Is Autologous Fecal Microbiota Transfer after Exclusive Enteral Nutrition in Pediatric Crohn’s Disease Patients Rational and Feasible? Data from a Feasibility Test
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility and Recruitment
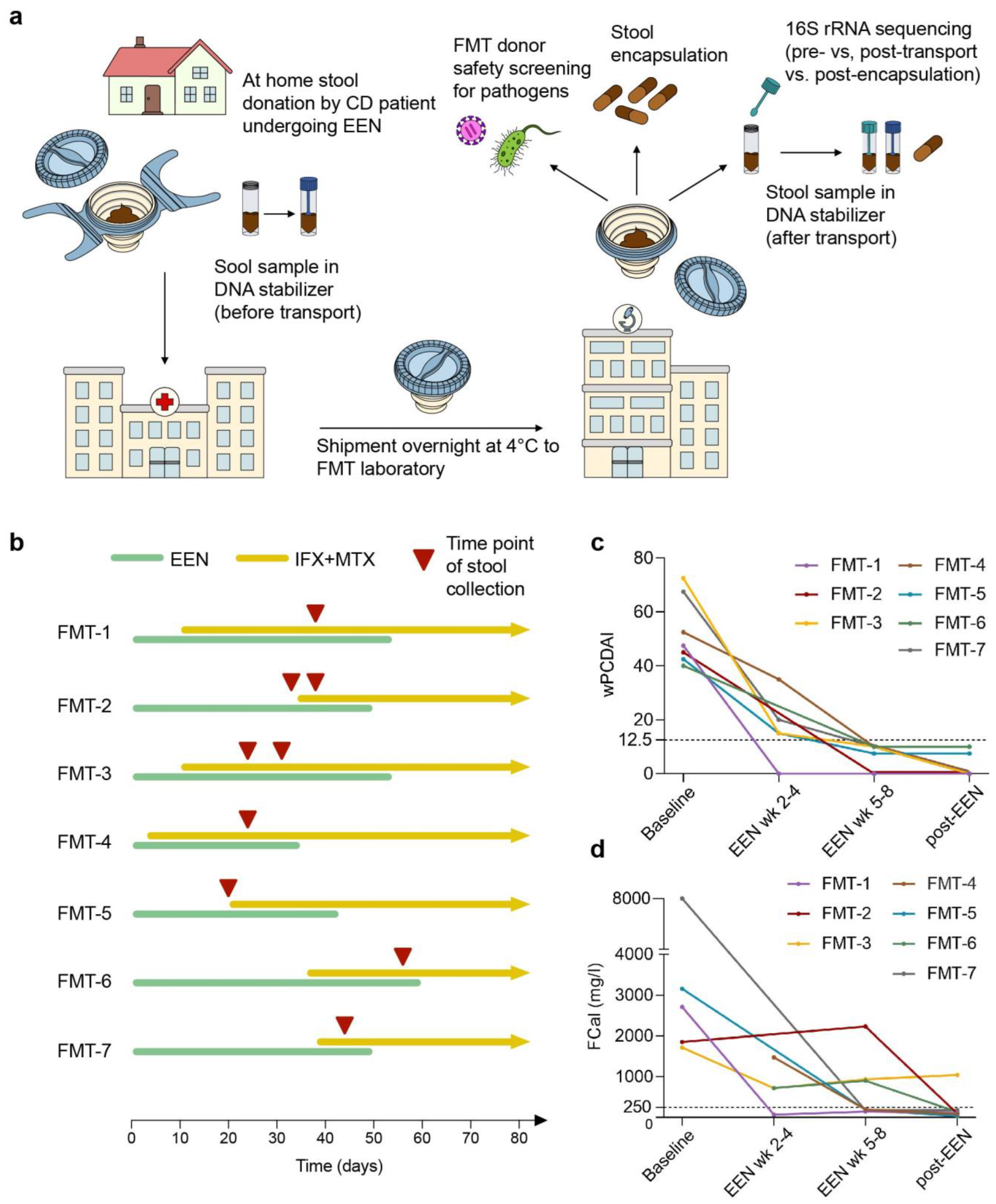
2.2. Study Design
2.3. 16S rRNA Sequencing
2.4. Analysis of Bacterial Composition
2.5. Calculation of Diversity Indices
2.6. Statistical Analysis
3. Results
3.1. Study Population
3.2. FMT Capsule Production Is Limited by Quantitative and Qualitative Deficits of Fecal Material
