Heat-Killed Lacticaseibacillus paracasei Repairs Lipopolysaccharide-Induced Intestinal Epithelial Barrier Damage via MLCK/MLC Pathway Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Bacterium Cultures and Preparation
2.3. Cell Culture
2.4. Cell Viability Assay
2.5. Measurement of Membrane Permeability of Caco-2 Cell Monolayers
2.6. Trans-Epithelial Electrical Resistance (TEER) Assay
2.7. Measurement of the Inflammatory Maker
2.8. Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR) Analysis
2.9. Western Blot Analysis
2.10. Immunofluorescence Analysis
2.11. Data Statistics
3. Results
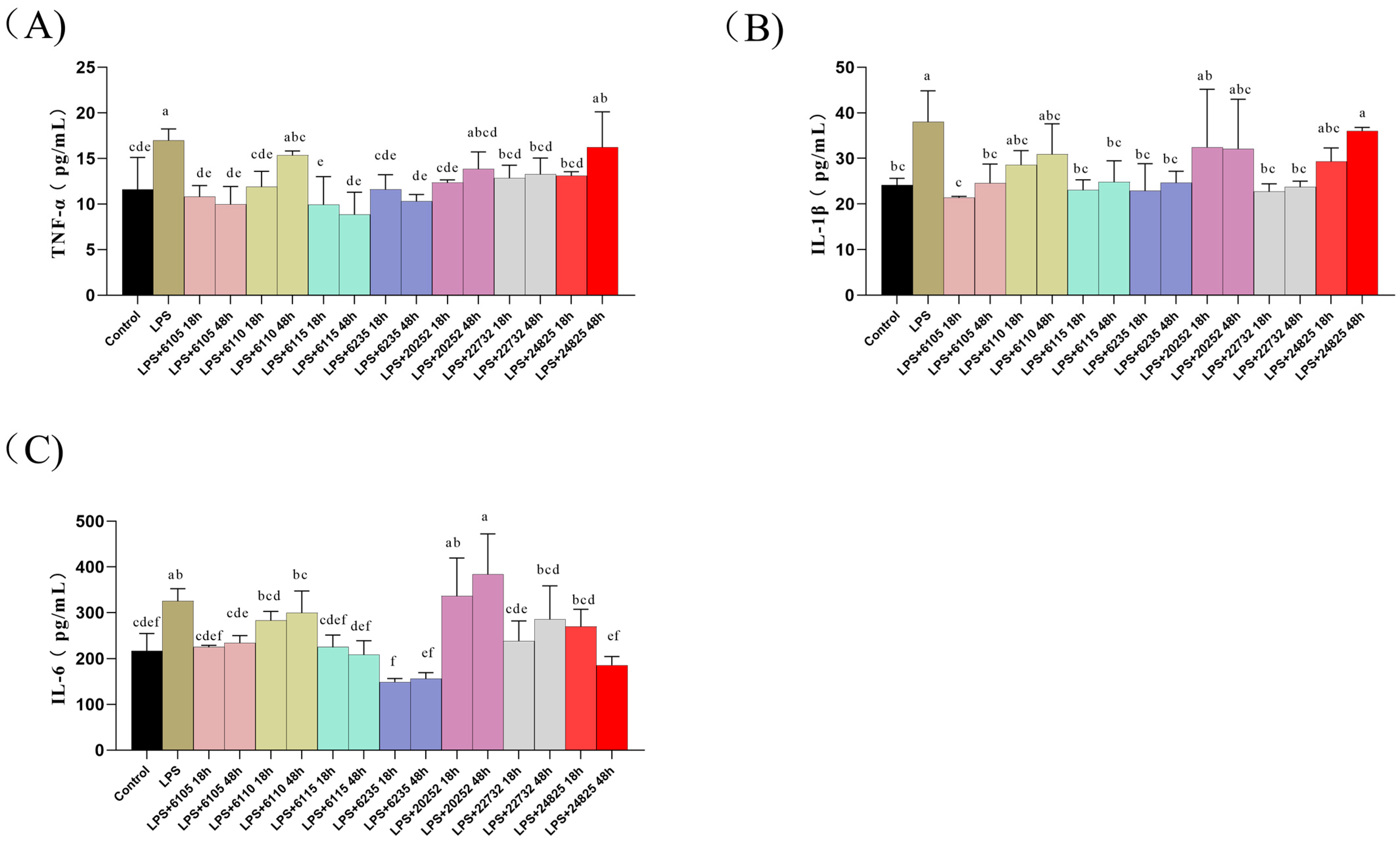
3.1. Effects of Nine HK-LP Strains on Pro-Inflammatory Factors Content
3.2. Effects of HK-LP on Caco-2 Cell Viability
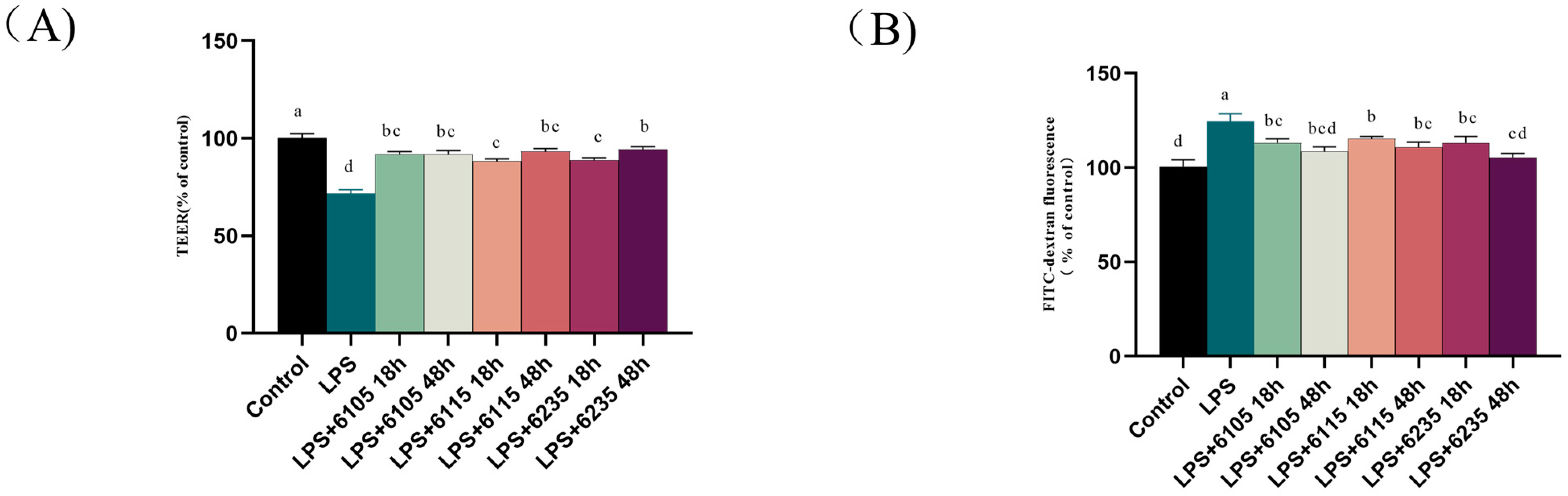
3.3. HK-LP Treatment Restored LPS-Induced Damage in Caco-2 Cells Monolayers
3.4. HK-LP Impacted LPS-Induced Inflammatory Factors in LPS-Induced Caco-2 Cell Monolayers
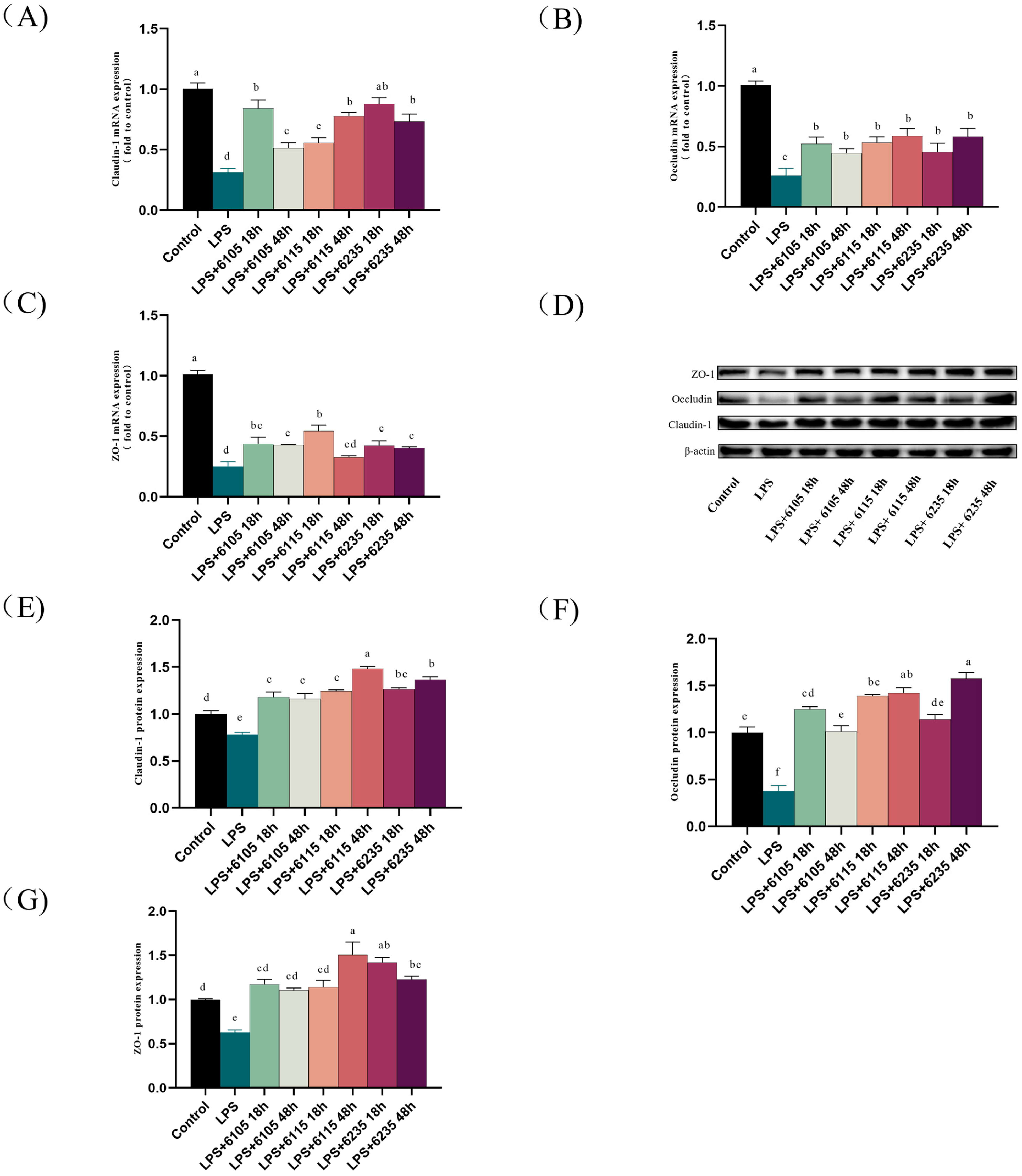
3.5. HK-LP Changed the Expression Levels of Tight Junction Proteins
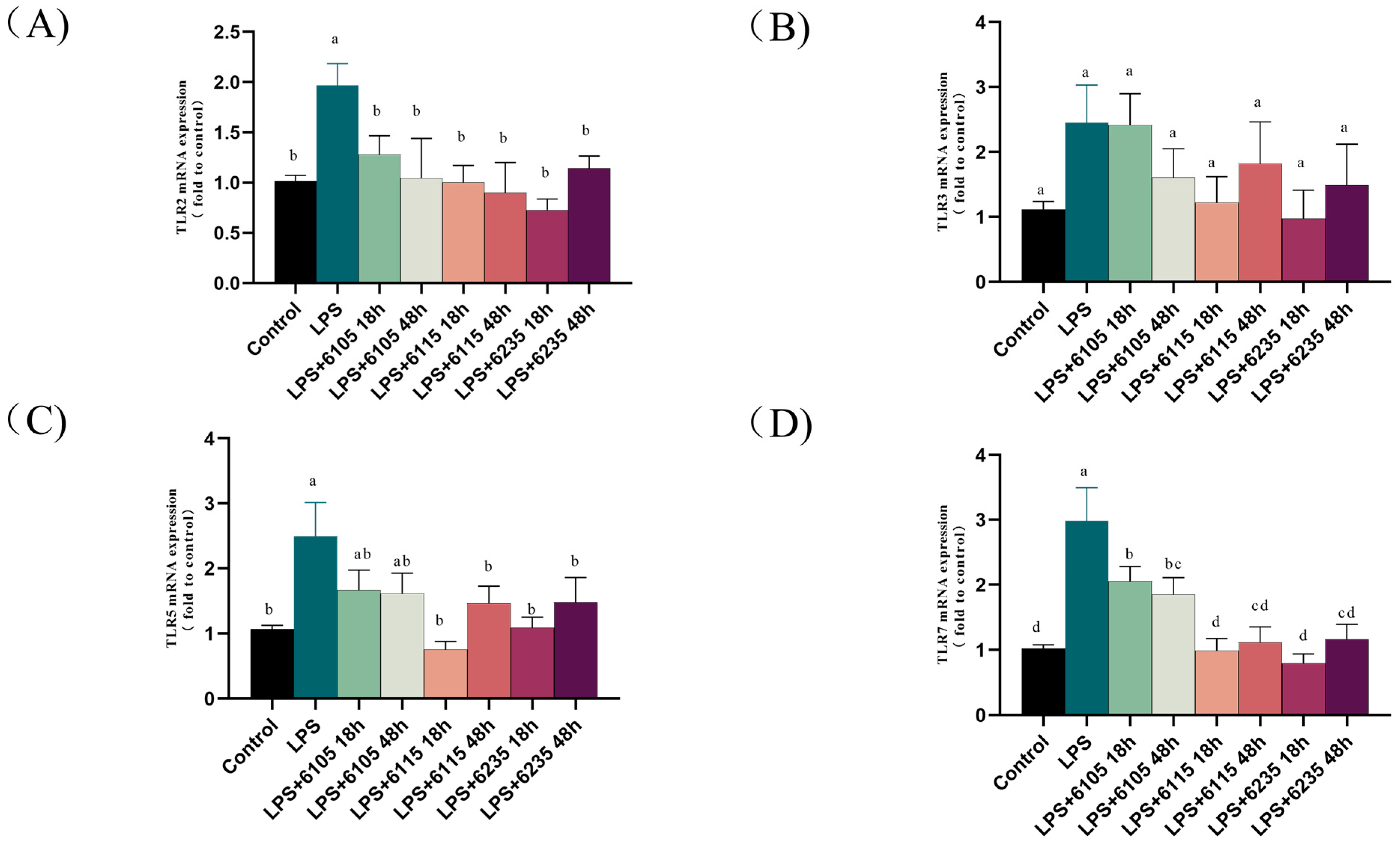
3.6. HK-LP Inhibited LPS-Induced TLRs in LPS-Induced Caco-2 Cell Monolayers
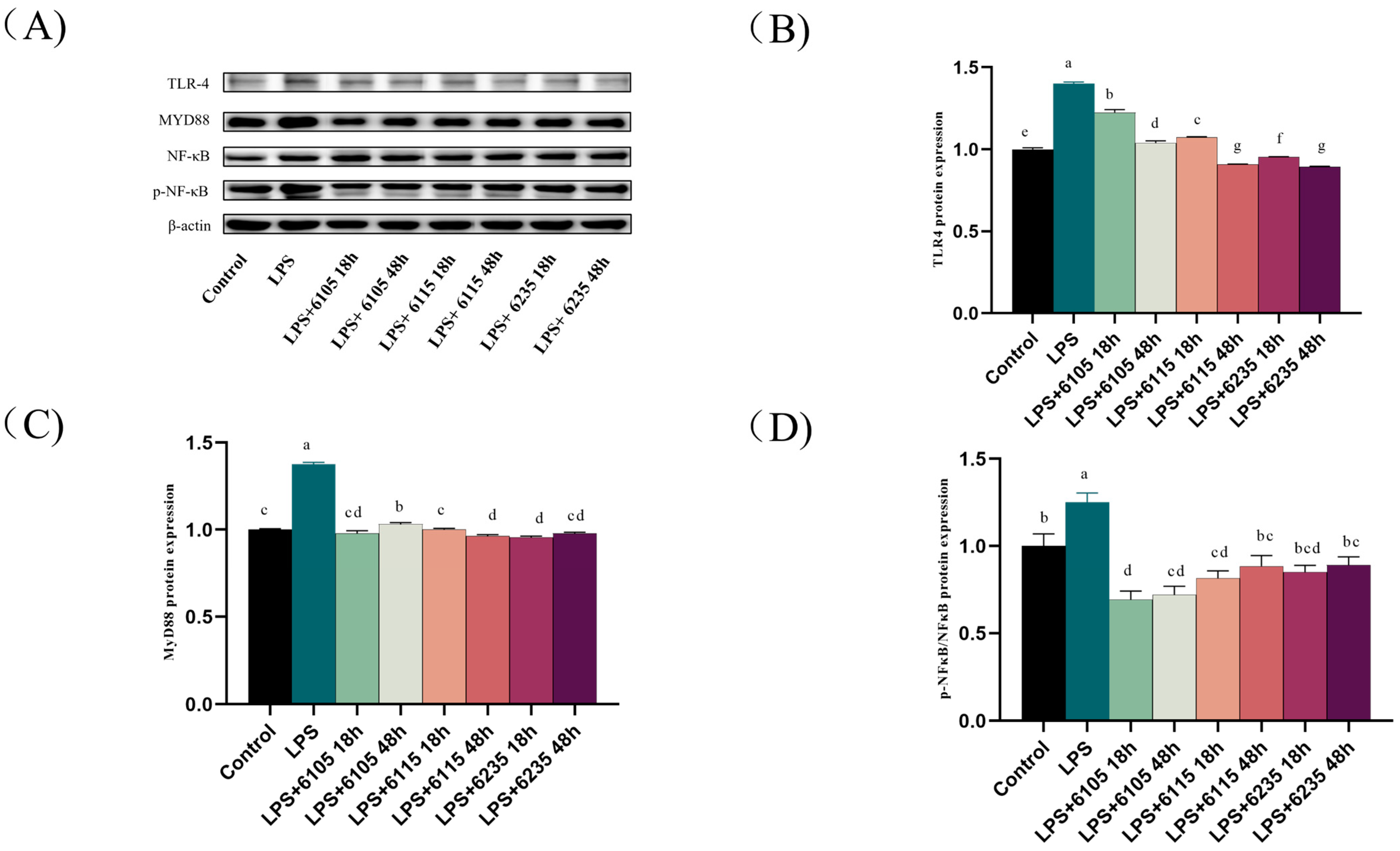
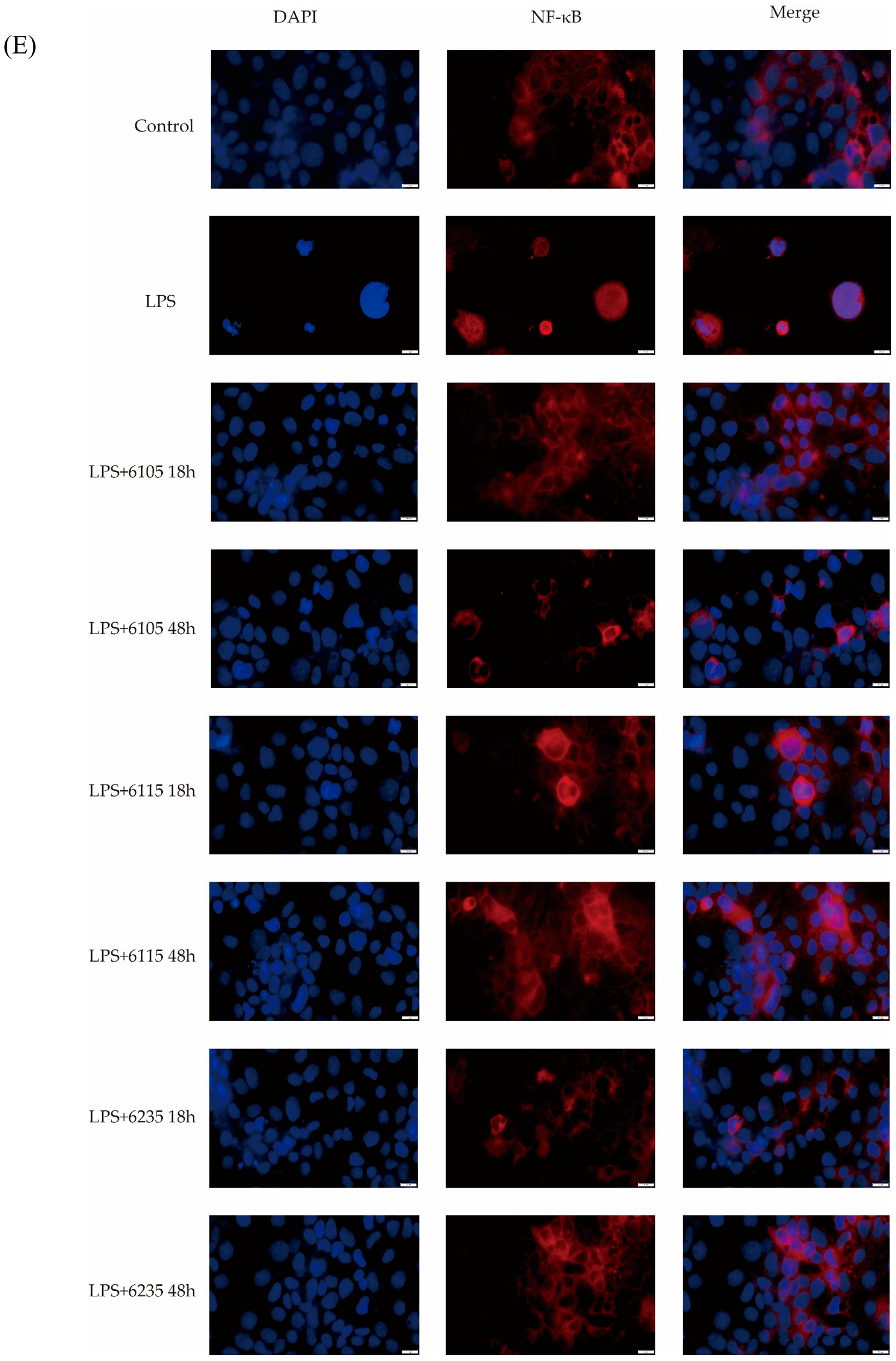
3.7. HK-LP Recovered TLR4/MyD88/NF-κB Signaling Pathway
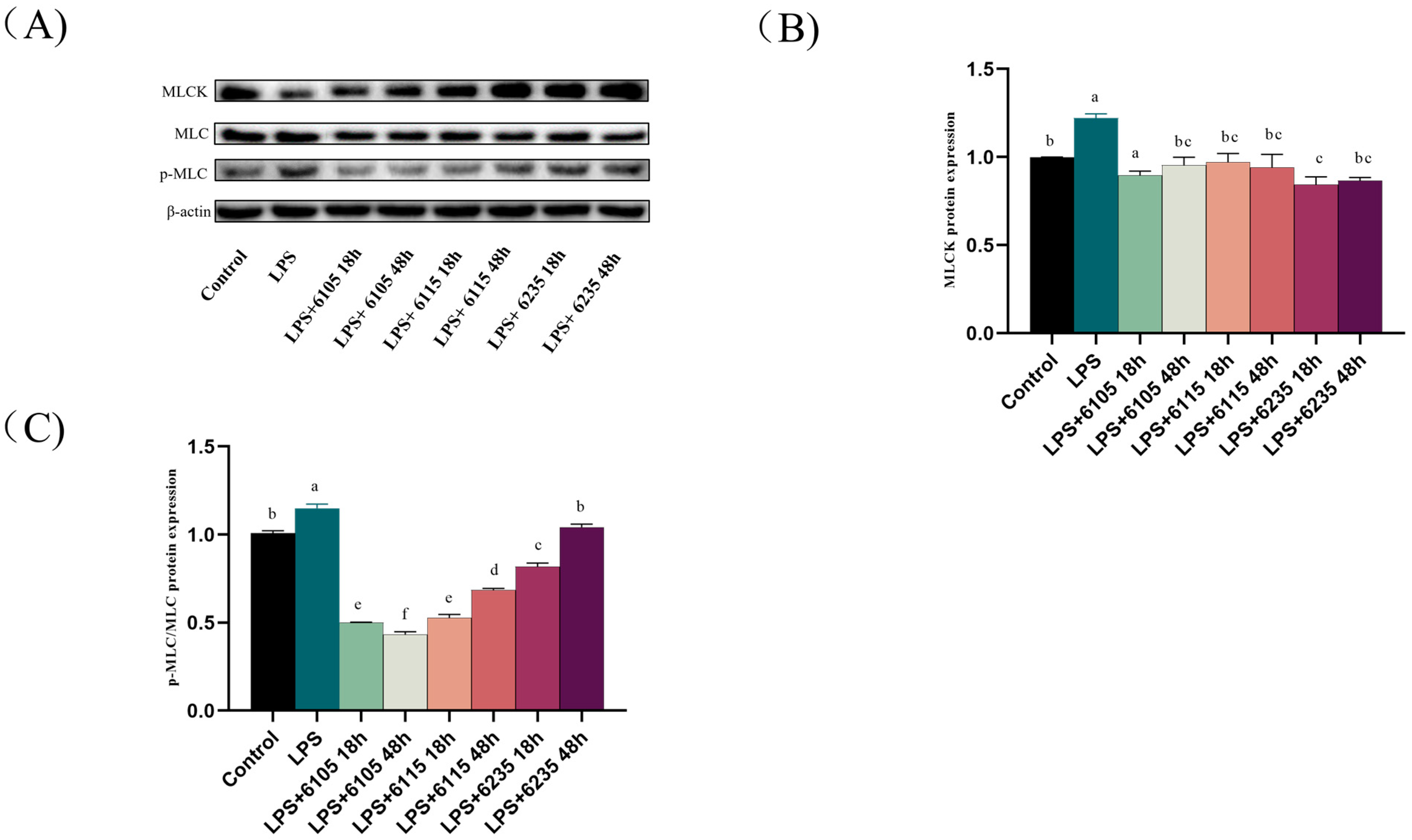
3.8. HK-LP Improved the Modulation of the MLCK/MLC Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, X.; Lin, C.; Zhen, W. Cancer care in China: A general review. Biomed. Imaging Interv. J. 2008, 4, e39. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef]
- Li, R.; Huang, X.; Yang, L.; Liang, X.; Huang, W.; Lai, K.P.; Zhou, L. Integrated Analysis Reveals the Targets and Mechanisms in Immunosuppressive Effect of Mesalazine on Ulcerative Colitis. Front. Nutr. 2022, 9, 867692. [Google Scholar] [CrossRef] [PubMed]
- Bashashati, M.; Rezaei, N.; Andrews, C.N.; Chen, C.-Q.; Daryani, N.E.; Sharkey, K.A.; Storr, M.A. Cytokines and irritable bowel syndrome: Where do we stand? Cytokine 2012, 57, 201–209. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Deng, Z. Imbalanced shift of cytokine expression between T helper 1 and T helper 2 (Th1/Th2) in intestinal mucosa of patients with post-infectious irritable bowel syndrome. BMC Gastroenterol. 2012, 12, 91. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Lucas, M.; Saz-Peiró, P.; Sebastián-Domingo, J.J. Irritable bowel syndrome immune hypothesis. Part two: The role of cytokines. Rev. Esp. Enferm. Dig. Organo Off. Soc. Esp. Patol. Dig. 2010, 102, 711–717. [Google Scholar] [CrossRef]
