Relationship between the Mediterranean Diet Score in Pregnancy and the Incidence of Asthma at 4 Years of Age: The Japan Environment and Children’s Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Data Collection
2.3. Calculation of the MD Score
2.4. Main Outcome
2.5. Statistical Analysis
3. Results
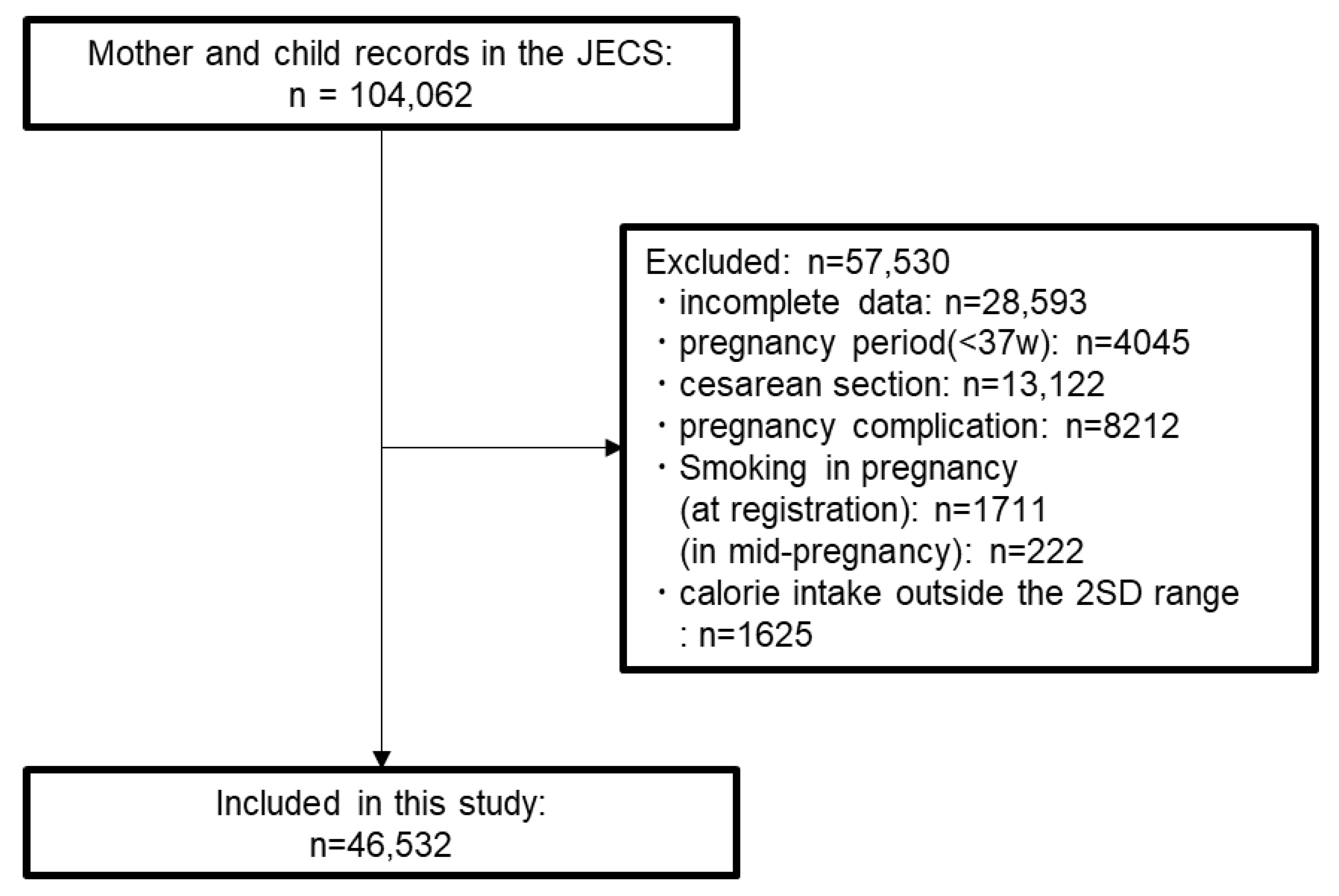
3.1. Participant Selection
3.2. Baseline Characteristics
3.3. Main Results
3.3.1. MDS Analysis
3.3.2. rMED Analysis
3.3.3. PMDS Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Trichopoulou, A.; Lagiou, P. Healthy Traditional Mediterranean Diet: An Expression of Culture, History, and Lifestyle. Nutr. Rev. 1997, 55, 383–389. [Google Scholar] [CrossRef]
- Keys, A. Coronary Heart Disease in Seven Countries. Nutrition 1997, 13, 249–253. [Google Scholar] [CrossRef]
- Tang, C.; Wang, X.; Qin, L.-Q.; Dong, J.-Y. Mediterranean Diet and Mortality in People with Cardiovascular Disease: A Meta-Analysis of Prospective Cohort Studies. Nutrients 2021, 13, 2623. [Google Scholar] [CrossRef]
- Chatzi, L.; Rifas-Shiman, S.L.; Georgiou, V.; Joung, K.E.; Koinaki, S.; Chalkiadaki, G.; Margioris, A.; Sarri, K.; Vassilaki, M.; Vafeiadi, M.; et al. Adherence to the Mediterranean Diet during Pregnancy and Offspring Adiposity and Cardiometabolic Traits in Childhood. Pediatr. Obes. 2017, 12, 47–56. [Google Scholar] [CrossRef]
- Crovetto, F.; Crispi, F.; Casas, R.; Martín-Asuero, A.; Borràs, R.; Vieta, E.; Estruch, R.; Gratacós, E.; Paules, C.; Nakaki, A.; et al. Effects of Mediterranean Diet or Mindfulness-Based Stress Reduction on Prevention of Small-for-Gestational Age Birth Weights in Newborns Born to At-Risk Pregnant Individuals. JAMA 2021, 326, 2150. [Google Scholar] [CrossRef]
- Al Wattar, B.H.; Dodds, J.; Placzek, A.; Beresford, L.; Spyreli, E.; Moore, A.; Gonzalez Carreras, F.J.; Austin, F.; Murugesu, N.; Roseboom, T.J.; et al. Mediterranean-Style Diet in Pregnant Women with Metabolic Risk Factors (ESTEEM): A Pragmatic Multicentre Randomised Trial. PLoS Med. 2019, 16, e1002857. [Google Scholar] [CrossRef]
- Rhee, D.K.; Ji, Y.; Hong, X.; Pearson, C.; Wang, X.; Caulfield, L.E. Mediterranean-Style Diet and Birth Outcomes in an Urban, Multiethnic, and Low-Income US Population. Nutrients 2021, 13, 1188. [Google Scholar] [CrossRef]
- Amati, F.; Hassounah, S.; Swaka, A. The Impact of Mediterranean Dietary Patterns During Pregnancy on Maternal and Offspring Health. Nutrients 2019, 11, 1098. [Google Scholar] [CrossRef] [PubMed]
- Chatzi, L.; Torrent, M.; Romieu, I.; Garcia-Esteban, R.; Ferrer, C.; Vioque, J.; Kogevinas, M.; Sunyer, J. Mediterranean Diet in Pregnancy Is Protective for Wheeze and Atopy in Childhood. Thorax 2008, 63, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Castro-Rodriguez, J.A.; Garcia-Marcos, L. What Are the Effects of a Mediterranean Diet on Allergies and Asthma in Children? Front. Pediatr. 2017, 5, 72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, J.; Fu, W.; Liu, S.; Gong, C.; Dai, J. Mediterranean Diet during Pregnancy and Childhood for Asthma in Children: A Systematic Review and Meta-analysis of Observational Studies. Pediatr. Pulmonol. 2019, 54, 949–961. [Google Scholar] [CrossRef]
