Interleukin-18 Is a Potential Biomarker Linking Dietary Fatty Acid Quality and Insulin Resistance: Results from a Cross-Sectional Study in Northern Italy
Abstract
1. Introduction
2. Subjects and Methods
2.1. Participants
2.2. Dietary Assessment
2.3. Biochemical Analysis
2.4. Metabolic Syndrome
2.5. Statistical Analysis
3. Results
3.1. Study Participant Characteristics
3.2. Relationship between IL-18 Circulating Levels, Insulin Resistance, Metabolic Syndrome and Body Composition
3.3. Dietary Fatty Acids and Their Impact on IL-18 Circulating Levels
3.4. Dietary Fatty Acid Intake and Insulin Resistance
3.5. Dietary Fatty Acid Quality as a Predictor of IL-18 Circulating Levels
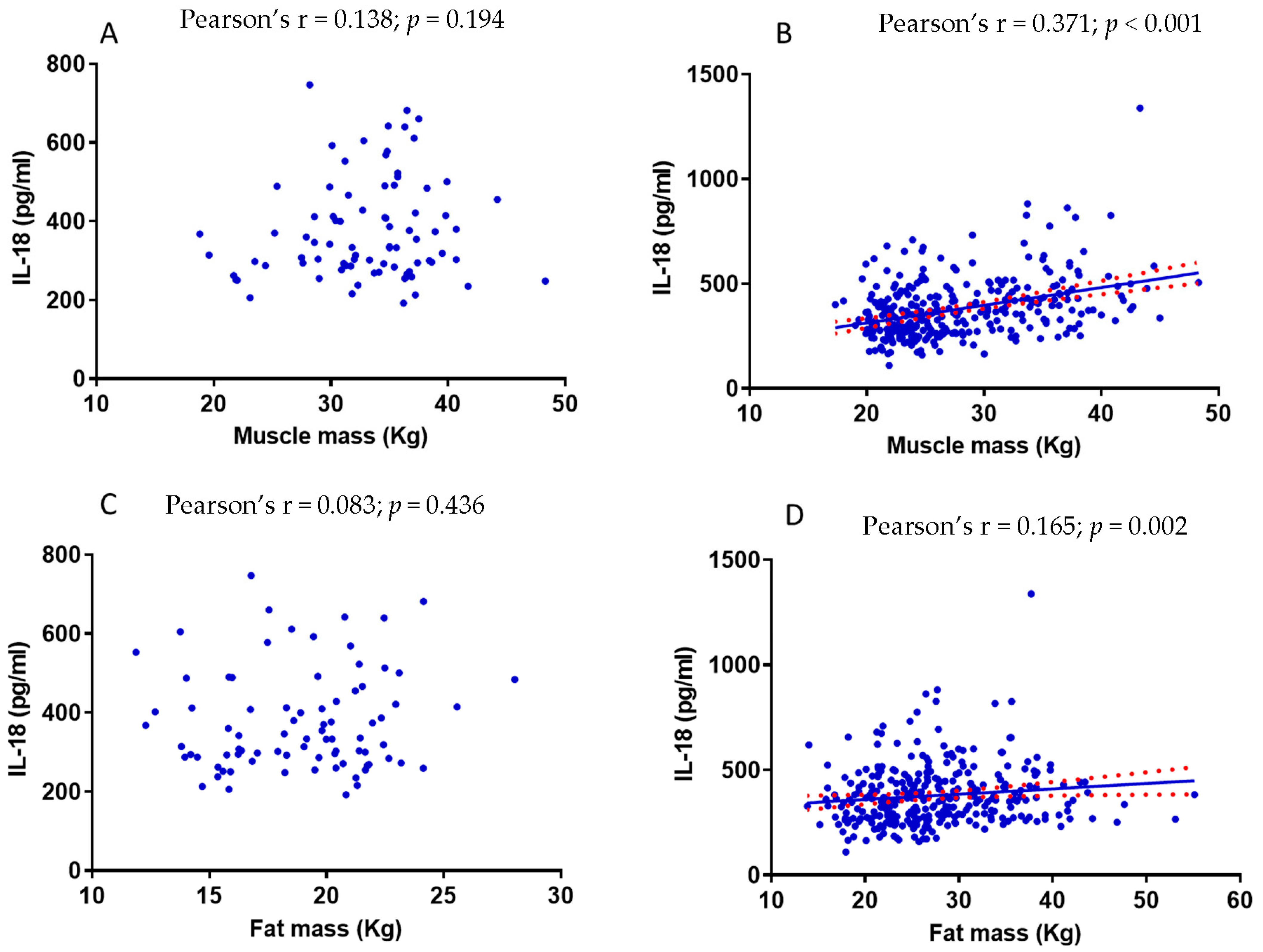
3.6. The Relationship between Muscle Mass and IL-18 Is Influenced by Fat Mass
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friedrich, M.J. Global Obesity Epidemic Worsening. JAMA 2017, 318, 603. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.V.; Fujioka, K.; Wilson, P.W.; Woodworth, K.A. Obesity: Why be concerned? Am. J. Med. 2009, 122, S4–S11. [Google Scholar] [CrossRef] [PubMed]
- Mazon, J.N.; de Mello, A.H.; Ferreira, G.K.; Rezin, G.T. The impact of obesity on neurodegenerative diseases. Life Sci. 2017, 182, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism 2019, 92, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Gregor, M.F.; Hotamisligil, G. Inflammatory Mechanisms in Obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef]
- de Luca, C.; Olefsky, J.M. Inflammation and insulin resistance. FEBS Lett. 2008, 582, 97–105. [Google Scholar] [CrossRef]
- Plomgaard, P.; Bouzakri, K.; Krogh-Madsen, R.; Mittendorfer, B.; Zierath, J.R.; Pedersen, B.K. Tumor necrosis factor-alpha induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes 2005, 54, 2939–2945. [Google Scholar] [CrossRef]
- Mohallem, R.; Aryal, U.K. Regulators of TNFalpha mediated insulin resistance elucidated by quantitative proteomics. Sci. Rep. 2020, 10, 20878. [Google Scholar] [CrossRef]
- Sergi, D.; Luscombe-Marsh, N.; Heilbronn, L.K.; Birch-Machin, M.; Naumovski, N.; Lionetti, L.; Proud, C.G.; Abeywardena, M.Y.; O’Callaghan, N. The Inhibition of Metabolic Inflammation by EPA Is Associated with Enhanced Mitochondrial Fusion and Insulin Signaling in Human Primary Myotubes. J. Nutr. 2021, 151, 810–819. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammatory pathways and insulin action. Int. J. Obes. Relat. Metab. Disord. 2003, 27, S53–S55. [Google Scholar] [CrossRef] [PubMed]
- Bruun, J.M.; Stallknecht, B.; Helge, J.W.; Richelsen, B. Interleukin-18 in plasma and adipose tissue: Effects of obesity, insulin resistance, and weight loss. Eur. J. Endocrinol. 2007, 157, 465–471. [Google Scholar] [CrossRef]
