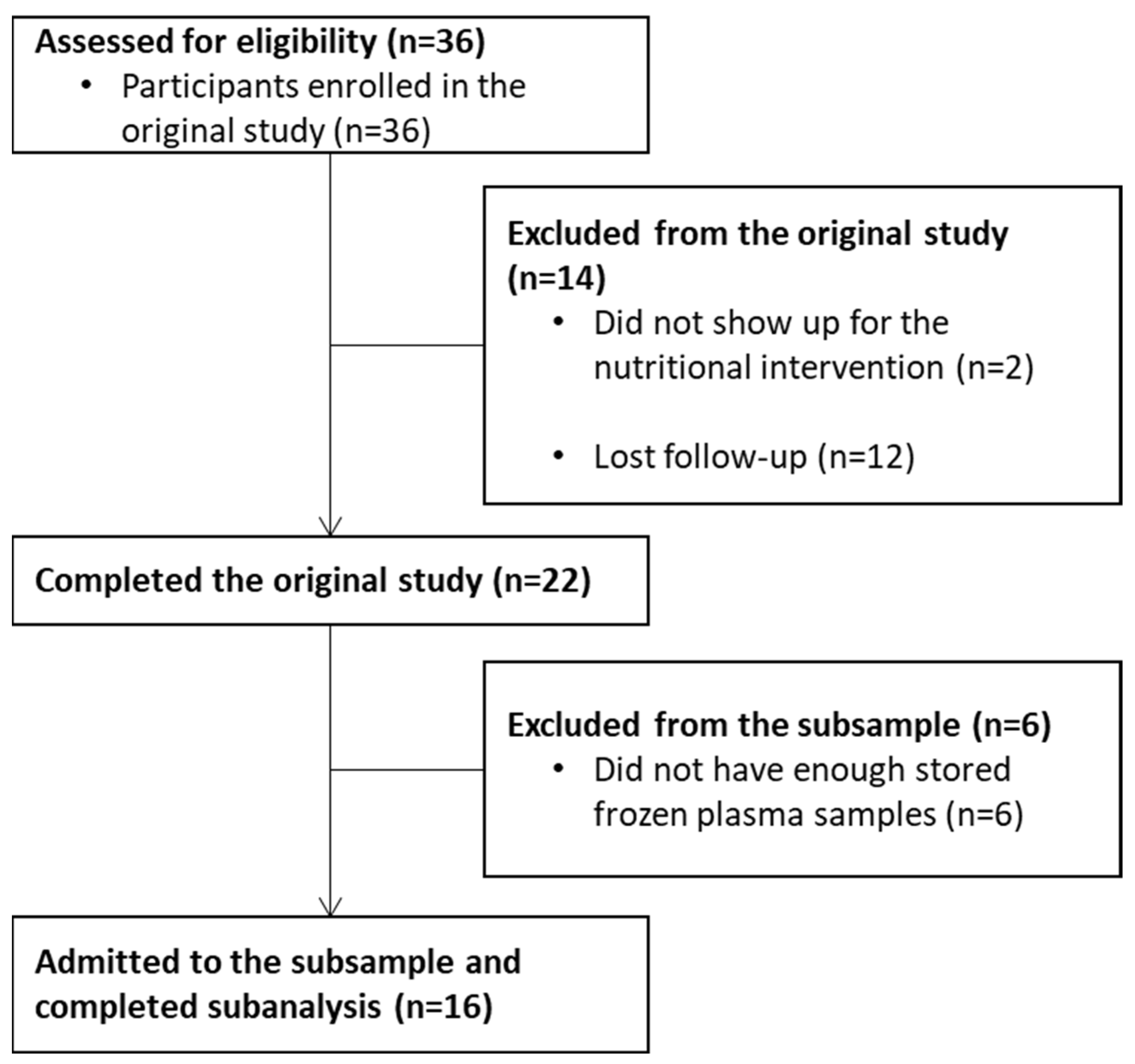
2.1. Study Design and Subjects
2.2. Blood Samples Management
2.3. Extracellular Vesicles Isolation
2.4. Light-Scattering Techniques
2.5. Antibody Array
2.6. Transmission Electron Microscopy (TEM)
2.7. Bone Densitometry and Body Composition Assessment
2.8. Statistical Analysis
3. Results
3.1. Changes in the Subsample after the Nutrition Intervention
3.2. Biophysical Properties of Plasma-Derived EVs
3.3. Associations between Bone Densitometry and Biophysical Properties of Plasma-Derived EVs
3.4. Associations between Biophysical Properties of Plasma-Derived EVs and Neoplasm Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Monroy-Cisneros, K.; Esparza-Romero, J.; Guevara-Torres, A.G.; Valencia, M.E.; Méndez-Estrada, R.O.; Tortoledo Ortiz, O.; Pacheco Moreno, B.I.; Astiazarán-García, H. Impacto Del Tratamiento Antineoplásico En El Estado Nutricional En Pacientes Con Cáncer de Mama. Nutr. Hosp. 2014, 30, 876–882. [Google Scholar]
- Fang, Q.; Huang, J.; Gan, L.; Shen, K.; Chen, X.; Wu, B. Weight Gain during Neoadjuvant Chemotherapy Is Associated with Worse Outcome among the Patients with Operable Breast Cancer. J. Breast Cancer 2019, 22, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, A.; Copson, E.; Eccles, D.; Durcan, L.; Howell, A.; Morris, J.; Howell, S.; Mcdiarmid, S.; Sellers, K.; Evans, D.G.; et al. Predictors of Weight Gain in a Cohort of Premenopausal Early Breast Cancer Patients Receiving Chemotherapy. Breast 2019, 45, 1–6. [Google Scholar] [CrossRef]
- Trestini, I.; Carbognin, L.; Monteverdi, S.; Zanelli, S.; de Toma, A.; Bonaiuto, C.; Nortilli, R.; Fiorio, E.; Pilotto, S.; Di, M.; et al. Clinical Implication of Changes in Body Composition and Weight in Patients with Early-Stage and Metastatic Breast Cancer. Crit. Rev. Oncol. Hematol. 2018, 129, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Crozier, J.; Moreno-Aspitia, A.; Ballman, K.; Dueck, A.; Pockaj, B.; Perez, E. Effect of Body Mass Index (BMI) on Tumor Characteristics and Disease-Free Survival in Patients from the HER2-Positive Adjuvant Trastuzumab Trial N9831. Cancer 2013, 119, 2447–2454. [Google Scholar] [CrossRef]
- Iwase, T.; Wang, X.; Shrimanker, T.V.; Kolonin, M.G.; Ueno, N.T. Body Composition and Breast Cancer Risk and Treatment: Mechanisms and Impact. Breast Cancer Res. Treat. 2021, 186, 273–283. [Google Scholar] [CrossRef]