- Biagi, C.; Di Nunzio, M.; Bordoni, A.; Gori, D.; Lanari, M. Effect of Adherence to Mediterranean Diet during Pregnancy on Children’s Health: A Systematic Review. Nutrients 2019, 11, 997. [Google Scholar] [CrossRef]
- Eckl, M.R.; Brouwer-Brolsma, E.M.; Küpers, L.K. Maternal Adherence to the Mediterranean Diet during Pregnancy: A Review of Commonly Used a Priori Indexes. Nutrients 2021, 13, 582. [Google Scholar] [CrossRef]
- Martínez-Galiano, J.; Olmedo-Requena, R.; Barrios-Rodríguez, R.; Amezcua-Prieto, C.; Bueno-Cavanillas, A.; Salcedo-Bellido, I.; Jimenez-Moleon, J.; Delgado-Rodríguez, M. Effect of Adherence to a Mediterranean Diet and Olive Oil Intake during Pregnancy on Risk of Small for Gestational Age Infants. Nutrients 2018, 10, 1234. [Google Scholar] [CrossRef]
- Michikawa, T.; Nitta, H.; Nakayama, S.F.; Yamazaki, S.; Isobe, T.; Tamura, K.; Suda, E.; Ono, M.; Yonemoto, J.; Iwai-Shimada, M.; et al. Baseline Profile of Participants in the Japan Environment and Children’s Study (JECS). J. Epidemiol. 2018, 28, 99–104. [Google Scholar] [CrossRef]
- Kawamoto, T.; Nitta, H.; Murata, K.; Toda, E.; Tsukamoto, N.; Hasegawa, M.; Yamagata, Z.; Kayama, F.; Kishi, R.; Ohya, Y.; et al. Rationale and Study Design of the Japan Environment and Children’s Study (JECS). BMC Public Health 2014, 14, 25. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Takachi, R.; Ishihara, J.; Ishii, Y.; Sasazuki, S.; Sawada, N.; Shinozawa, Y.; Tanaka, J.; Kato, E.; Kitamura, K.; et al. Validity of Short and Long Self-Administered Food Frequency Questionnaires in Ranking Dietary Intake in Middle-Aged and Elderly Japanese in the Japan Public Health Center-Based Prospective Study for the Next Generation (JPHC-NEXT) Protocol Area. J. Epidemiol. 2016, 26, 420–432. [Google Scholar] [CrossRef]
- Asher, M.I.; Keil, U.; Anderson, H.R.; Beasley, R.; Crane, J.; Martinez, F.; Mitchell, E.A.; Pearce, N.; Sibbald, B.; Stewart, A.W.; et al. International Study of Asthma and Allergies in Childhood (ISAAC): Rationale and Methods. Eur. Respir. J. 1995, 8, 483–491. [Google Scholar] [CrossRef]
- Weiland, S.K. Phase II of the International Study of Asthma and Allergies in Childhood (ISAAC II): Rationale and Methods. Eur. Respir. J. 2004, 24, 406–412. [Google Scholar] [CrossRef]