- Hung, J.; McQuillan, B.M.; Chapman, C.M.; Thompson, P.L.; Beilby, J.P. Elevated interleukin-18 levels are associated with the metabolic syndrome independent of obesity and insulin resistance. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1268–1273. [Google Scholar] [CrossRef]
- Zirlik, A.; Abdullah, S.M.; Gerdes, N.; MacFarlane, L.; Schonbeck, U.; Khera, A.; McGuire, D.K.; Vega, G.L.; Grundy, S.; Libby, P.; et al. Interleukin-18, the metabolic syndrome, and subclinical atherosclerosis: Results from the Dallas Heart Study. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2043–2049. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Thomas, R.; Kochumon, S.; Sindhu, S. Increased adipose tissue expression of IL-18R and its ligand IL-18 associates with inflammation and insulin resistance in obesity. Immun. Inflamm. Dis. 2017, 5, 318–335. [Google Scholar] [CrossRef]
- Van Guilder, G.P.; Hoetzer, G.L.; Greiner, J.J.; Stauffer, B.L.; Desouza, C.A. Influence of metabolic syndrome on biomarkers of oxidative stress and inflammation in obese adults. Obesity 2006, 14, 2127–2131. [Google Scholar] [CrossRef]
- Rabkin, S.W. The role of interleukin 18 in the pathogenesis of hypertension-induced vascular disease. Nat. Clin. Pract. Cardiovasc. Med. 2009, 6, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.; Collins, M.; Jennings, C.; van der Merwe, L.; Soderstrom, I.; Olsson, T.; Levitt, N.S.; Lambert, E.V.; Goedecke, J.H. The association of interleukin-18 genotype and serum levels with metabolic risk factors for cardiovascular disease. Eur. J. Endocrinol. 2007, 157, 633–640. [Google Scholar] [CrossRef]
- Espinola-Klein, C.; Rupprecht, H.J.; Bickel, C.; Lackner, K.; Genth-Zotz, S.; Post, F.; Munzel, T.; Blankenberg, S.; Athero Gene, I. Impact of inflammatory markers on cardiovascular mortality in patients with metabolic syndrome. Eur. J. Cardiovasc. Prev. Rehabil. 2008, 15, 278–284. [Google Scholar] [CrossRef]
- Presta, I.; Andreozzi, F.; Succurro, E.; Marini, M.A.; Laratta, E.; Lauro, R.; Hribal, M.L.; Perticone, F.; Sesti, G. IL-18 gene polymorphism and metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2009, 19, e5–e6. [Google Scholar] [CrossRef]
- Hart, M.J.; Torres, S.J.; McNaughton, S.A.; Milte, C.M. Dietary patterns and associations with biomarkers of inflammation in adults: A systematic review of observational studies. Nutr. J. 2021, 20, 24. [Google Scholar] [CrossRef]
- Bonaccio, M.; Cerletti, C.; Iacoviello, L.; de Gaetano, G. Mediterranean diet and low-grade subclinical inflammation: The Moli-sani study. Endocr. Metab. Immune Disord. Drug Targets 2015, 15, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Christ, A.; Lauterbach, M.; Latz, E. Western Diet and the Immune System: An Inflammatory Connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef] [PubMed]
