Effect of a Multispecies Synbiotic Supplementation on Body Composition, Antioxidant Status, and Gut Microbiomes in Overweight and Obese Subjects: A Randomized, Double-Blind, Placebo-Controlled Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Body Composition Assessment
2.4. Biochemical Assessment
2.5. Gut Microbiome Analysis
2.6. Dietary Assessment
2.7. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Body Composition Measurement
3.3. Biochemical Blood Profiles
3.4. Dietary Measurement
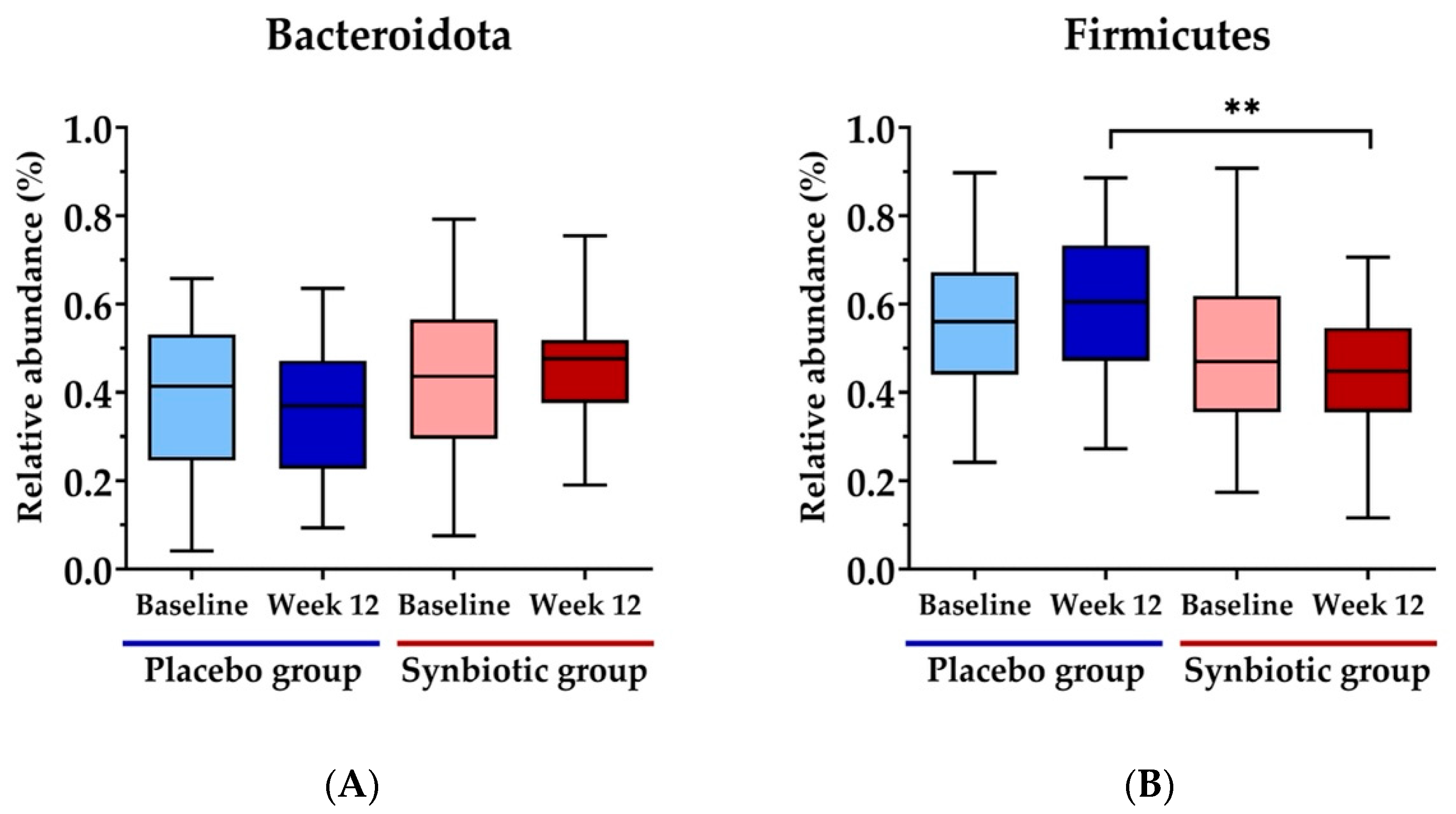
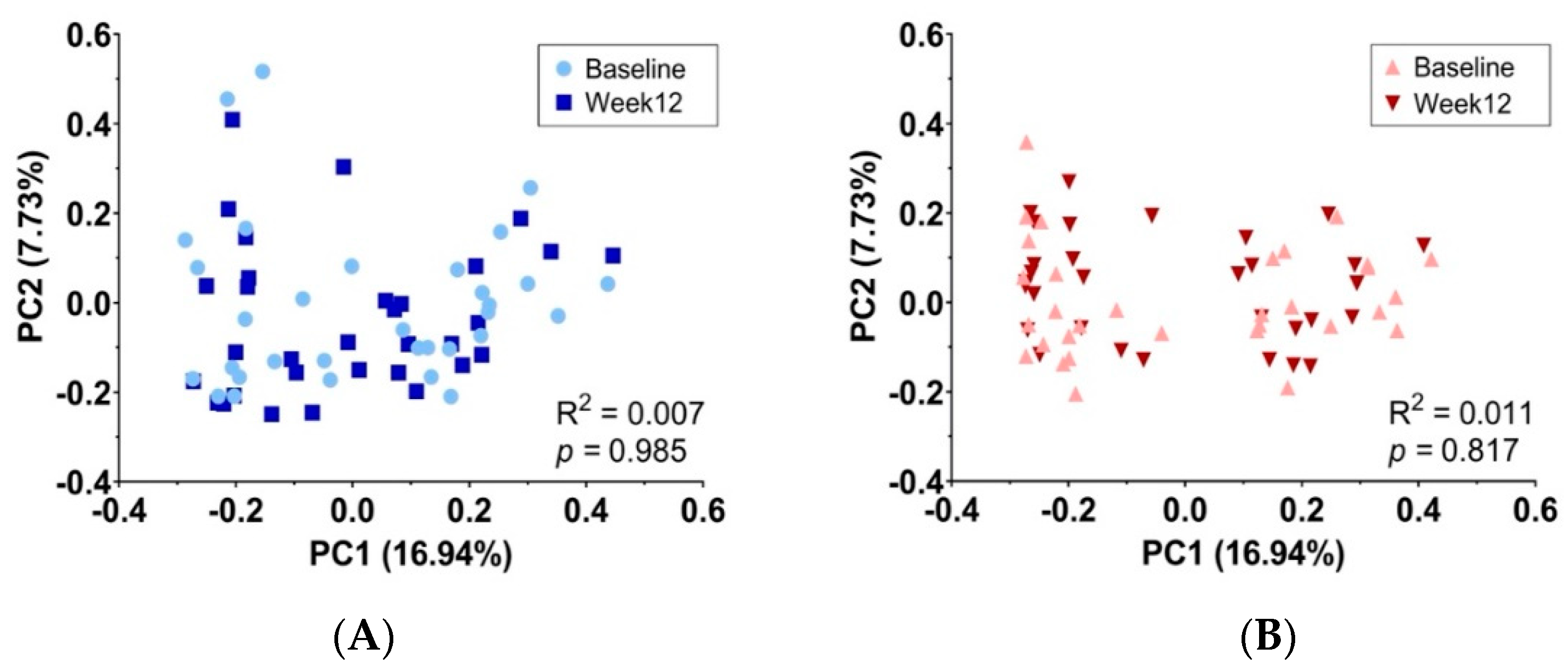
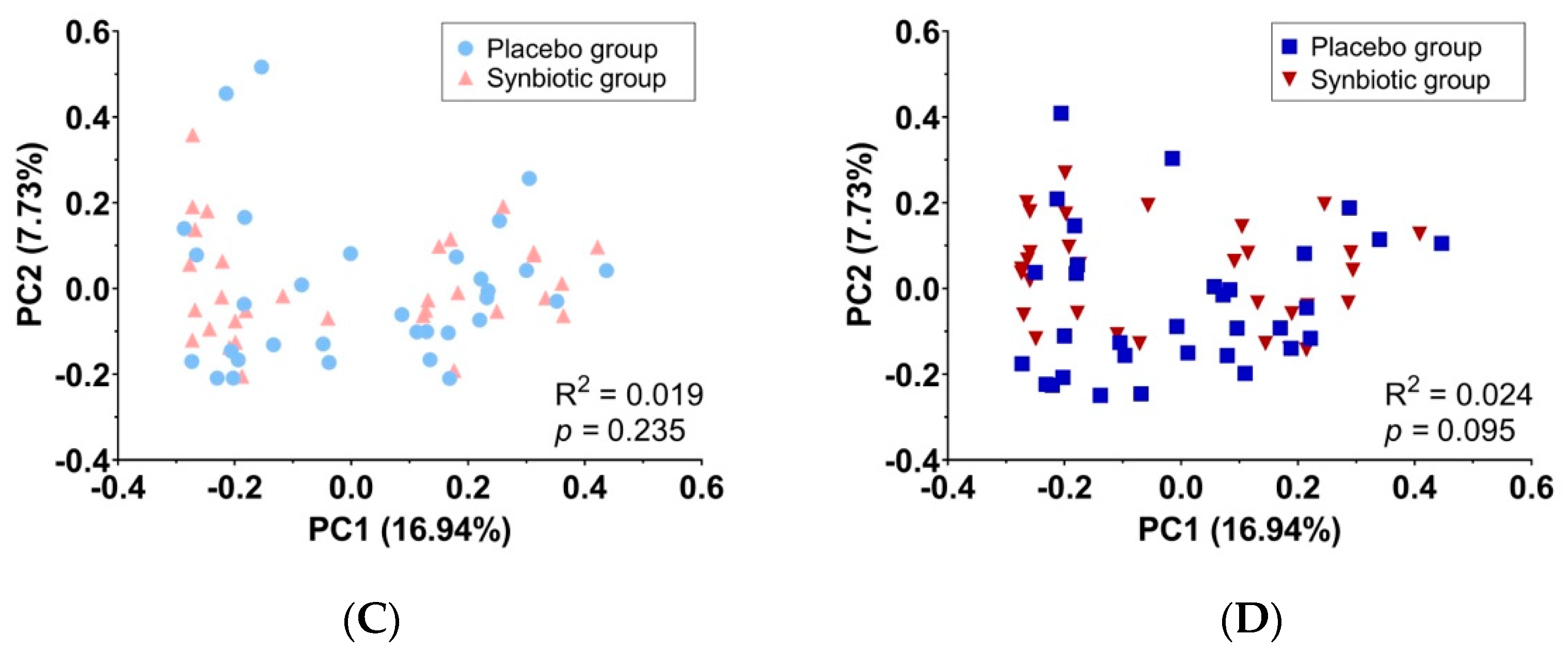
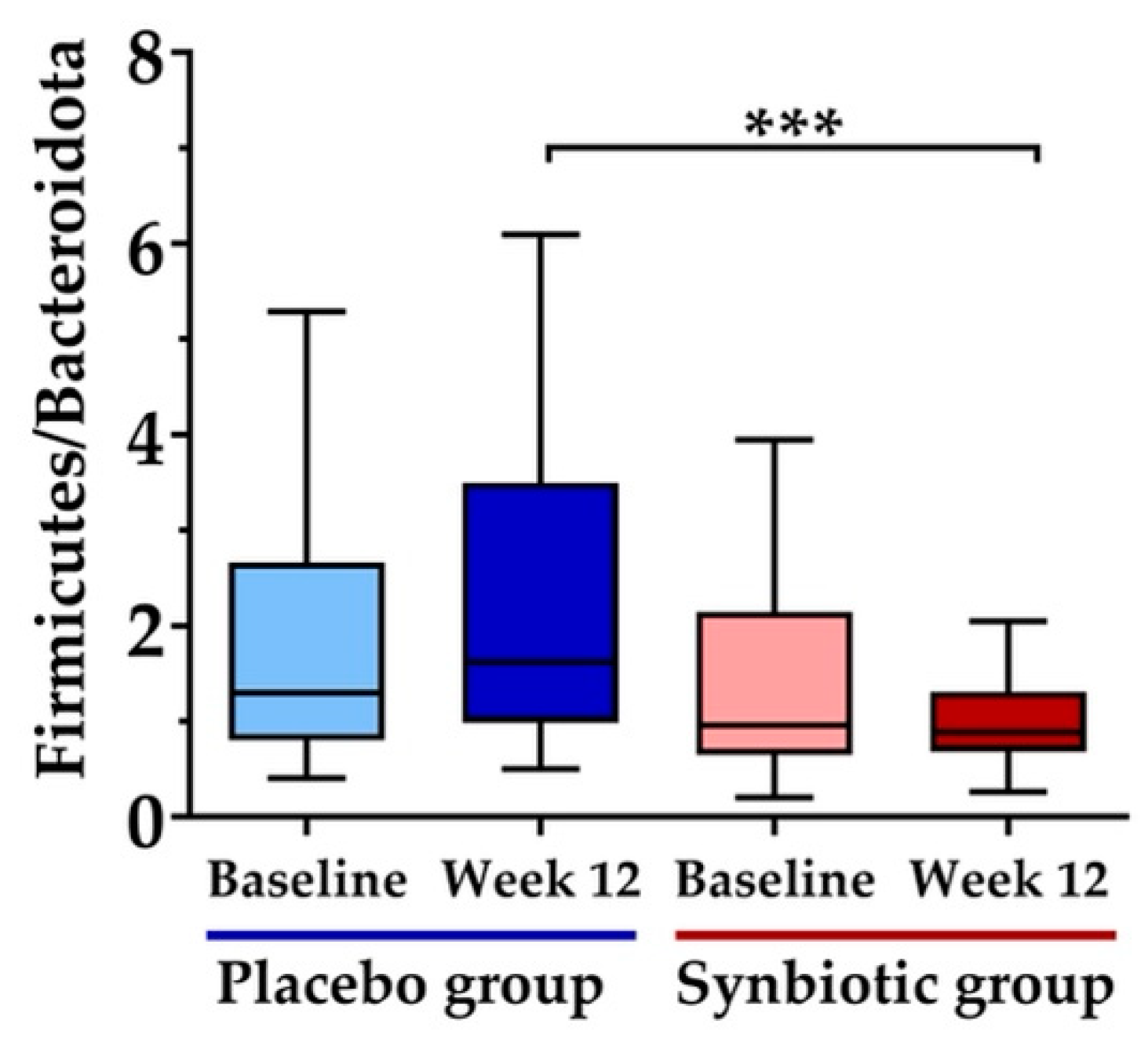
3.5. Gut Microbiota Measurement
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simo, L.P.; Agbor, V.N.; Temgoua, F.Z.; Fozeu, L.C.F.; Bonghaseh, D.T.; Mbonda, A.G.N.; Yurika, R.; Dotse-Gborgbortsi, W.; Mbanya, D. Prevalence and factors associated with overweight and obesity in selected health areas in a rural health district in Cameroon: A cross-sectional analysis. BMC Public Health 2021, 21, 475. [Google Scholar] [CrossRef] [PubMed]
- Amaha, N.D. Ethiopian progress towards achieving the global nutrition targets of 2025: Analysis of sub-national trends and progress inequalities. BMC Res. Notes 2020, 13, 559. [Google Scholar] [CrossRef]
- Biswas, T.; Townsend, N.; Magalhaes, R.S.; Islam, M.S.; Hasan, M.M.; Mamun, A. Current progress and future directions in the double burden of malnutrition among women in South and Southeast Asian countries. CND 2019, 3, nzz026. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, N.; Asada, R.; Saito, A.; Kanemoto, S.; Imaizumi, K. Obesity-induced endoplasmic reticulum stress causes chronic inflammation in adipose tissue. Sci. Rep. 2012, 2, 799. [Google Scholar] [CrossRef]
- Suriyaprom, K.; Kaewprasert, S.; Putpadungwipon, P.; Namjuntra, P.; Klongthalay, S. Association of antioxidant status and inflammatory markers with metabolic syndrome in Thais. J. Health Popul. Nutr. 2019, 38, 1. [Google Scholar] [CrossRef]
- Adenan, D.M.; Jaafar, Z.; Jayapalan, J.J.; Aziz, A.A. Plasma antioxidants and oxidative stress status in obese women: Correlation with cardiopulmonary response. PeerJ 2020, 8, e9230. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [PubMed]
- Furet, J.-P.; Kong, L.-C.; Tap, J.; Poitou, C.; Basdevant, A.; Bouillot, J.-L.; Mariat, D.; Corthier, G.; Doré, J.; Henegar, C. Differential adaptation of human gut microbiota to bariatric surgery–induced weight loss: Links with metabolic and low-grade inflammation markers. Diabetes 2010, 59, 3049–3057. [Google Scholar] [CrossRef]