- Darkoh, C.; Comer, L.; Zewdie, G.; Harold, S.; Snyder, N.; Dupont, H.L. Chemotactic chemokines are important in the pathogenesis of irritable bowel syndrome. PLoS ONE 2014, 9, e93144. [Google Scholar] [CrossRef]
- Wang, G.; Wang, H.; Jin, Y.; Xiao, Z.; Umar Yaqoob, M.; Lin, Y.; Chen, H.; Wang, M. Galactooligosaccharides as a protective agent for intestinal barrier and its regulatory functions for intestinal microbiota. Food Res. Int. 2022, 155, 111003. [Google Scholar] [CrossRef]
- Xie, C.; Zhang, Y.; Wang, H.H.; Matsumoto, A.; Nakamura, A.; Ishikawa, R.; Yoshiyama, S.; Hayakawa, K.; Kohama, K.; Gao, Y. Calcium regulation of non-kinase and kinase activities of recombinant myosin light-chain kinase and its mutants. IUBMB Life 2009, 61, 1092–1098. [Google Scholar] [CrossRef]
- Nenci, A.; Becker, C.; Wullaert, A.; Gareus, R.; van Loo, G.; Danese, S.; Huth, M.; Nikolaev, A.; Neufert, C.; Madison, B.; et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature 2007, 446, 557–561. [Google Scholar] [CrossRef]
- Anderson, R.C.; Cookson, A.L.; McNabb, W.C.; Park, Z.; McCann, M.J.; Kelly, W.J.; Roy, N.C. Lactobacillus plantarum MB452 enhances the function of the intestinal barrier by increasing the expression levels of genes involved in tight junction formation. BMC Microbiol. 2010, 10, 316. [Google Scholar] [CrossRef]
- Yu, P.; Ke, C.; Guo, J.; Zhang, X.; Li, B. Lactobacillus plantarum L15 Alleviates Colitis by Inhibiting LPS-Mediated NF-κB Activation and Ameliorates DSS-Induced Gut Microbiota Dysbiosis. Front. Immunol. 2020, 11, 575173. [Google Scholar] [CrossRef] [PubMed]
- Merenstein, D.J.; Tan, T.P.; Molokin, A.; Smith, K.H.; Roberts, R.F.; Shara, N.M.; Mete, M.; Sanders, M.E.; Solano-Aguilar, G. Safety of Bifidobacterium animalis subsp. lactis (B. lactis) strain BB-12-supplemented yogurt in healthy adults on antibiotics: A phase I safety study. Gut Microbes 2015, 6, 66–77. [Google Scholar] [CrossRef] [PubMed]
- König, J.; Wells, J.; Cani, P.D.; García-Ródenas, C.L.; MacDonald, T.; Mercenier, A.; Whyte, J.; Troost, F.; Brummer, R.J. Human Intestinal Barrier Function in Health and Disease. Clin. Transl. Gastroenterol. 2016, 7, e196. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Singh, P.; Nataraj, B.H.; Kokkiligadda, A.; Naithani, H.; Azmal Ali, S.; Behare, P.V.; Nagpal, R. Fostering next-generation probiotics in human gut by targeted dietary modulation: An emerging perspective. Food Res. Int. 2021, 150, 110716. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Castro-Herrera, V.M.; Rasmussen, C.; Wellejus, A.; Miles, E.A.; Calder, P.C. In Vitro Effects of Live and Heat-Inactivated Bifidobacterium animalis Subsp. Lactis, BB-12 and Lactobacillus rhamnosus GG on Caco-2 Cells. Nutrients 2020, 12, 1719. [Google Scholar] [CrossRef]