- Limon-Miro, A.T.; Lopez-Teros, V.; Astiazaran-Garcia, H. Dietary Guidelines for Breast Cancer Patients: A Critical Review. Adv. Nutr. 2017, 8, 613–623. [Google Scholar] [CrossRef]
- Taverna, S.; Giusti, I.; D’ascenzo, S.; Pizzorno, L.; Dolo, V. Breast Cancer Derived Extracellular Vesicles in Bone Metastasis Induction and Their Clinical Implications as Biomarkers. Int. J. Mol. Sci. 2020, 21, 3573. [Google Scholar] [CrossRef]
- Brook, N.; Brook, E.; Dharmarajan, A.; Dass, C.R.; Chan, A. Breast Cancer Bone Metastases: Pathogenesis and Therapeutic Targets. Int. J. Biochem. Cell. Biol. 2018, 96, 63–78. [Google Scholar] [CrossRef]
- Hadji, P. Cancer Treatment-Induced Bone Loss in Women with Breast Cancer. Bonekey Rep. 2015, 4, 692. [Google Scholar] [CrossRef] [PubMed]
- Shemanko, C.; Cong, Y.; Forsyth, A. What Is Breast in the Bone? Int. J. Mol. Sci. 2016, 17, 1764. [Google Scholar] [CrossRef] [PubMed]
- Kelly, O.J.; Gilman, J.C.; Boschiero, D.; Ilich, J.Z. Osteosarcopenic Obesity: Current Knowledge, Revised Identification Criteria and Treatment Principles. Nutrients 2019, 11, 747. [Google Scholar] [CrossRef] [PubMed]
- Samuelson, I.; Vidal-Puig, A.J. Fed-EXosome: Extracellular Vesicles and Cell–Cell Communication in Metabolic Regulation. Essays Biochem. 2018, 62, 165–175. [Google Scholar]
- Kumar, D.N.; Chaudhuri, A.; Aqil, F.; Dehari, D.; Munagala, R.; Singh, S.; Gupta, R.C.; Agrawal, A.K. Exosomes as Emerging Drug Delivery and Diagnostic Modality for Breast Cancer: Recent Advances in Isolation and Application. Cancers 2022, 14, 1435. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Caponnetto, F.; Manini, I.; Skrap, M.; Palmai-pallag, T.; di Loreto, C.; Beltrami, A.P.; Cesselli, D.; Ferrari, E. Size-Dependent Cellular Uptake of Exosomes. Nanomedicine 2016, 13, 1011–1020. [Google Scholar] [CrossRef]
- Rupert, D.L.M.; Claudio, V.; Lässer, C.; Bally, M. Methods for the Physical Characterization and Quantification of Extracellular Vesicles in Biological Samples. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 3164–3179. [Google Scholar] [CrossRef]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of Secretion and Uptake of Exosomes and Other Extracellular Vesicles for Cell-to-Cell Communication. Nat. Cell. Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef]
- Mendivil-Alvarado, H.; Sosa-León, L.A.; Carvajal-Millan, E.; Astiazaran-Garcia, H. Malnutrition and Biomarkers: A Journey through Extracellular Vesicles. Nutrients 2022, 14, 1002. [Google Scholar] [CrossRef]