- Hadi, A.; Alizadeh, K.; Hajianfar, H.; Mohammadi, H.; Miraghajani, M. Efficacy of synbiotic supplementation in obesity treatment: A systematic review and meta-analysis of clinical trials. Crit. Rev. Food Sci. Nutr. 2020, 60, 584–596. [Google Scholar] [CrossRef]
- Núñez-Sánchez, M.A.; Herisson, F.M.; Cluzel, G.L.; Caplice, N.M. Metabolic syndrome and synbiotic targeting of the gut microbiome. Curr. Opin. Food Sci. 2021, 41, 60–69. [Google Scholar] [CrossRef]
- Rabiei, S.; Hedayati, M.; Rashidkhani, B.; Saadat, N.; Shakerhossini, R. The effects of synbiotic supplementation on body mass index, metabolic and inflammatory biomarkers, and appetite in patients with metabolic syndrome: A triple-blind randomized controlled trial. J. Diet. Suppl. 2019, 16, 294–306. [Google Scholar] [CrossRef]
- Fernandes, R.; do Rosario, V.A.; Mocellin, M.C.; Kuntz, M.G.; Trindade, E.B. Effects of inulin-type fructans, galacto-oligosaccharides and related synbiotics on inflammatory markers in adult patients with overweight or obesity: A systematic review. Clin. Nutr. 2017, 36, 1197–1206. [Google Scholar] [CrossRef]
- Hibberd, A.; Yde, C.; Ziegler, M.; Honoré, A.; Saarinen, M.; Lahtinen, S.; Stahl, B.; Jensen, H.; Stenman, L. Probiotic or synbiotic alters the gut microbiota and metabolism in a randomised controlled trial of weight management in overweight adults. Benef. Microbes 2019, 10, 121–135. [Google Scholar] [CrossRef]
- Perna, S.; Ilyas, Z.; Giacosa, A.; Gasparri, C.; Peroni, G.; Faliva, M.A.; Rigon, C.; Naso, M.; Riva, A.; Petrangolini, G. Is probiotic supplementation useful for the management of body weight and other anthropometric measures in adults affected by overweight and obesity with metabolic related diseases? A systematic review and meta-analysis. Nutrients 2021, 13, 666. [Google Scholar] [CrossRef]
- Gomes, A.C.; de Sousa, R.G.M.; Botelho, P.B.; Gomes, T.L.N.; Prada, P.O.; Mota, J.F. The additional effects of a probiotic mix on abdominal adiposity and antioxidant Status: A double-blind, randomized trial. Obesity 2017, 25, 30–38. [Google Scholar] [CrossRef]