- Mileti, E.; Matteoli, G.; Iliev, I.D.; Rescigno, M.; Fritz, J.R.H. Comparison of the Immunomodulatory Properties of Three Probiotic Strains of Lactobacilli Using Complex Culture Systems: Prediction for In Vivo Efficacy. PLoS ONE 2009, 4, e7056. [Google Scholar] [CrossRef]
- Ibnou-Zekri, N.; Blum, S.; Schiffrin, E.J.; von der Weid, T. Divergent patterns of colonization and immune response elicited from two intestinal Lactobacillus strains that display similar properties in vitro. Infect. Immun. 2003, 71, 428–436. [Google Scholar] [CrossRef]
- Peña, J.A.; Rogers, A.B.; Ge, Z.; Ng, V.; Li, S.Y.; Fox, J.G.; Versalovic, J. Probiotic Lactobacillus spp. Diminish Helicobacter hepaticus-Induced Inflammatory Bowel Disease in Interleukin-10-Deficient Mice. ASM J. 2005, 73, 912–920. [Google Scholar] [CrossRef]
- Chen, C.-L.; Hsu, P.-Y.; Pan, T.-M. Therapeutic effects of Lactobacillus paracasei subsp. paracasei NTU 101 powder on dextran sulfate sodium-induced colitis in mice. J. Food Drug Anal. 2019, 27, 83–92. [Google Scholar] [CrossRef]
- Maehata, H.; Arai, S.; Iwabuchi, N.; Abe, F. Immuno-modulation by heat-killed Lacticaseibacillus paracasei MCC1849 and its application to food products. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211008291. [Google Scholar] [CrossRef]
- Wang, S.; Ahmadi, S.; Nagpal, R.; Jain, S.; Mishra, S.P.; Kavanagh, K.; Zhu, X.; Wang, Z.; McClain, D.A.; Kritchevsky, S.B.; et al. Lipoteichoic acid from the cell wall of a heat killed Lactobacillus paracasei D3-5 ameliorates aging-related leaky gut, inflammation and improves physical and cognitive functions: From C. elegans to mice. GeroScience 2020, 42, 333–352. [Google Scholar] [CrossRef] [PubMed]
- Speciale, A.; Muscarà, C.; Molonia, M.S.; Toscano, G.; Cimino, F.; Saija, A. In Vitro Protective Effects of a Standardized Extract From Cynara Cardunculus L. Leaves Against TNF-α-Induced Intestinal Inflammation. Front. Pharm. 2022, 13, 809938. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Negoro, R.; Yamashita, T.; Kawai, K.; Ichikawa, M.; Mori, T.; Nakatsu, N.; Harada, K.; Ito, S.; Yamada, H.; et al. Generation of Human iPSC-Derived Intestinal Epithelial Cell Monolayers by CDX2 Transduction. Cell Mol. Gastroenterol. Hepatol. 2019, 8, 513–526. [Google Scholar] [CrossRef]
- Gong, S.; Zheng, J.; Zhang, J.; Wang, Y.; Xie, Z.; Wang, Y.; Han, J. Taxifolin ameliorates lipopolysaccharide-induced intestinal epithelial barrier dysfunction via attenuating NF-kappa B/MLCK pathway in a Caco-2 cell monolayer model. Food Res. Int. 2022, 158, 111502. [Google Scholar] [CrossRef] [PubMed]