- Limon-Miro, A.T.; Lopez-Teros, V.; Astiazaran-Garcia, H. Dynamic Macronutrient Meal-Equivalent Menu Method: Towards Individual Nutrition Intervention Programs. Methods Protoc. 2019, 2, 78. [Google Scholar] [CrossRef] [PubMed]
- Limon-Miro, A.T.; Valencia, M.E.; Lopez-Teros, V.; Alemán-Mateo, H.; Méndez-Estrada, R.O.; Pacheco-Moreno, B.I.; Astiazaran-Garcia, H. An Individualized Food-Based Nutrition Intervention Reduces Visceral and Total Body Fat While Preserving Skeletal Muscle Mass in Breast Cancer Patients under Antineoplastic Treatment. Clin. Nutr. 2021, 40, 4394–4403. [Google Scholar] [CrossRef] [PubMed]
- Limon-Miro, A.T.; Valencia, M.E.; Lopez-Teros, V.; Guzman-Leon, A.E.; Mendivil-Alvarado, H.; Astiazaran-Garcia, H. Bioelectric Impedance Vector Analysis (BIVA) in Breast Cancer Patients: A Tool for Research and Clinical Practice. Medicina 2019, 55, 663. [Google Scholar] [CrossRef] [PubMed]
- Vanitallie, T.B.; Yang, M.-U.; Heymsfield, S.B.; Funk, R.C.; Boileau, R.A. Height-Normalized Mass: Potentially Useful Indicators of Nutritional Status. Am. J. Clin. Nutr. 1990, 52, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, C.L. Osteoporosis: A Long-Term and Late-Effect of Breast Cancer Treatments. Cancers 2020, 12, 3094. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, C.L.; Manola, J.; Leboff, M. Ovarian Failure after Adjuvant Chemotherapy Is Associated with Rapid Bone Loss in Women with Early-Stage Breast Cancer. J. Clin. Oncol. 2001, 19, 3306–3311. [Google Scholar] [CrossRef]
- Bainbridge, K.E.; Sowers, M.F.; Crutchfield, M.; Lin, X.; Jannausch, M.; Harlow, S.D. Natural History of Bone Loss over 6 Years among Premenopausal and Early Postmenopausal Women. Am. J. Epidemiol. 2002, 156, 410–417. [Google Scholar] [CrossRef]
- Verweij, F.J.; Balaj, L.; Boulanger, C.M.; Carter, D.R.F.; Compeer, E.B.; D’Angelo, G.; el Andaloussi, S.; Goetz, J.G.; Gross, J.C.; Hyenne, V.; et al. The Power of Imaging to Understand Extracellular Vesicle Biology in Vivo. Nat. Methods 2021, 18, 1013–1026. [Google Scholar] [CrossRef]
- Ansari, S.; de Wildt, B.W.M.; Vis, M.A.M.; de Korte, C.E.; Ito, K.; Hofmann, S.; Yuana, Y. Matrix Vesicles: Role in Bone Mineralization and Potential Use as Therapeutics. Pharmaceuticals 2021, 14, 289. [Google Scholar] [CrossRef]