- Abu Khaled, M.; McCutcheon, M.; Reddy, S.; Pearman, P.; Hunter, G.; Weinsier, R. Electrical impedance in assessing human body composition: The BIA method. Am. J. Clin. Nutr. 1988, 47, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Chusak, C.; Thilavech, T.; Adisakwattana, S. Consumption of Mesona chinensis attenuates postprandial glucose and improves antioxidant status induced by a high carbohydrate meal in overweight subjects. Am. J. Chin. Med. 2014, 42, 315–336. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Chu, C.Y.; Chu, K.O.; Choy, K.W.; Khaw, K.S.; Rogers, M.S.; Pang, C.P. Trolox-equivalent antioxidant capacity assay versus oxygen radical absorbance capacity assay in plasma. Clin. Chem. 2004, 50, 952–954. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Jitvaropas, R.; Mayuramart, O.; Sawaswong, V.; Kaewsapsak, P.; Payungporn, S. Classification of salivary bacteriome in asymptomatic COVID-19 cases based on long-read nanopore sequencing. Exp. Biol. Med. 2022, 247, 1937–1946. [Google Scholar] [CrossRef] [PubMed]
- Wick, R.R.; Judd, L.M.; Holt, K.E. Performance of neural network basecalling tools for Oxford Nanopore sequencing. Genome. Biol. 2019, 20, 129. [Google Scholar] [CrossRef]
- Abenavoli, L.; Scarpellini, E.; Colica, C.; Boccuto, L.; Salehi, B.; Sharifi-Rad, J.; Aiello, V.; Romano, B.; De Lorenzo, A.; Izzo, A.A. Gut microbiota and obesity: A role for probiotics. Nutrients 2019, 11, 2690. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pérez, H.; Ciuffreda, L.; Flores, C. NanoCLUST: A species-level analysis of 16S rRNA nanopore sequencing data. J. Bioinform. 2021, 37, 1600–1601. [Google Scholar] [CrossRef]
- Cole, J.R.; Chai, B.; Marsh, T.L.; Farris, R.J.; Wang, Q.; Kulam, S.; Chandra, S.; McGarrell, D.M.; Schmidt, T.M.; Garrity, G.M. The Ribosomal Database Project (RDP-II): Previewing a new autoaligner that allows regular updates and the new prokaryotic taxonomy. Nucleic. Acids Res. 2003, 31, 442–443. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Liu, P.; Zhou, G.; Xia, J. Using MicrobiomeAnalyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nat. Protoc. 2020, 15, 799–821. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Yi, K.; Hansana, V.; Kim, J.-M.; Kim, Y. Comparison of nutrient intake in Lao PDR by the Korean CAN-pro and Thailand INMUCAL analysis programs. Prev. Nutr. Food Sci. 2021, 26, 40. [Google Scholar] [CrossRef]
- Davis, C.D. The gut microbiome and its role in obesity. Nutr. Today 2016, 51, 167. [Google Scholar] [CrossRef]
- Amabebe, E.; Robert, F.O.; Agbalalah, T.; Orubu, E.S.F. Microbial dysbiosis-induced obesity: Role of gut microbiota in homoeostasis of energy metabolism. Br. J. Nutr. 2020, 123, 1127–1137. [Google Scholar] [CrossRef]