- Ottman, N.; Reunanen, J.; Meijerink, M.; Pietilä, T.E.; Kainulainen, V.; Klievink, J.; Huuskonen, L.; Aalvink, S.; Skurnik, M.; Boeren, S.; et al. Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function. PLoS ONE 2017, 12, e0173004. [Google Scholar] [CrossRef]
- Huo, J.; Li, M.; Wei, J.; Wang, Y.; Hao, W.; Sun, W.; Wu, J.; Huang, M. RNA-seq based elucidation of mechanism underlying the protective effect of Huangshui polysaccharide on intestinal barrier injury in Caco-2 cells. Food Res. Int. 2022, 162, 112175. [Google Scholar] [CrossRef]
- Paddison, P.J.; Silva, J.M.; Conklin, D.S.; Schlabach, M.; Li, M.; Aruleba, S.; Balija, V.; O’Shaughnessy, A.; Gnoj, L.; Scobie, K.; et al. A resource for large-scale RNA-interference-based screens in mammals. Nature 2004, 428, 427–431. [Google Scholar] [CrossRef]
- Karthikeyan, R.S.; Priya, J.L.; Leal, S.M., Jr.; Toska, J.; Rietsch, A.; Prajna, V.; Pearlman, E.; Lalitha, P. Host response and bacterial virulence factor expression in Pseudomonas aeruginosa and Streptococcus pneumoniae corneal ulcers. PLoS ONE 2013, 8, e64867. [Google Scholar] [CrossRef]
- Mesci, P.; Macia, A.; LaRock, C.N.; Tejwani, L.; Fernandes, I.R.; Suarez, N.A.; Zanotto, P.M.d.A.; Beltrão-Braga, P.C.B.; Nizet, V.; Muotri, A.R. Modeling neuro-immune interactions during Zika virus infection. Hum. Mol. Genet. 2018, 27, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Na, B.H.; Hoang, T.X.; Kim, J.Y. Hsp90 Inhibition Reduces TLR5 Surface Expression and NF-κB Activation in Human Myeloid Leukemia THP-1 Cells. Biomed. Res. Int. 2018, 2018, 4319369. [Google Scholar] [CrossRef]
- Chattergoon, M.A.; Latanich, R.; Quinn, J.; Winter, M.E.; Buckheit, R.W., 3rd; Blankson, J.N.; Pardoll, D.; Cox, A.L. HIV and HCV activate the inflammasome in monocytes and macrophages via endosomal Toll-like receptors without induction of type 1 interferon. PLoS Pathog. 2014, 10, e1004082. [Google Scholar] [CrossRef] [PubMed]
- Corpetti, C.; Del Re, A.; Seguella, L.; Palenca, I.; Rurgo, S.; De Conno, B.; Pesce, M.; Sarnelli, G.; Esposito, G. Cannabidiol inhibits SARS-Cov-2 spike (S) protein-induced cytotoxicity and inflammation through a PPARγ-dependent TLR4/NLRP3/Caspase-1 signaling suppression in Caco-2 cell line. Phytother. Res. PTR 2021, 35, 6893–6903. [Google Scholar] [CrossRef]
- Da Silva, M.; Jaggers, G.K.; Verstraeten, S.V.; Erlejman, A.G.; Fraga, C.G.; Oteiza, P.I. Large procyanidins prevent bile-acid-induced oxidant production and membrane-initiated ERK1/2, p38, and Akt activation in Caco-2 cells. Free Radic Biol. Med. 2012, 52, 151–159. [Google Scholar] [CrossRef]