- Ellwood, P.; Asher, M.I.; Beasley, R.; Clayton, T.O.; Stewart, A.W. Phase Three Manual of the International Study of Asthma and Allergies in Childhood (ISAAC). Int. J. Tuberc. Lung Dis. 2000, 9, 10–16. [Google Scholar]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean Diet and Survival in a Greek Population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Buckland, G.; Gonzalez, C.A.; Agudo, A.; Vilardell, M.; Berenguer, A.; Amiano, P.; Ardanaz, E.; Arriola, L.; Barricarte, A.; Basterretxea, M.; et al. Adherence to the Mediterranean Diet and Risk of Coronary Heart Disease in the Spanish EPIC Cohort Study. Am. J. Epidemiol. 2009, 170, 1518–1529. [Google Scholar] [CrossRef] [PubMed]
- Descotes, J.; Choquet-Kastylevsky, G. Gell and Coombs’s Classification: Is It Still Valid? Toxicology 2001, 158, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Justiz Vaillant, A.A.; Vashisht, R.; Zito, P.M. Immediate Hypersensitivity Reactions; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Tsuge, M.; Ikeda, M.; Matsumoto, N.; Yorifuji, T.; Tsukahara, H. Current Insights into Atopic March. Children 2021, 8, 1067. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the Freely Available Easy-to-Use Software ‘EZR’ for Medical Statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Yamamoto-Hanada, K.; Yang, L.; Ishitsuka, K.; Ayabe, T.; Mezawa, H.; Konishi, M.; Shoda, T.; Matsumoto, K.; Saito, H.; Ohya, Y. Allergic Profiles of Mothers and Fathers in the Japan Environment and Children’s Study (JECS): A Nationwide Birth Cohort Study. World Allergy Organ. J. 2017, 10, 24. [Google Scholar] [CrossRef]
- Burke, H.; Leonardi-Bee, J.; Hashim, A.; Pine-Abata, H.; Chen, Y.; Cook, D.G.; Britton, J.R.; McKeever, T.M. Prenatal and Passive Smoke Exposure and Incidence of Asthma and Wheeze: Systematic Review and Meta-Analysis. Pediatrics 2012, 129, 735–744. [Google Scholar] [CrossRef]
- Van Neerven, R.J.J.; Savelkoul, H. Nutrition and Allergic Diseases. Nutrients 2017, 9, 762. [Google Scholar] [CrossRef]
- Federico, M.J.; McFarlane, A.E.; Szefler, S.J.; Abrams, E.M. The Impact of Social Determinants of Health on Children with Asthma. J. Allergy Clin. Immunol. Pract. 2020, 8, 1808–1814. [Google Scholar] [CrossRef]
- Miller, C.B.; Benny, P.; Riel, J.; Boushey, C.; Perez, R.; Khadka, V.; Qin, Y.; Maunakea, A.K.; Lee, M.-J. Adherence to Mediterranean Diet Impacts Gastrointestinal Microbial Diversity throughout Pregnancy. BMC Pregnancy Childbirth 2021, 21, 558. [Google Scholar] [CrossRef]
- Du, T.; Lei, A.; Zhang, N.; Zhu, C. The Beneficial Role of Probiotic Lactobacillus in Respiratory Diseases. Front. Immunol. 2022, 13, 908010. [Google Scholar] [CrossRef]
- Shao, Y.; Forster, S.C.; Tsaliki, E.; Vervier, K.; Strang, A.; Simpson, N.; Kumar, N.; Stares, M.D.; Rodger, A.; Brocklehurst, P.; et al. Stunted Microbiota and Opportunistic Pathogen Colonization in Caesarean-Section Birth. Nature 2019, 574, 117–121. [Google Scholar] [CrossRef]
| Type1 Allergy | Mothers (n = 46,532) | Children (n = 46,532) |
|---|---|---|