- Bengmark, S. Bioecologic control of the gastrointestinal tract: The role of flora and supplemented probiotics and synbiotics. Gastroenterol. Clin. N. Am. 2005, 34, 413–436. [Google Scholar] [CrossRef]
- Timmerman, H.M.; Koning, C.J.; Mulder, L.; Rombouts, F.M.; Beynen, A.C. Monostrain, multistrain and multispecies probiotics—A comparison of functionality and efficacy. Int. J. Food Microbiol. 2004, 96, 219–233. [Google Scholar] [CrossRef]
- Pengrattanachot, N.; Thongnak, L.; Lungkaphin, A. The impact of prebiotic fructooligosaccharides on gut dysbiosis and inflammation in obesity and diabetes related kidney disease. Food Funct. 2022, 13, 5925–5945. [Google Scholar] [CrossRef] [PubMed]
- Hadi, A.; Sepandi, M.; Marx, W.; Moradi, S.; Parastouei, K. Clinical and psychological responses to synbiotic supplementation in obese or overweight adults: A randomized clinical trial. Complement. Ther. Med. 2019, 47, 102216. [Google Scholar] [CrossRef]
- Sudha, M.R.; Ahire, J.; Jayanthi, N.; Tripathi, A.; Nanal, S. Effect of multi-strain probiotic (UB0316) in weight management in overweight/obese adults: A 12-week double blind, randomised, placebo-controlled study. Benef. Microbes 2019, 10, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Ipar, N.; Aydogdu, S.D.; Yildirim, G.K.; Inal, M.; Gies, I.; Vandenplas, Y.; Dinleyici, E. Effects of synbiotic on anthropometry, lipid profile and oxidative stress in obese children. Benef. Microbes 2015, 6, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, D.; Sharma, S. Abdominal obesity, adipokines and non-communicable diseases. J. Steroid. Biochem. Mol. Biol. 2020, 203, 105737. [Google Scholar] [CrossRef]
- Rabiei, S.; Shakerhosseini, R.; Saadat, N. The effects of symbiotic therapy on anthropometric measures, body composition and blood pressure in patient with metabolic syndrome: A triple blind RCT. Med. J. Islam. Repub. Iran. 2015, 29, 213. [Google Scholar] [PubMed]
- Minami, J.; Iwabuchi, N.; Tanaka, M.; Yamauchi, K.; Xiao, J.-z.; Abe, F.; Sakane, N. Effects of Bifidobacterium breve B-3 on body fat reductions in pre-obese adults: A randomized, double-blind, placebo-controlled trial. Biosci. Microbiota Food Health 2018, 37, 67–75. [Google Scholar] [CrossRef]
- Kim, J.; Yun, J.M.; Kim, M.K.; Kwon, O.; Cho, B. Lactobacillus gasseri BNR17 supplementation reduces the visceral fat accumulation and waist circumference in obese adults: A randomized, double-blind, placebo-controlled trial. J. Med. Food 2018, 21, 454–461. [Google Scholar] [CrossRef]