- Yao, M.; Yao, Y.; Qin, B.; Pan, M.; Ju, X.; Xu, F.; Wang, L. Screening and identification of high bioavailable oligopeptides from rapeseed napin (Brassica napus) protein-derived hydrolysates via Caco-2/HepG2 co-culture model. Food Res. Int. 2022, 155, 111101. [Google Scholar] [CrossRef]
- Martorell, P.; Alvarez, B.; Llopis, S.; Navarro, V.; Ortiz, P.; Gonzalez, N.; Balaguer, F.; Rojas, A.; Chenoll, E.; Ramón, D.; et al. Heat-Treated Bifidobacterium longum CECT-7347: A Whole-Cell Postbiotic with Antioxidant, Anti-Inflammatory, and Gut-Barrier Protection Properties. Antioxidants 2021, 10, 536. [Google Scholar] [CrossRef]
- Jam, S.A.M.; Talebi, M.; Alipour, B.; Khosroushahi, A.Y. The therapeutic effect of potentially probiotic Lactobacillus paracasei on dimethylhydrazine induced colorectal cancer in rats. Food Biosci. 2021, 41, 101097. [Google Scholar] [CrossRef]
- Cifre, M.; Palou, A.; Oliver, P. Impaired CPT1A Gene Expression Response to Retinoic Acid Treatment in Human PBMC as Predictor of Metabolic Risk. Nutrients 2020, 12, 2269. [Google Scholar] [CrossRef]
- Yu, H.R.; Sheen, J.M.; Hou, C.Y.; Lin, I.C.; Huang, L.T.; Tain, Y.L.; Cheng, H.H.; Lai, Y.J.; Lin, Y.J.; Tiao, M.M.; et al. Effects of Maternal Gut Microbiota-Targeted Therapy on the Programming of Nonalcoholic Fatty Liver Disease in Dams and Fetuses, Related to a Prenatal High-Fat Diet. Nutrients 2022, 14, 4004. [Google Scholar] [CrossRef] [PubMed]
- Borchardt, R.T.; Hidalgo, I.J.; Raub, T.J. Characterization of the Human Colon Carcinoma Cell Line (Caco-2) as a Model System for Intestinal Epithelial Permeability, Gastroenterology, 96, 736–749, 1989—The Backstory. AAPS J. 2011, 13, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Erickson, R.H.; Gum, J.R.; Yoshioka, M.; Gum, E.; Kim, Y.S. Biosynthesis of Alkaline Phosphatase During Differentiation of the Human Colon Cancer Cell Line Caco-2. Gastroenterology 1990, 98, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Pinton, P.; Nougayrède, J.P.; Del Rio, J.C.; Moreno, C.; Marin, D.E.; Ferrier, L.; Bracarense, A.P.; Kolf-Clauw, M.; Oswald, I.P. The food contaminant deoxynivalenol, decreases intestinal barrier permeability and reduces claudin expression. Toxicol. Appl. Pharmacol. 2009, 237, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Li, Y.; Wan, Y.; Hu, T.; Liu, L.; Yang, S.; Gong, Z.; Zeng, Q.; Wei, Y.; Yang, W.; et al. A Novel Postbiotic From Lactobacillus rhamnosus GG With a Beneficial Effect on Intestinal Barrier Function. Front. Microbiol. 2019, 10, 477. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Tsai, Y.-C.; Wang, S.-Y.; Chen, Y.-P.; Chen, M.-J. Coculture Strategy for Developing Lactobacillus paracasei PS23 Fermented Milk with Anti-Colitis Effect. Foods 2021, 10, 2337. [Google Scholar] [CrossRef]