- Troseid, M.; Arnesen, H.; Hjerkinn, E.M.; Seljeflot, I. Serum levels of interleukin-18 are reduced by diet and n-3 fatty acid intervention in elderly high-risk men. Metabolism 2009, 58, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Sergi, D.; Luscombe-Marsh, N.; Naumovski, N.; Abeywardena, M.; O’Callaghan, N. Palmitic Acid, but Not Lauric Acid, Induces Metabolic Inflammation, Mitochondrial Fragmentation, and a Drop in Mitochondrial Membrane Potential in Human Primary Myotubes. Front. Nutr. 2021, 8, 663838. [Google Scholar] [CrossRef]
- Zhou, H.; Urso, C.J.; Jadeja, V. Saturated Fatty Acids in Obesity-Associated Inflammation. J. Inflamm. Res. 2020, 13, 1–14. [Google Scholar] [CrossRef]
- Mu, L.; Mukamal, K.J.; Naqvi, A.Z. Erythrocyte saturated fatty acids and systemic inflammation in adults. Nutrition 2014, 30, 1404–1408. [Google Scholar] [CrossRef]
- Santamarina, A.B.; Pisani, L.P.; Baker, E.J.; Marat, A.D.; Valenzuela, C.A.; Miles, E.A.; Calder, P.C. Anti-inflammatory effects of oleic acid and the anthocyanin keracyanin alone and in combination: Effects on monocyte and macrophage responses and the NF-kappaB pathway. Food Funct. 2021, 12, 7909–7922. [Google Scholar] [CrossRef]
- Sears, B.; Perry, M. The role of fatty acids in insulin resistance. Lipids Health Dis. 2015, 14, 121. [Google Scholar] [CrossRef]
- Palomer, X.; Pizarro-Delgado, J.; Barroso, E.; Vazquez-Carrera, M. Palmitic and Oleic Acid: The Yin and Yang of Fatty Acids in Type 2 Diabetes Mellitus. Trends Endocrinol. Metab. 2018, 29, 178–190. [Google Scholar] [CrossRef]
- Yong, K.; Mori, T.; Chew, G.; Beilin, L.J.; Puddey, I.; Watts, G.F.; Irish, A.; Dogra, G.; Boudville, N.; Lim, W. The Effects of OMEGA-3 Fatty Acid Supplementation Upon Interleukin-12 and Interleukin-18 in Chronic Kidney Disease Patients. J. Ren. Nutr. 2019, 29, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.M.; Sergi, D.; Colombari, S.; Capatti, E.; Situlin, R.; Biolo, G.; Di Girolamo, F.G.; Lazzer, S.; Šimunič, B.; Pišot, R. Dietary Acid Load but Not Mediterranean Diet Adherence Score Is Associated with Metabolic and Cardiovascular Health State: A Population Observational Study from Northern Italy. Front. Nutr. 2022, 617, 828587. [Google Scholar] [CrossRef]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.K.; Huh, J.H.; Yoo, J.S.; Kim, J.W.; Lee, K.J. HOMA-estimated insulin resistance as an independent prognostic factor in patients with acute pancreatitis. Sci. Rep. 2019, 9, 14894. [Google Scholar] [CrossRef]
- Teng, K.T.; Chang, C.Y.; Chang, L.F.; Nesaretnam, K. Modulation of obesity-induced inflammation by dietary fats: Mechanisms and clinical evidence. Nutr. J. 2014, 13, 12. [Google Scholar] [CrossRef]
- Reilly, S.M.; Saltiel, A.R. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 2017, 13, 633–643. [Google Scholar] [CrossRef]