- DiBaise, J.K.; Frank, D.N.; Mathur, R. Impact of the gut microbiota on the development of obesity: Current concepts. Am. J. Gastroenterol. Suppl. 2012, 1, 22. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Mathur, R.; Barlow, G.M. Obesity and the microbiome. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 1087–1099. [Google Scholar] [CrossRef] [PubMed]
- Finotello, F.; Mastrorilli, E.; Di Camillo, B. Measuring the diversity of the human microbiota with targeted next-generation sequencing. Brief. Bioinform. 2018, 19, 679–692. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- De La Cuesta-Zuluaga, J.; Corrales-Agudelo, V.; Carmona, J.; Abad, J.; Escobar, J. Body size phenotypes comprehensively assess cardiometabolic risk and refine the association between obesity and gut microbiota. Int. J. Obes. 2018, 42, 424–432. [Google Scholar] [CrossRef]
- Stanislawski, M.A.; Dabelea, D.; Lange, L.A.; Wagner, B.D.; Lozupone, C.A. Gut microbiota phenotypes of obesity. NPJ Biofilms Microbiomes 2019, 5, 18. [Google Scholar] [CrossRef]
- Lozupone, C.; Knight, R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef]
- Sergeev, I.N.; Aljutaily, T.; Walton, G.; Huarte, E. Effects of synbiotic supplement on human gut microbiota, body composition and weight loss in obesity. Nutrients 2020, 12, 222. [Google Scholar] [CrossRef]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
- Crovesy, L.; Masterson, D.; Rosado, E.L. Profile of the gut microbiota of adults with obesity: A systematic review. Eur. J. Clin. Nutr. 2020, 74, 1251–1262. [Google Scholar] [CrossRef]
- Marvasti, F.E.; Moshiri, A.; Taghavi, M.S.; Riazi, S.; Taati, M.; Sadati, S.F.; Ghaheri, A.; Masoomi, M.; Vaziri, F.; Fateh, A. The first report of differences in gut microbiota composition between obese and normal weight Iranian subjects. Iran. Biomed. J. 2020, 24, 148. [Google Scholar]
- Hassan, N.E.; El Shebini, S.M.; El-Masry, S.A.; Ahmed, N.H.; Kamal, A.N.; Ismail, A.S.; Alian, K.M.; Mostafa, M.I.; Selim, M.; Afify, M.A. Brief overview of dietary intake, some types of gut microbiota, metabolic markers and research opportunities in sample of Egyptian women. Sci. Rep. 2022, 12, 17291. [Google Scholar] [CrossRef]
- Pekkala, S.; Munukka, E.; Kong, L.; Pöllänen, E.; Autio, R.; Roos, C.; Wiklund, P.; Fischer-Posovszky, P.; Wabitsch, M.; Alen, M. Toll-like receptor 5 in obesity: The role of gut microbiota and adipose tissue inflammation. Obesity 2015, 23, 581–590. [Google Scholar] [CrossRef]