- Karczewski, J.; Troost, F.J.; Konings, I.; Dekker, J.; Kleerebezem, M.; Brummer, R.J.; Wells, J.M. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G851–G859. [Google Scholar] [CrossRef]
- Mayer, M.J.; D’Amato, A.; Colquhoun, I.J.; Gall, G.L.; Narbad, A. Identification of Genes Required for Glucan Exopolysaccharide Production in Lactobacillus johnsonii Suggests a Novel Biosynthesis Mechanism. Appl. Environ. Microbiol. 2020, 86, e02808–e02819. [Google Scholar] [CrossRef]
- Wang, Y.L.; Fang, M.; Wang, X.M.; Liu, W.Y.; Zheng, Y.J.; Wu, X.B.; Tao, R. Proinflammatory effects and molecular mechanisms of interleukin-17 in intestinal epithelial cell line HT-29. World J. Gastroenterol. 2014, 20, 17924–17931. [Google Scholar] [CrossRef]
- Strober, W.; Fuss, I.J. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology 2011, 140, 1756–1767. [Google Scholar] [CrossRef]
- Matter, K.; Balda, M.S. Signalling to and from tight junctions. Nat. Rev. Mol. Cell Biol. 2003, 4, 225–236. [Google Scholar] [CrossRef]
- Shen, L.; Weber, C.R.; Raleigh, D.R.; Yu, D.; Turner, J.R. Tight junction pore and leak pathways: A dynamic duo. Annu. Rev. Physiol. 2011, 73, 283–309. [Google Scholar] [CrossRef] [PubMed]
- Obata, Y.; Takahashi, D.; Ebisawa, M.; Kakiguchi, K.; Yonemura, S.; Jinnohara, T.; Kanaya, T.; Fujimura, Y.; Ohmae, M.; Hase, K.; et al. Epithelial cell-intrinsic Notch signaling plays an essential role in the maintenance of gut immune homeostasis. J. Immunol. 2012, 188, 2427–2436. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Bian, C.; Luo, Z.; Guille, C.; Ogunrinde, E.; Wu, J.; Zhao, M.; Fitting, S.; Kamen, D.L.; Oates, J.C.; et al. Progesterone decreases gut permeability through upregulating occludin expression in primary human gut tissues and Caco-2 cells. Sci. Rep. 2019, 9, 8367. [Google Scholar] [CrossRef]
- Brenchley, J.M.; Douek, D.C. Microbial translocation across the GI tract. Annu. Rev. Immunol. 2012, 30, 149–173. [Google Scholar] [CrossRef]
- Kim, S.; Shin, Y.C.; Kim, T.Y.; Kim, Y.; Lee, Y.S.; Lee, S.H.; Kim, M.N.; Eunju, O.; Kim, K.S.; Kweon, M.N. Mucin degrader Akkermansia muciniphila accelerates intestinal stem cell-mediated epithelial development. Gut Microbes 2021, 13, 1892441. [Google Scholar] [CrossRef] [PubMed]
- Edelblum, K.L.; Turner, J.R. The tight junction in inflammatory disease: Communication breakdown. Curr. Opin. Pharmacol. 2009, 9, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Rogers, A.P.; Mileto, S.J.; Lyras, D. Impact of enteric bacterial infections at and beyond the epithelial barrier. Nat. Rev. Microbiol. 2022, 21, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Meredith, T.C.; Kahne, D. On the essentiality of lipopolysaccharide to Gram-negative bacteria. Curr. Opin. Microbiol. 2013, 16, 779–785. [Google Scholar] [CrossRef]