- Ciardiello, C.; Migliorino, R.; Leone, A.; Budillon, A. Large Extracellular Vesicles: Size Matters in Tumor Progres-sion. Cytokine Growth Factor. Rev. 2020, 51, 69–74. [Google Scholar] [CrossRef]
- Li, N.; Huang, Z.; Zhang, X.; Song, X.; Xiao, Y. Reflecting Size Differences of Exosomes by Using the Combination of Membrane-Targeting Viscosity Probe and Fluorescence Lifetime Imaging Microscopy. Anal. Chem. 2019, 91, 15308–15316. [Google Scholar] [CrossRef]
- Kato, K.; Kobayashi, M.; Hanamura, N.; Akagi, T.; Kosaka, N.; Ochiya, T.; Ichiki, T. Electrokinetic Evaluation of Individual Exosomes by On-Chip Microcapillary Electrophoresis with Laser Dark-Field Microscopy. Jpn. J. Appl. Phys. 2013, 52, 06GK10. [Google Scholar] [CrossRef]
- Yokoi, A.; Ochiya, T. Exosomes and Extracellular Vesicles: Rethinking the Essential Values in Cancer Biology. Semin. Cancer Biol. 2021, 74, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Woo, H.; Lee, C.; Min, Y.; Kumar, S.; Sunkara, V.; Jo, H.; Lee, Y.J.; Kim, J.; Ha, H.K.; et al. EV-Ident: Identi-fying Tumor-Speci Fi c Extracellular Vesicles by Size Fractionation and Single-Vesicle Analysis. Anal. Chem. 2020, 92, 6010–6018. [Google Scholar] [CrossRef] [PubMed]
- Bondar, O.V.; Saifullina, D.v.; Shakhmaeva, I.I.; Mavlyutova, I.I.; Abdullin, T.I. Monitoring of the Zeta Potential of Human Cells upon Reduction in Their Viability and Interaction with Polymers. Acta Nat. 2012, 4, 78–81. [Google Scholar] [CrossRef]
- Hartsuiker, L.; Zeijen, N.J.L.; Terstappen, L.W.M.M.; Otto, C. A Comparison of Breast Cancer Tumor Cells with Varying Expression of the Her2/Neu Receptor by Raman Microspectroscopic Imaging. Analyst 2010, 135, 3220–3226. [Google Scholar] [CrossRef]
- Kim, I.C.; Lee, J.H.; Bang, G.; Choi, S.H.; Kim, Y.H.; Kim, K.P.; Kim, H.K.; Ro, J. Lipid Profiles for HER2-Positive Breast Cancer. Anticancer Res. 2013, 33, 2467–2472. [Google Scholar]
- Mukerjee, S.; Saeedan, A.S.; Ansari, M.N. Polyunsaturated Fatty Acids Mediated Regulation of Membrane Bio-chemistry and Tumor Cell Membrane Integrity. Membranes 2021, 11, 479. [Google Scholar] [CrossRef]
- Ferrara, D.; Montecucco, F.; Dallegri, F.; Carbone, F. Impact of Different Ectopic Fat Depots on Cardiovascular and Metabolic Diseases. J. Cell. Physiol. 2019, 234, 21630–21641. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Zhang, Q.; Tuo, H.; Bin, L.; Mu, Z.; Ju, T.; Xiong, K. How Does Temperature Play a Role in the Storage of Extracellular Vesicles? J. Cell. Physiol. 2020, 235, 7663–7680. [Google Scholar] [CrossRef]