- Hassan, N.E.; El-Masry, S.A.; Nageeb, A.; El Hussieny, M.S.; Khalil, A.; Aly, M.; Selim, M.; Alian, K.; Rasheed, E.A.; Wahed, M.M.A. Linking gut microbiota, metabolic syndrome and metabolic health among a sample of obese Egyptian females. Open. Access. Maced. J. Med. Sci. 2021, 9, 1123–1131. [Google Scholar] [CrossRef]
- McMurray, F.; Patten, D.A.; Harper, M.E. Reactive oxygen species and oxidative stress in obesity—Recent findings and empirical approaches. Obesity 2016, 24, 2301–2310. [Google Scholar] [CrossRef]
- Manna, P.; Jain, S.K. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: Causes and therapeutic strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef]
- Sepidarkish, M.; Farsi, F.; Akbari-Fakhrabadi, M.; Namazi, N.; Almasi-Hashiani, A.; Hagiagha, A.M.; Heshmati, J. The effect of vitamin D supplementation on oxidative stress parameters: A systematic review and meta-analysis of clinical trials. Pharmacol. Res. 2019, 139, 141–152. [Google Scholar] [CrossRef]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, Á.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef]
- Soleimani, A.; Motamedzadeh, A.; Zarrati Mojarrad, M.; Bahmani, F.; Amirani, E.; Ostadmohammadi, V.; Tajabadi-Ebrahimi, M.; Asemi, Z. The effects of synbiotic supplementation on metabolic status in diabetic patients undergoing hemodialysis: A randomized, double-blinded, placebo-controlled trial. Probiotics Antimicrob. Proteins 2019, 11, 1248–1256. [Google Scholar] [CrossRef]
- Chaiyasut, C.; Sivamaruthi, B.S.; Kesika, P.; Khongtan, S.; Khampithum, N.; Thangaleela, S.; Peerajan, S.; Bumrungpert, A.; Chaiyasut, K.; Sirilun, S. Synbiotic supplementation improves obesity index and metabolic biomarkers in Thai obese adults: A randomized clinical trial. Foods 2021, 10, 1580. [Google Scholar] [CrossRef]
- Roshan, H.; Ghaedi, E.; Rahmani, J.; Barati, M.; Najafi, M.; Karimzedeh, M.; Nikpayam, O. Effects of probiotics and synbiotic supplementation on antioxidant status: A meta-analysis of randomized clinical trials. Clin. Nutr. ESPEN 2019, 30, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, M.; Hashemi, T.; Shakeri, H.; Abedi, F.; Sabihi, S.-S.; Alizadeh, S.-A.; Asemi, Z. Synbiotic food consumption reduces levels of triacylglycerols and VLDL, but not cholesterol, LDL, or HDL in plasma from pregnant women. Lipids 2014, 49, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Bander, Z.A.; Nitert, M.D.; Mousa, A.; Naderpoor, N. The gut microbiota and inflammation: An overview. Int. J. Environ. Res. Public Health 2020, 17, 7618. [Google Scholar] [CrossRef]