| One or more (%) | 23,176 (49.8) | 11,293 (24.3) |
| Asthma (%) | 4324 (9.3) | 3815 (8.2) |
| Food allergy (%) | 2050 (4.4) | 2616 (5.6) |
| Atopic dermatitis (%) | 7394 (15.9) | 3954 (8.5) |
| Allergic conjunctivitis (%) | 4661 (10.0) | 1223 (2.6) |
| Allergic rhinitis (%) | 16,930 (36.4) | 3526 (7.6) |
| All Children | Maternal Allergies Group | No Maternal Allergies Group | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Type1 Allergy | MDS High (n = 13,336) | MDS Low (n = 33,196) | p | MDS High (n = 6634) | MDS Low (n = 16,542) | p | MDS High (n = 6702) | MDS Low (n = 16,654) | p |
| One or more (%) | 3182 (23.9) | 8111 (24.4) | 0.196 | 1941 (29.3) | 4933 (29.8) | 0.406 | 1241 (18.5) | 3178 (19.1) | 0.327 |
| Asthma (%) | 1060 (7.9) | 2755 (8.3) | 0.219 | 641 (9.7) | 1706 (10.3) | 0.144 | 419 (6.3) | 1049 (6.3) | 0.917 |
| Food allergy (%) | 747 (5.6) | 1869 (5.6) | 0.921 | 449 (6.8) | 1132 (6.8) | 0.860 | 298 (4.4) | 737 (4.4) | 0.972 |
| Atopic dermatitis (%) | 1157 (8.7) | 2797 (8.4) | 0.392 | 729 (11.0) | 1719 (10.4) | 0.189 | 428 (6.4) | 1078 (6.5) | 0.830 |
| Allergic conjunctivitis (%) | 358 (2.7) | 865 (2.6) | 0.654 | 246 (3.7) | 578 (3.5) | 0.450 | 112 (1.7) | 287 (1.7) | 0.824 |
| Allergic rhinitis (%) | 981 (7.4) | 2545 (7.7) | 0.260 | 648 (9.8) | 1662 (10.0) | 0.537 | 333 (5.0) | 883 (5.3) | 0.315 |
| All Children | Maternal Allergies Group | No Maternal Allergies Group | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Type1 Allergy | rMED High (n = 4476) | rMED Low (n = 42,056) | p | rMED High (n = 2265) | rMED Low (n = 20,911) | p | rMED High (n = 2211) | rMED Low (n = 21,145) | p |
| One or more (%) | 1117 (25.0) | 10,176 (24.2) | 0.268 | 704 (31.1) | 6170 (29.5) | 0.125 | 413 (18.7) | 4006 (18.9) | 0.783 |
| Asthma (%) | 364 (8.1) | 3451 (8.2) | 0.887 | 222 (9.8) | 2125 (10.2) | 0.614 | 142 (6.4) | 1326 (6.3) | 0.816 |
| Food allergy (%) | 266 (5.9) | 2350 (5.6) | 0.344 | 178 (7.9) | 1403 (6.7) | 0.043 | 88 (4.0) | 947 (4.5) | 0.303 |
| Atopic dermatitis (%) | 390 (8.7) | 3564 (8.5) | 0.606 | 253 (11.2) | 2195 (10.5) | 0.340 | 137 (6.2) | 1369 (6.5) | 0.645 |
| Allergic conjunctivitis (%) | 131 (2.9) | 1092 (2.6) | 0.206 | 96 (4.2) | 728 (3.5) | 0.074 | 35 (1.6) | 364 (1.7) | 0.695 |
| Allergic rhinitis (%) | 354 (7.9) | 3172 (7.5) | 0.395 | 249 (11.0) | 2061 (9.9) | 0.093 | 105 (4.7) | 1111 (5.3) | 0.333 |
| All Children | Maternal Allergies Group | No Maternal Allergies Group | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Type1 Allergy | PMDS High (n = 31,920) | PMDS Low (n = 14,612) | p | PMDS High (n = 16,109) | PMDS Low (n = 7067) | p | PMDS High (n = 15,811) | PMDS Low (n = 7545) | p |
| One or more (%) | 7646 (24.0) | 3647 (25.0) | 0.020 | 4735 (29.4) | 2139 (30.3) | 0.185 | 2911 (18.4) | 1508 (20.0) | 0.004 |
| Asthma (%) | 2533 (7.9) | 1282 (8.8) | 0.002 | 1598 (9.9) | 749 (10.6) | 0.120 | 935 (5.9) | 533 (7.1) | 0.0008 |