| Clinical Characteristics | Premenopausal BCPs (n = 9) | Postmenopausal BCPs (n = 7) |
|---|---|---|
| Age (years old) | 39 ± 5 | 61 ± 5 |
| Body weight (kg) | 75.5 ± 16 | 68.7 ± 14 |
| Body Mass Index (kg/m2) | 29.3 ± 6 | 26.7 ± 4 |
| Normal weight (n, (%)) | 2 (22.2) | 4 (57.1) |
| Overweight (n, (%)) | 3 (33.3) | 0 (0) |
| Obese (n, (%)) | 4 (44.4) | 3 (42.9) |
| Fat Mass Index (kg/m2) | 12.1 ± 3 | 10.7 ± 3 |
| Abdominal fat (kg) | 2.8 ± 1 | 2.4 ± 2 |
| ASMI (kg/m2) | 5.6 ± 1 | 4.9 ± 1 |
| Physical activity level according to IPAQ (n, (%)) | ||
| Low physical activity level | 5 (55.6) | 5 (71.4) |
| Moderate physical activity level | 4 (44.4) | 2 (28.6) |
| Tumor grade (n, (%)) | ||
| Grade 1 | 1 (11.1) | 1 (14.3) |
| Grade 2 | 6 (66.7) | 6 (85.7) |
| Grade 3 | 2 (22.2) | 0 (0) |
| Molecular type of tumor (n, (%)) | ||
| Luminal A | 3 (33.3) | 4 (57.1) |
| Luminal B | 4 (44.4) | 2 (28.6) |
| HER2/Neu | 1 (11.1) | 1 (14.3) |
| Triple negative | 1 (11.1) | 0 (0) |
| Anatomical Regions | Premenopausal BCPs (n = 9) | Postmenopausal BCPs (n = 7) | ||||
|---|---|---|---|---|---|---|
| Bone Densitometry Parameters | Before | After | p * | Before | After | p * |
| Total body scan | ||||||
| BMC (g) | 2078.7 (487.5) | 2119.2 (457.9) | 0.95 | 2001.2 (613.3) | 1989.4 (810.6) | 0.31 |
| BMD (g/cm2) | 1.17 (0.10) | 1.17 (0.11) | 0.67 | 1.18 (0.38) | 1.17 (0.27) | 1.00 |
| Lumbar spine scan | ||||||
| BMC (g) | 54.80 (9.91) | 57.92 (1.90) | 0.95 | 48.70 (11.57) | 51.13 (10.97) | 1.00 |
| BMD (g/cm2) | 1.02 (0.11) | 0.98 (0.09) | 0.02 | 0.96 (0.29) | 0.914 (0.25) | 0.06 |
| Z-score (SD) | 0.10 (1.20) | -0.20 (1.00) | 0.02 | 0.70 (1.60) | 0.60 (1.90) | 0.12 |
| T-score (SD) | - | - | - | −0.70 (1.90) | −1.20 (2.40) | 0.14 |
| Proximal femur scan | ||||||
| Total hip BMC (g) | 34.42 (4.24) | 33.74 (5.98) | 0.76 | 26.63 (7.97) | 26.95 (6.45) | 0.86 |
| Total hip BMD (g/cm2) | 1.02 (0.08) | 1.00 (0.05) | 0.01 | 0.82 (0.15) | 0.80 (0.12) | 0.04 |
| Total hip Z-score (SD) | 0.50 (0.06) | 0.60 (0.50) | 0.14 | 0.10 (2.10) | 0.00 (1.00) | 0.04 |
| Total hip T-score (SD) | - | - | - | −1.00 (1.20) | −1.10 (0.90) | 0.04 |
| Femoral neck BMC (g) | 3.50 (0.94) | 3.95 (0.54) | 0.28 | 2.83 (1.06) | 2.70 (0.86) | 0.12 |