- Ferrarese, R.; Ceresola, E.; Preti, A.; Canducci, F. Probiotics, prebiotics and synbiotics for weight loss and metabolic syndrome in the microbiome era. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7588–7605. [Google Scholar]


| Parameters | Placebo Group (n = 31) | Synbiotic Group (n = 32) | p-Value |
|---|---|---|---|
| Sex (male/female) | 12/19 | 14/18 | |
| Age (years) | 33.69 ± 6.54 | 32.35 ± 6.53 | 0.422 |
| Height (cm) | 163.41 ± 10.71 | 163.74 ± 7.16 | 0.885 |
| Weight (kg) | 72.56 ± 13.22 | 73.86 ± 12.03 | 0.685 |
| BMI (kg/m2) | 27.00 ± 2.84 | 27.47 ± 3.30 | 0.547 |
| Waist circumference (cm) | 90.72 ± 10.37 | 91.01 ± 9.76 | 0.909 |
| Percentage of body fat (%) | 34.44 ± 8.45 | 35.85 ± 8.79 | 0.519 |
| Parameters | Baseline | Week 6 | Week 12 |
|---|---|---|---|
| Body weight (kg) | |||
| Placebo group | 73.86 ± 12.03 Aa | 73.93 ± 12.17 Aa | 73.82 ± 12.28 Aa |
| Synbiotic group | 72.56 ± 13.22 Aa | 72.55 ± 13.06 Aa | 72.10 ± 12.85 Aa |
| Body mass index (kg/m2) | |||
| Placebo group | 27.47 ± 3.30 Aa | 27.50 ± 3.37 Aa | 27.46 ± 3.43 Aa |
| Synbiotic group | 27.00 ± 2.84 Aa | 27.01 ± 2.88 Aa | 26.85 ± 2.91 Aa |
| Waist circumference (cm) | |||
| Placebo group | 91.01 ± 9.76 Aa | 90.25 ± 9.68 Aa | 91.85 ± 9.11 Aa |
| Synbiotic group | 90.72 ± 10.37 Aa | 89.18 ± 10.34 Ab | 88.39 ± 8.93 Ab |
| Percentage of body fat (%) | |||
| Placebo group | 35.85 ± 8.79 Aa | 34.66 ± 8.14 Ab | 35.23 ± 8.62 Aa |
| Synbiotic group | 34.44 ± 8.45 Aa | 34.47 ± 8.11 Aa | 33.02 ± 8.26 Ab |
| Parameters | Baseline | Week 6 | Week 12 |
|---|---|---|---|
| Fasting plasma glucose (mg/dL) | |||
| Placebo group | 87.87 ± 7.54 Aa | 86.52 ± 8.08 Aa | 86.55 ± 8.24 Aa |
| Synbiotic group | 89.66 ± 9.39 Aa | 88.06 ± 10.03 Aa | 88.69 ± 11.73 Aa |
| Total cholesterol (mg/dL) | |||
| Placebo group | 189.52 ± 42.78 Aa | 191.13 ± 38.30 Aa | 198.00 ± 34.71 Aa |
| Synbiotic group | 205.69 ± 31.64 Aa | 213.34 ± 41.26 Aa | 209.53 ± 34.24 Aa |
| Triglyceride (mg/dL) | |||
| Placebo group | 90.10 ± 51.53 Aa | 119.16 ± 73.57 Ab | 123.61 ± 67.02 Ab |
| Synbiotic group | 91.72 ± 52.96 Aa | 107.75 ± 53.27 Ab | 113.94 ± 69.59 Ab |
| HDL (mg/dL) | |||
| Placebo group | 49.81 ± 12.28 Aa | 49.68 ± 12.55 Aa | 51.58 ± 12.03 Aa |