- Bagarolli, R.A.; Tobar, N.; Oliveira, A.G.; Araújo, T.G.; Carvalho, B.M.; Rocha, G.Z.; Vecina, J.F.; Calisto, K.; Guadagnini, D.; Prada, P.O.; et al. Probiotics modulate gut microbiota and improve insulin sensitivity in DIO mice. J. Nutr. Biochem. 2017, 50, 16–25. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Gaynor, R.B. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J. Clin. Investig. 2001, 107, 135–142. [Google Scholar] [CrossRef]
- Sun, S.-C. The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef]
- Di Tommaso, N.; Gasbarrini, A.; Ponziani, F.R. Intestinal Barrier in Human Health and Disease. Int. J. Environ. Res. Public Health 2021, 18, 12836. [Google Scholar] [CrossRef] [PubMed]
- Adachi, O.; Kawai, T.; Takeda, K.; Matsumoto, M.; Tsutsui, H.; Sakagami, M.; Nakanishi, K.; Akira, S. Targeted Disruption of the MyD88 Gene Results in Loss of IL-1- and IL-18-Mediated Function. Immunity 1998, 9, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Grantham, E.K.; Warden, A.S.; McCarthy, G.S.; DaCosta, A.; Mason, S.; Blednov, Y.; Mayfield, R.D.; Harris, R.A. Role of toll-like receptor 7 (TLR7) in voluntary alcohol consumption. Brain Behav. Immun. 2020, 89, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Yue, Y.; Ma, C.; Dong, L.; Chen, F. Pasteurized Akkermansia muciniphila Ameliorate the LPS-Induced Intestinal Barrier Dysfunction via Modulating AMPK and NF-kappaB through TLR2 in Caco-2 Cells. Nutrients 2022, 14, 764. [Google Scholar] [CrossRef]
- Frasca, D.; Romero, M.; Diaz, A.; Garcia, D.; Thaller, S.; Blomberg, B.B. B Cells with a Senescent-Associated Secretory Phenotype Accumulate in the Adipose Tissue of Individuals with Obesity. Int. J. Mol. Sci. 2021, 22, 1839. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, I.J.; Raub, T.J.; Borchardt, R.T. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 1989, 96, 736–749. [Google Scholar] [CrossRef] [PubMed]
- Weber, E.W.; Han, F.; Tauseef, M.; Birnbaumer, L.; Mehta, D.; Muller, W.A. TRPC6 is the endothelial calcium channel that regulates leukocyte transendothelial migration during the inflammatory response. J. Exp. Med. 2015, 212, 1883–1899. [Google Scholar] [CrossRef]
- Wu, F.; Guo, X.; Xu, J.; Wang, W.; Li, B.; Huang, Q.; Su, L.; Xu, Q. Role of myosin light chain and myosin light chain kinase in advanced glycation end product-induced endothelial hyperpermeability in vitro and in vivo %J. Diabetes Vasc. Dis. Res. Off. J. Int. Soc. Diabetes Vasc. Dis. 2016, 13, 137–144. [Google Scholar] [CrossRef]