- Solinas, G.; Karin, M. JNK1 and IKKbeta: Molecular links between obesity and metabolic dysfunction. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2010, 24, 2596–2611. [Google Scholar] [CrossRef]
- Krogh-Madsen, R.; Plomgaard, P.; Moller, K.; Mittendorfer, B.; Pedersen, B.K. Influence of TNF-alpha and IL-6 infusions on insulin sensitivity and expression of IL-18 in humans. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E108–E114. [Google Scholar] [CrossRef]
- Hivert, M.F.; Sun, Q.; Shrader, P.; Mantzoros, C.S.; Meigs, J.B.; Hu, F.B. Circulating IL-18 and the risk of type 2 diabetes in women. Diabetologia 2009, 52, 2101–2108. [Google Scholar] [CrossRef]
- Thorand, B.; Kolb, H.; Baumert, J.; Koenig, W.; Chambless, L.; Meisinger, C.; Illig, T.; Martin, S.; Herder, C. Elevated levels of interleukin-18 predict the development of type 2 diabetes: Results from the MONICA/KORA Augsburg Study, 1984–2002. Diabetes 2005, 54, 2932–2938. [Google Scholar] [CrossRef]
- Ruiz-Nunez, B.; Dijck-Brouwer, D.A.; Muskiet, F.A. The relation of saturated fatty acids with low-grade inflammation and cardiovascular disease. J. Nutr. Biochem. 2016, 36, 1–20. [Google Scholar] [CrossRef]
- Baer, D.J.; Judd, J.T.; Clevidence, B.A.; Tracy, R.P. Dietary fatty acids affect plasma markers of inflammation in healthy men fed controlled diets: A randomized crossover study. Am. J. Clin. Nutr. 2004, 79, 969–973. [Google Scholar] [CrossRef]
- Weaver, K.L.; Ivester, P.; Seeds, M.; Case, L.D.; Arm, J.P.; Chilton, F.H. Effect of dietary fatty acids on inflammatory gene expression in healthy humans. J. Biol. Chem. 2009, 284, 15400–15407. [Google Scholar] [CrossRef] [PubMed]
- Ravaut, G.; Legiot, A.; Bergeron, K.F.; Mounier, C. Monounsaturated Fatty Acids in Obesity-Related Inflammation. Int. J. Mol. Sci. 2020, 22, 330. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, J.; Costa, G.; Renaud, J.; Moitie, A.; Glemet, H.; Sergi, D.; Martinoli, M.G. The Neuroinflammatory and Neurotoxic Potential of Palmitic Acid Is Mitigated by Oleic Acid in Microglial Cells and Microglial-Neuronal Co-cultures. Mol. Neurobiol. 2021, 58, 3000–3014. [Google Scholar] [CrossRef] [PubMed]
- Salvado, L.; Coll, T.; Gomez-Foix, A.M.; Salmeron, E.; Barroso, E.; Palomer, X.; Vazquez-Carrera, M. Oleate prevents saturated-fatty-acid-induced ER stress, inflammation and insulin resistance in skeletal muscle cells through an AMPK-dependent mechanism. Diabetologia 2013, 56, 1372–1382. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Li, L.; Liu, Y.; Pu, J.; Zhang, S.; Yu, J.; Zhao, J.; Liu, P. Oleate blocks palmitate-induced abnormal lipid distribution, endoplasmic reticulum expansion and stress, and insulin resistance in skeletal muscle. Endocrinology 2011, 152, 2206–2218. [Google Scholar] [CrossRef]