| Synbiotic group | 51.16 ± 12.87 Aa | 50.16 ± 13.10 Aa | 50.66 ± 10.62 Aa |
| LDL (mg/dL) | |||
| Placebo group | 126.13 ± 38.50 Aa | 127.55 ± 36.73 Aa | 122.84 ± 31.10 Aa |
| Synbiotic group | 141.03 ± 30.69 Aa | 153.38 ± 34.96 Bb | 135.84 ± 32.61 Aa |
| Parameters | Baseline | Week 6 | Week 12 |
|---|---|---|---|
| TEAC (µmol/L) | |||
| Placebo group | 1312.59 ± 155.59 Aa | 1303.81 ± 173.58 Aa | 1254.42 ± 195.54 Aa |
| Synbiotic group | 1265.07 ± 70.35 Aa | 1307.78 ± 170.22 Aa | 1340.34 ± 70.25 Bb |
| Thiol (mmol/L) | |||
| Placebo group | 38.78 ± 8.46 Aa | 38.47 ± 6.07 Aa | 42.12 ± 5.73 Ab |
| Synbiotic group | 39.93 ± 5.50 Aa | 39.69 ± 3.92 Aa | 42.52 ± 5.05 Ab |
| MDA (µmol/L) | |||
| Placebo group | 0.29 ± 0.31 Aa | 0.61 ± 0.47 Ab | 1.82 ± 0.94 Ab |
| Synbiotic group | 0.50 ± 0.44 Ba | 0.27 ± 0.49 Ba | 0.11 ± 0.11 Bb |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oraphruek, P.; Chusak, C.; Ngamukote, S.; Sawaswong, V.; Chanchaem, P.; Payungporn, S.; Suantawee, T.; Adisakwattana, S. Effect of a Multispecies Synbiotic Supplementation on Body Composition, Antioxidant Status, and Gut Microbiomes in Overweight and Obese Subjects: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2023, 15, 1863. https://doi.org/10.3390/nu15081863
Oraphruek P, Chusak C, Ngamukote S, Sawaswong V, Chanchaem P, Payungporn S, Suantawee T, Adisakwattana S. Effect of a Multispecies Synbiotic Supplementation on Body Composition, Antioxidant Status, and Gut Microbiomes in Overweight and Obese Subjects: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients. 2023; 15(8):1863. https://doi.org/10.3390/nu15081863
Chicago/Turabian StyleOraphruek, Piyarat, Charoonsri Chusak, Sathaporn Ngamukote, Vorthon Sawaswong, Prangwalai Chanchaem, Sunchai Payungporn, Tanyawan Suantawee, and Sirichai Adisakwattana. 2023. "Effect of a Multispecies Synbiotic Supplementation on Body Composition, Antioxidant Status, and Gut Microbiomes in Overweight and Obese Subjects: A Randomized, Double-Blind, Placebo-Controlled Study" Nutrients 15, no. 8: 1863. https://doi.org/10.3390/nu15081863
APA StyleOraphruek, P., Chusak, C., Ngamukote, S., Sawaswong, V., Chanchaem, P., Payungporn, S., Suantawee, T., & Adisakwattana, S. (2023). Effect of a Multispecies Synbiotic Supplementation on Body Composition, Antioxidant Status, and Gut Microbiomes in Overweight and Obese Subjects: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients, 15(8), 1863. https://doi.org/10.3390/nu15081863