| Femoral neck BMD (g/cm2) | 0.88 (0.25) | 0.16 (0.28) | 0.28 | 0.69 (0.13) | 0.69 (0.13) | 0.02 |
| Femoral neck Z-score (SD) | 0.20 (1.50) | 1.50 (0.33) | 0.33 | 0.00 (1.10) | −0.30 (0.80) | 0.04 |
| Femoral neck T-score (SD) | - | - | - | −1.50 (1.2) | −1.50 (0.80) | 0.34 |
| Predicted Bone Densitometry Parameter | Model 1 * | Model 2 ** | ||
|---|---|---|---|---|
| Explanatory Variables | β | p | β | p |
| Lumbar spine BMD (g/cm2) | ||||
| Large plasma-derived EVs Dh (nm) | 0.00007 | 0.01 | 0.0001 | <0.01 |
| Body weight (kg) | 0.00637 | <0.01 | 0.006 | <0.01 |
| Age (years old) | - | −0.003 | 0.63 | |
| Menopausal status | ||||
| Premenopausal | - | Ref. | ||
| Postmenopausal | - | 0.035 | 0.78 | |
| Lumbar spine Z-score (SD) | ||||
| Large plasma-derived EVs %I (%) | −0.00449 | <0.01 | −0.00461 | <0.01 |
| Fat Mass Index (kg/m2) | 0.15649 | <0.01 | 0.16050 | <0.01 |
| Age (years old) | - | −0.03274 | 0.50 | |
| Menopausal status | ||||
| Premenopausal | - | Ref. | ||
| Postmenopausal | - | 1.404 | 0.22 | |
| Femoral neck BMC (g) | ||||
| Large plasma-derived EVs Dh (nm) | −0.00074 | <0.01 | −0.00072 | <0.01 |
| Age (years old) | −0.05672 | <0.01 | −0.08105 | 0.03 |
| Coffee consumption (cups/day) | 0.15564 | <0.01 | 0.08087 | 0.09 |
| Body weight (kg) | - | 0.02871 | 0.01 | |
| Menopausal status | ||||
| Premenopausal | - | Ref. | ||
| Postmenopausal | - | 0.93931 | 0.30 | |
| Total hip BMD (g/cm2) | ||||
| Large plasma-derived EVs %I (%) | −0.00021 | <0.01 | −0.00019 | 0.02 |
| Time of measurement | ||||
| Before treatment | Ref. | Ref. | ||
| Six months of treatment | −0.01758 | <0.01 | −0.015513 | <0.01 |
| Menopausal status | ||||
| Premenopausal | Ref. | Ref. | ||
| Postmenopausal | −0.15982 | <0.01 | −0.20204 | 0.05 |
| Age (years old) | - | 0.00237 | 0.26 | |
| Body weight (kg) | - | 0.00117 | <0.01 | |
| Predicted Biophysical Property | Model 1 * | Model 2 ** | ||
|---|---|---|---|---|
| Explanatory Variables | β | p | β | p |
| Large plasma-derived EVs Dh (nm) | ||||
| Molecular type of tumor | ||||
| Luminal A | −417.67 | <0.01 | −438.68 | <0.01 |
| Luminal B | −336.86 | 0.02 | −331.04 | 0.04 |
| HER2/Neu | −485.90 | <0.01 | −480.47 | <0.01 |
| Triple negative | Ref. | Ref. | ||
| Physical activity (min/week) | 0.44 | 0.03 | 0.43 | 0.06 |
| Storage time (months) | - | 0.00 | 0.99 | |
| Temperature at measurement (°C) | - | 0.54 | 0.58 | |
| Small plasma-derived EVs Dh (nm) | ||||