| Food allergy (%) | 1772 (5.6) | 844 (5.8) | 0.340 | 1080 (6.7) | 501 (7.1) | 0.297 | 692 (4.4) | 343 (4.5) | 0.579 |
| Atopic dermatitis (%) | 2687 (8.4) | 1267 (8.7) | 0.373 | 1695 (10.5) | 753 (10.7) | 0.779 | 992 (6.3) | 514 (6.8) | 0.124 |
| Allergic conjunctivitis (%) | 844 (2.6) | 379 (2.6) | 0.776 | 581 (3.6) | 243 (3.4) | 0.550 | 263 (1.7) | 136 (1.8) | 0.476 |
| Allergic rhinitis (%) | 2393 (7.5) | 1133 (7.8) | 0.340 | 1600 (9.9) | 710 (10.0) | 0.807 | 793 (5.0) | 423 (5.6) | 0.062 |
| Median [g/Day] | ||
|---|---|---|
| Food Item | Previous Study | Current Study |
| Vegetable | 499.6 | 158.0 |
| Legume | 6.7 | 52.1 |
| Fruit and nut | 356.3 | 106.8 |
| Cereal | 139.7 | 431.5 |
| Fish | 18.8 | 27.1 |
| Dairy product | 191.1 | 172.1 |
| Meat | 89.8 | 57.4 |
| All Children | |||
|---|---|---|---|
| Type 1 Allergy | PMDSnM High (n = 23,149) | PMDSnM Low (n = 23,383) | p |
| One or more (%) | 5735 (24.8) | 5558 (23.8) | 0.012 |
| Asthma (%) | 1926 (8.3) | 1889 (8.1) | 0.351 |
| Food allergy (%) | 1303 (5.6) | 1313 (5.6) | 0.965 |
| Atopic dermatitis (%) | 2032 (8.8) | 1922 (8.2) | 0.032 |
| Allergic conjunctivitis (%) | 638 (2.8) | 585 (2.5) | 0.092 |
| Allergic rhinitis (%) | 1806 (7.8) | 1720 (7.4) | 0.072 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakano, K.; Kuraoka, S.; Oda, M.; Ohba, T.; Mitsubuchi, H.; Nakamura, K.; Katoh, T.; the Japan Environment and Children’s Study (JECS) Group. Relationship between the Mediterranean Diet Score in Pregnancy and the Incidence of Asthma at 4 Years of Age: The Japan Environment and Children’s Study. Nutrients 2023, 15, 1772. https://doi.org/10.3390/nu15071772
Nakano K, Kuraoka S, Oda M, Ohba T, Mitsubuchi H, Nakamura K, Katoh T, the Japan Environment and Children’s Study (JECS) Group. Relationship between the Mediterranean Diet Score in Pregnancy and the Incidence of Asthma at 4 Years of Age: The Japan Environment and Children’s Study. Nutrients. 2023; 15(7):1772. https://doi.org/10.3390/nu15071772
Chicago/Turabian StyleNakano, Kaita, Shohei Kuraoka, Masako Oda, Takashi Ohba, Hiroshi Mitsubuchi, Kimitoshi Nakamura, Takahiko Katoh, and the Japan Environment and Children’s Study (JECS) Group. 2023. "Relationship between the Mediterranean Diet Score in Pregnancy and the Incidence of Asthma at 4 Years of Age: The Japan Environment and Children’s Study" Nutrients 15, no. 7: 1772. https://doi.org/10.3390/nu15071772
APA StyleNakano, K., Kuraoka, S., Oda, M., Ohba, T., Mitsubuchi, H., Nakamura, K., Katoh, T., & the Japan Environment and Children’s Study (JECS) Group. (2023). Relationship between the Mediterranean Diet Score in Pregnancy and the Incidence of Asthma at 4 Years of Age: The Japan Environment and Children’s Study. Nutrients, 15(7), 1772. https://doi.org/10.3390/nu15071772