- Sergi, D.; Morris, A.C.; Kahn, D.E.; McLean, F.H.; Hay, E.A.; Kubitz, P.; MacKenzie, A.; Martinoli, M.G.; Drew, J.E.; Williams, L.M. Palmitic acid triggers inflammatory responses in N42 cultured hypothalamic cells partially via ceramide synthesis but not via TLR4. Nutr. Neurosci. 2018, 23, 321–334. [Google Scholar] [CrossRef]
- Lim, J.H.; Gerhart-Hines, Z.; Dominy, J.E.; Lee, Y.; Kim, S.; Tabata, M.; Xiang, Y.K.; Puigserver, P. Oleic acid stimulates complete oxidation of fatty acids through protein kinase A-dependent activation of SIRT1-PGC1alpha complex. J. Biol. Chem. 2013, 288, 7117–7126. [Google Scholar] [CrossRef]
- Bertrand, C.; Pignalosa, A.; Wanecq, E.; Rancoule, C.; Batut, A.; Deleruyelle, S.; Lionetti, L.; Valet, P.; Castan-Laurell, I. Effects of dietary eicosapentaenoic acid (EPA) supplementation in high-fat fed mice on lipid metabolism and apelin/APJ system in skeletal muscle. PLoS ONE 2013, 8, e78874. [Google Scholar] [CrossRef] [PubMed]
- Makinen, S.; Nguyen, Y.H.; Skrobuk, P.; Koistinen, H.A. Palmitate and oleate exert differential effects on insulin signalling and glucose uptake in human skeletal muscle cells. Endocr. Connect. 2017, 6, 331–339. [Google Scholar] [CrossRef]
- Luukkonen, P.K.; Sadevirta, S.; Zhou, Y.; Kayser, B.; Ali, A.; Ahonen, L.; Lallukka, S.; Pelloux, V.; Gaggini, M.; Jian, C.; et al. Saturated Fat Is More Metabolically Harmful for the Human Liver Than Unsaturated Fat or Simple Sugars. Diabetes Care 2018, 41, 1732–1739. [Google Scholar] [CrossRef] [PubMed]
- Funaki, M. Saturated fatty acids and insulin resistance. J. Med. Investig. 2009, 56, 88–92. [Google Scholar] [CrossRef]
- Abbott, K.A.; Burrows, T.L.; Acharya, S.; Thota, R.N.; Garg, M.L. DHA-enriched fish oil reduces insulin resistance in overweight and obese adults. Prostaglandins Leukot. Essent. Fat. Acids 2020, 159, 102154. [Google Scholar] [CrossRef] [PubMed]
- Fretts, A.M.; Jensen, P.N.; Hoofnagle, A.N.; McKnight, B.; Howard, B.V.; Umans, J.; Sitlani, C.M.; Siscovick, D.S.; King, I.B.; Djousse, L.; et al. Plasma ceramides containing saturated fatty acids are associated with risk of type 2 diabetes. J. Lipid Res. 2021, 62, 100119. [Google Scholar] [CrossRef]
- Rosqvist, F.; Kullberg, J.; Stahlman, M.; Cedernaes, J.; Heurling, K.; Johansson, H.E.; Iggman, D.; Wilking, H.; Larsson, A.; Eriksson, O.; et al. Overeating Saturated Fat Promotes Fatty Liver and Ceramides Compared with Polyunsaturated Fat: A Randomized Trial. J. Clin. Endocrinol. Metab. 2019, 104, 6207–6219. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Leung, J.C.K.; Chan, L.Y.Y.; Yiu, W.H.; Tang, S.C.W. A global perspective on the crosstalk between saturated fatty acids and Toll-like receptor 4 in the etiology of inflammation and insulin resistance. Prog. Lipid Res. 2020, 77, 101020. [Google Scholar] [CrossRef]


| Subjects, number | 403 |
| Female, number (%) | 230 (57) |
| Age (years) | 66 ± 5 |
| Systolic blood pressure (mmHg) | 138 ± 19 |