- Meyer, B.K.; Pray-Grant, M.G.; Vanden Heuvel, J.P.; Perdew, G.H. Hepatitis B virus X-associated protein 2 is a subunit of the unliganded aryl hydrocarbon receptor core complex and exhibits transcriptional enhancer activity. Mol. Cell. Biol. 1998, 18, 978–988. [Google Scholar] [CrossRef]
- Kost, E.R.; Mutch, D.G.; Herzog, T.J. Interferon-γ and Tumor Necrosis Factor-α Induce Synergistic Cytolytic Effects in Ovarian Cancer Cell Lines—Roles of the TR60 and TR80 Tumor Necrosis Factor Receptors. Gynecol. Oncol. 1999, 72, 392–401. [Google Scholar] [CrossRef] [PubMed]


| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| IL-4 | TCTTTGCTGCCTCCAAGAACA | GTAGAACTGCCGGAGCACAG |
| IL-10 | CGCTAGAACCAAGCTGTCCT | CACATGCGCCTTGATGTCTG |
| TGF-β | AGCAACAATTCCTGGCGATACCTC | TCAACCACTGCCGCACAACTC |
| IL-1β | TGACGGACCCCAAAAGATGA | TCTCCACAGCCACAATGAGT |
| IL-6 | TGAAGCACCCACCAATACAA | CCAACCTCAGAAAGCAGCTT |
| TNF-α | CCCTCACACTCAGATCATCTTCT | CTACGACGTGGGCTACAG |
| iNos | GGAGCGAGTTGTGGATTG | CCAGGAAGTAGGTGAGGG |
| ZO-1 | GGATGTTTATCGCATTGTA | AAGAGCCCAGTTTTCCATTGTA |
| Occludin | TCTAGGACGCAGCAGATTGG | TGGACTTTCAAGAGGCCTGG |
| Claudin | AGTTAGGAGCCTTGATGCCG | GCACAGGGAGTAGGATACGC |
| TLR2 | CTTCACTCAGGAGCAGCAAGCA | ACACCAGTGCTGTCCTGTGACA |
| TLR3 | GCGCTAAAAAGTGAAGAACTGGAT | GCTGGACATTGTTCAGAAAGAGG |
| TLR5 | CCTTACAGCGAACCTCATCCAC | TCCACTACAGGAGGAGAAGCGA |
| TLR7 | CTTTGGACCTCAGCCACAACCA | CGCAACTGGAAGGCATCTTGTAG |
| GAPDH | TGGAGAAACCTGCCAAGTATGA | TGGAAGAATGGGAGTTGCTGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Z.; Zhang, G.; Liu, R.; Wang, Y.; Tsapieva, A.N.; Zhang, L.; Han, J. Heat-Killed Lacticaseibacillus paracasei Repairs Lipopolysaccharide-Induced Intestinal Epithelial Barrier Damage via MLCK/MLC Pathway Activation. Nutrients 2023, 15, 1758. https://doi.org/10.3390/nu15071758
Xie Z, Zhang G, Liu R, Wang Y, Tsapieva AN, Zhang L, Han J. Heat-Killed Lacticaseibacillus paracasei Repairs Lipopolysaccharide-Induced Intestinal Epithelial Barrier Damage via MLCK/MLC Pathway Activation. Nutrients. 2023; 15(7):1758. https://doi.org/10.3390/nu15071758
Chicago/Turabian StyleXie, Zhixin, Gongsheng Zhang, Rongxu Liu, Yucong Wang, Anna N. Tsapieva, Lili Zhang, and Jianchun Han. 2023. "Heat-Killed Lacticaseibacillus paracasei Repairs Lipopolysaccharide-Induced Intestinal Epithelial Barrier Damage via MLCK/MLC Pathway Activation" Nutrients 15, no. 7: 1758. https://doi.org/10.3390/nu15071758
APA StyleXie, Z., Zhang, G., Liu, R., Wang, Y., Tsapieva, A. N., Zhang, L., & Han, J. (2023). Heat-Killed Lacticaseibacillus paracasei Repairs Lipopolysaccharide-Induced Intestinal Epithelial Barrier Damage via MLCK/MLC Pathway Activation. Nutrients, 15(7), 1758. https://doi.org/10.3390/nu15071758