| Molecular type of tumor | ||||
| HER2/Neu | −44.55 | 0.04 | −46.17 | 0.06 |
| Triple negative | Ref. | Ref. | ||
| Storage time (months) | - | −0.65 | 0.60 | |
| Temperature at measurement (°C) | - | 2.81 | 0.97 | |
| ζ potential (mV) | ||||
| HER2 status | ||||
| Overexpressed | −1.59 | <0.01 | −1.68 | <0.01 |
| Normally expressed | Ref. | Ref. | ||
| Ki-67 | ||||
| ≤15% | Ref. | Ref. | ||
| >15% | −0.93 | 0.02 | −1.15 | 0.02 |
| Abdominal fat (kg) | 0.42 | 0.01 | 0.42 | 0.01 |
| Storage time (months) | - | 0.03 | 0.39 | |
| Temperature at measurement (°C) | - | −2.19 | 0.43 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coronado-Alvarado, C.D.; Limon-Miro, A.T.; Mendivil-Alvarado, H.; Lizardi-Mendoza, J.; Carvajal-Millan, E.; Méndez-Estrada, R.O.; González-Ríos, H.; Astiazaran-Garcia, H. Biophysical Parameters of Plasma-Derived Extracellular Vesicles as Potential Biomarkers of Bone Disturbances in Breast Cancer Patients Receiving an Individualized Nutrition Intervention. Nutrients 2023, 15, 1963. https://doi.org/10.3390/nu15081963
Coronado-Alvarado CD, Limon-Miro AT, Mendivil-Alvarado H, Lizardi-Mendoza J, Carvajal-Millan E, Méndez-Estrada RO, González-Ríos H, Astiazaran-Garcia H. Biophysical Parameters of Plasma-Derived Extracellular Vesicles as Potential Biomarkers of Bone Disturbances in Breast Cancer Patients Receiving an Individualized Nutrition Intervention. Nutrients. 2023; 15(8):1963. https://doi.org/10.3390/nu15081963
Chicago/Turabian StyleCoronado-Alvarado, Carlos D., Ana Teresa Limon-Miro, Herminia Mendivil-Alvarado, Jaime Lizardi-Mendoza, Elizabeth Carvajal-Millan, Rosa Olivia Méndez-Estrada, Humberto González-Ríos, and Humberto Astiazaran-Garcia. 2023. "Biophysical Parameters of Plasma-Derived Extracellular Vesicles as Potential Biomarkers of Bone Disturbances in Breast Cancer Patients Receiving an Individualized Nutrition Intervention" Nutrients 15, no. 8: 1963. https://doi.org/10.3390/nu15081963
APA StyleCoronado-Alvarado, C. D., Limon-Miro, A. T., Mendivil-Alvarado, H., Lizardi-Mendoza, J., Carvajal-Millan, E., Méndez-Estrada, R. O., González-Ríos, H., & Astiazaran-Garcia, H. (2023). Biophysical Parameters of Plasma-Derived Extracellular Vesicles as Potential Biomarkers of Bone Disturbances in Breast Cancer Patients Receiving an Individualized Nutrition Intervention. Nutrients, 15(8), 1963. https://doi.org/10.3390/nu15081963