| Diastolic blood pressure (mmHg) | 85 ± 10 |
| Body mass index (kg/m2) | 26.5 ± 3.7 |
| Waist circumference (cm) | 92.1 ± 10.3 |
| Fat-free mass (%) | 64.6 ± 6.6 |
| Fat-free mass (kg) | 46.2 ± 9.3 |
| Fat mass (%) | 35.4 ± 6.6 |
| Fat mass (kg) | 25.5 ± 7.2 |
| Muscle mass (kg) | 28.8 ± 6.5 |
| Glucose (mg/dL) | 96.3 ± 10.6 |
| Insulin (U/L) | 9.0 ± 5.0 |
| HOMA-IR | 2.2 ± 1.4 |
| Total cholesterol (mg/dL) | 218.8 ± 37.7 |
| HDL cholesterol (mg/dL) | 67.6 ± 17.3 |
| LDL cholesterol (mg/dL) | 131.6 ± 33.0 |
| Triglycerides (mg/dL) | 98.5 ± 42.6 |
| hsCPR (mg/dL) | 0.207 ± 0.306 |
| IL-18 (pg/mL) | 377.6 ± 139.1 |
| MetS, number (%) | 49 (12.2) |
| Subjects therapy: | |
| Antihypertensive drugs, number (%) | 109 (27) |
| Beta blockers, number (%) | 24 (6) |
| Hypolipidemic therapy, number (%) | 64 (15.9) |
| HOMA-IR | IL-18 | |||
|---|---|---|---|---|
| rho | p Value | rho | p Value | |
| Age (years) | 0.048 | 0.346 | 0.025 | 0.617 |
| Systolic blood pressure (mmHg) | 0.148 | 0.003 | 0.086 | 0.084 |
| Diastolic blood pressure (mmHg) | 0.130 | 0.010 | 0.136 | 0.006 |
| Body mass index (kg/mq) | 0.515 | <0.001 | 0.176 | <0.001 |
| Waist circumference (cm) | 0.503 | <0.001 | 0.232 | <0.001 |
| Fat-free mass (%) | −0.224 | <0.001 | 0.085 | 0.089 |
| Fat-free mass (Kg) | 0.287 | <0.001 | 0.266 | <0.001 |
| Fat mass (%) | 0.224 | <0.001 | −0.085 | 0.089 |
| Fat mass (Kg) | 0.458 | <0.001 | 0.124 | 0.013 |
| Muscle mass (Kg) | 0.304 | <0.001 | 0.292 | <0.001 |
| Glucose (mg/dL) | 0.529 | <0.001 | 0.180 | <0.001 |
| Insulin (U/L) | 0.979 | <0.001 | 0.234 | <0.001 |
| HOMA-IR | - | - | 0.247 | <0.001 |
| Total cholesterol (mg/dL) | −0.081 | 0.106 | −0.138 | 0.006 |
| LDL cholesterol (mg/dL) | −0.033 | 0.514 | −0.066 | 0.188 |
| HDL cholesterol (mg/dL) | −0.266 | <0.001 | −0.217 | <0.001 |
| Triglycerides (mg/dL) | 0.320 | <0.001 | 0.104 | 0.039 |
| IL-18 (pg/mL) | 0.247 | <0.001 | - | - |
| hsCRP (mg/L) | 0.206 | <0.001 | 0.202 | <0.001 |
| HOMA-IR | IL-18 | |||
|---|---|---|---|---|
| rho | p Value | rho | p Value | |
| Total calories (kcal/day) | −0.003 | 0.958 | 0.083 | 0.097 |
| Alcohol (kcal/day) | 0.018 | 0.724 | 0.037 | 0.462 |
| Protein (g/day) | −0.005 | 0.917 | 0.007 | 0.889 |
| Lipid (g/day) | 0.034 | 0.496 | 0.031 | 0.541 |
| Available carbohydrates (g/day) | −0.044 | 0.380 | 0.071 | 0.154 |
| Starch (g/day) | 0.070 | 0.166 | 0.061 | 0.218 |
| Total fibre (g/day) | −0.153 | 0.002 | −0.066 | 0.186 |
| Cholesterol (mg/day) | 0.114 | 0.023 | 0.051 | 0.311 |
| SFA (g/day) | 0.068 | 0.174 | 0.091 | 0.068 |
| MUFA (g/day) | −0.048 | 0.336 | −0.029 | 0.556 |
| PUFA (g/day) | −0.019 | 0.704 | −0.015 | 0.760 |
| MUFA/SFA ratio | −0.163 | 0.001 | −0.167 | 0.001 |
| PUFA/SFA ratio | −0.092 | 0.068 | −0.142 | 0.004 |
| (MUFA + PUFA)/SFA ratio | −0.166 | 0.001 | −0.169 | <0.001 |
| C20:5 EPA (g/day) | −0.094 | 0.061 | −0.100 | 0.045 |
| C22:6 DHA (g/day) | −0.128 | 0.011 | −0.137 | 0.006 |
| Omega-3/SFA ratio | −0.166 | 0.001 | −0.204 | <0.001 |
| Omega-6/Omega-3 ratio | 0.129 | 0.011 | 0.095 | 0.057 |
| Mediterranean diet adherence | −0.084 | 0.094 | −0.101 | 0.044 |
| Model | R2 | p Value Model | Predictor | Unstandardized B Coefficient | p Value |
|---|---|---|---|---|---|
| 1 | 0.257 | <0.001 | Waist circumference (cm) | 0.011 | <0.001 |
| 2 | 0.311 | <0.001 | Waist circumference (cm) | 0.010 | <0.001 |
| Triglycerides (mg/dL) | 0.270 | 0.001 | |||
| 3 | 0.334 | <0.001 | Waist circumference (cm) | 0.005 | 0.001 |
| Triglycerides (mg/dL) | 0.285 | 0.002 | |||
| IL-18 (pg/mL) | 0.017 | 0.031 |
| Model | R2 | p Value Model | Predictor | Unstandardized B Coefficient | p Value |
|---|---|---|---|---|---|
| 1 | 0.126 | <0.001 | Muscle Mass (kg) | 0.007 | <0.001 |
| 2 | 0.182 | <0.001 | Muscle Mass (kg) | 0.007 | <0.001 |
| Omega-3/SFA Ratio | −0.073 | 0.003 | |||
| 3 | 0.211 | <0.001 | Muscle Mass (kg) | 0.005 | 0.006 |
| Omega-3/SFA Ratio | 0.285 | 0.002 | |||
| HDL Cholesterol (mg/dL) | 0.017 | 0.027 | |||
| 4 | 0.239 | <0.001 | Muscle Mass (kg) | 0.006 | 0.001 |
| Omega-3/SFA Ratio | −0.069 | 0.001 | |||
| Cholesterol HDL (mg/dL) | −0.001 | 0.011 | |||
| Total Cholesterol (mg/dL) | −0.027 | 0.027 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sergi, D.; Sanz, J.M.; Lazzer, S.; Brombo, G.; Zuliani, G.; Biolo, G.; Šimunič, B.; Pišot, R.; Dalla Nora, E.; Passaro, A. Interleukin-18 Is a Potential Biomarker Linking Dietary Fatty Acid Quality and Insulin Resistance: Results from a Cross-Sectional Study in Northern Italy. Nutrients 2023, 15, 1782. https://doi.org/10.3390/nu15071782
Sergi D, Sanz JM, Lazzer S, Brombo G, Zuliani G, Biolo G, Šimunič B, Pišot R, Dalla Nora E, Passaro A. Interleukin-18 Is a Potential Biomarker Linking Dietary Fatty Acid Quality and Insulin Resistance: Results from a Cross-Sectional Study in Northern Italy. Nutrients. 2023; 15(7):1782. https://doi.org/10.3390/nu15071782
Chicago/Turabian StyleSergi, Domenico, Juana Maria Sanz, Stefano Lazzer, Gloria Brombo, Giovanni Zuliani, Gianni Biolo, Boštjan Šimunič, Rado Pišot, Edoardo Dalla Nora, and Angelina Passaro. 2023. "Interleukin-18 Is a Potential Biomarker Linking Dietary Fatty Acid Quality and Insulin Resistance: Results from a Cross-Sectional Study in Northern Italy" Nutrients 15, no. 7: 1782. https://doi.org/10.3390/nu15071782
APA StyleSergi, D., Sanz, J. M., Lazzer, S., Brombo, G., Zuliani, G., Biolo, G., Šimunič, B., Pišot, R., Dalla Nora, E., & Passaro, A. (2023). Interleukin-18 Is a Potential Biomarker Linking Dietary Fatty Acid Quality and Insulin Resistance: Results from a Cross-Sectional Study in Northern Italy. Nutrients, 15(7), 1782. https://doi.org/10.3390/nu15071782