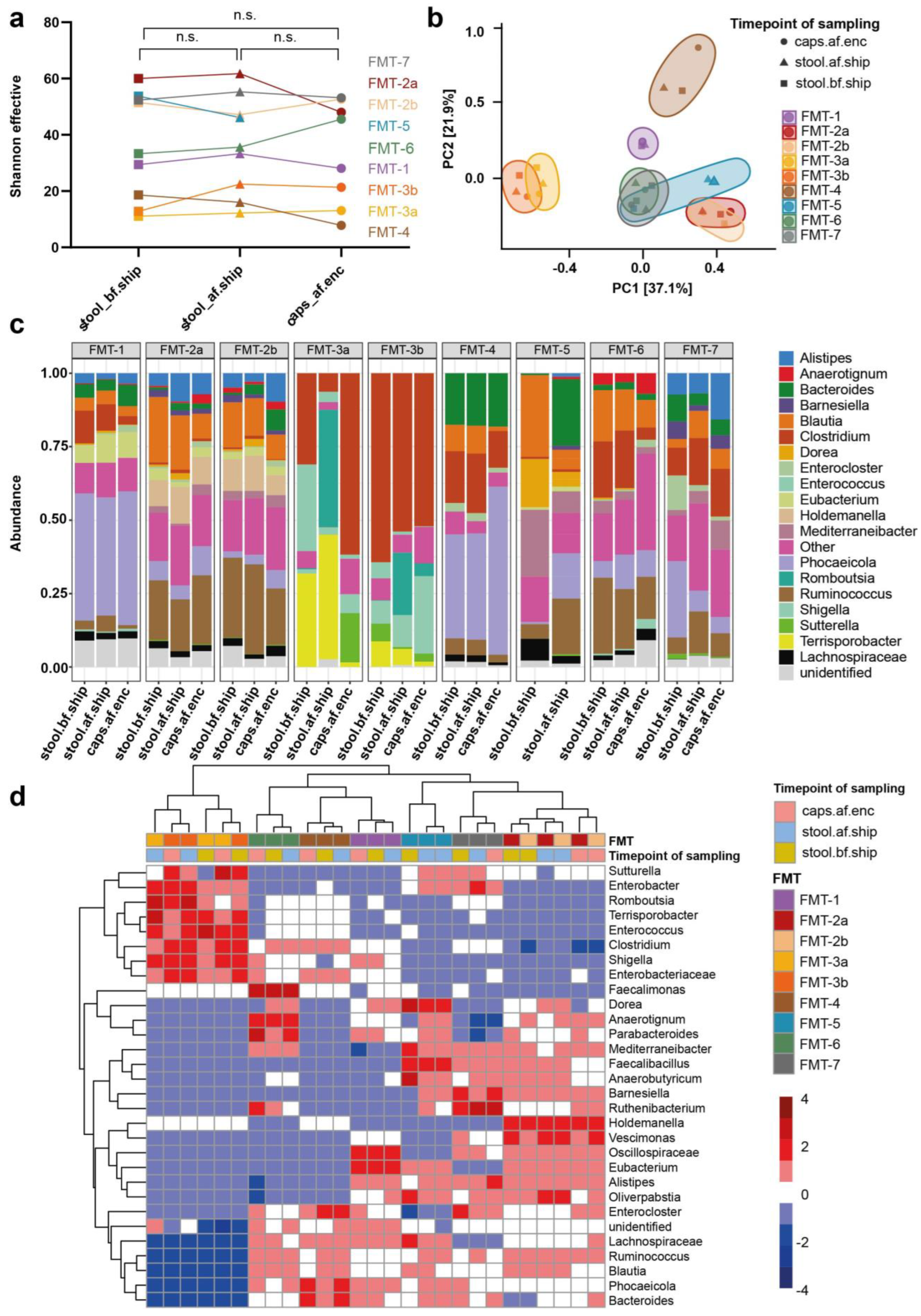
3.3. Transport and Processing of Stool Donations into FMT Capsules Induce Minor Changes in Microbial Composition
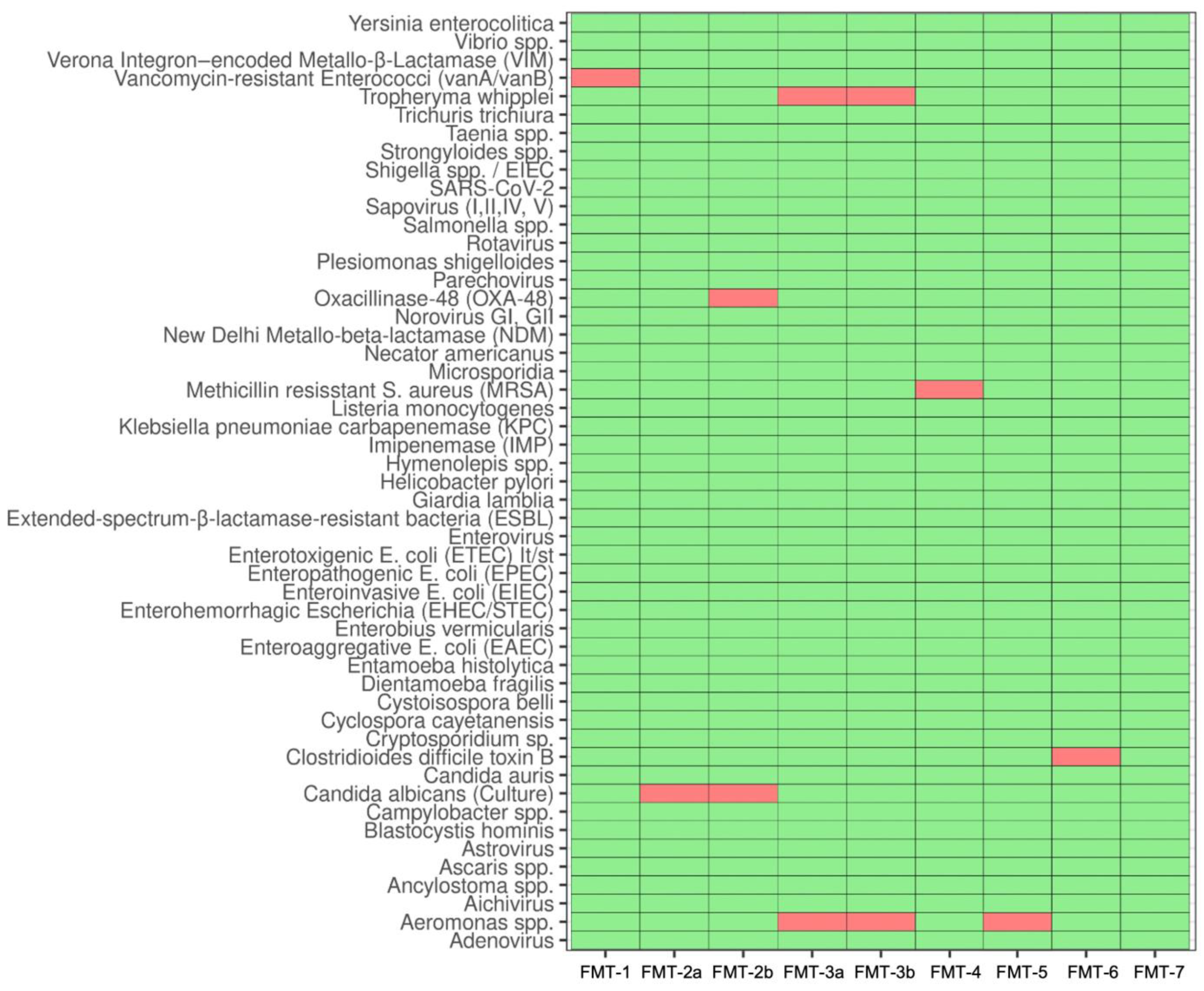
3.4. Safety Screening Reveals High Prevalence of Pathogen Colonization
3.5. Stool Donations from Pediatric CD Patients under EEN Are Not Suitable for Autologous FMT Capsule Production
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kelly, C.R.; Yen, E.F.; Grinspan, A.M.; Kahn, S.A.; Atreja, A.; Lewis, J.D.; Moore, T.A.; Rubin, D.T.; Kim, A.M.; Serra, S.; et al. Fecal Microbiota Transplantation Is Highly Effective in Real-World Practice: Initial Results From the FMT National Registry. Gastroenterology 2021, 160, 183–192.e3. [Google Scholar] [CrossRef]
- Aggarwala, V.; Mogno, I.; Li, Z.; Yang, C.; Britton, G.J.; Chen-Liaw, A.; Mitcham, J.; Bongers, G.; Gevers, D.; Clemente, J.C.; et al. Precise Quantification of Bacterial Strains after Fecal Microbiota Transplantation Delineates Long-Term Engraftment and Explains Outcomes. Nat. Microbiol. 2021, 6, 1309–1318. [Google Scholar] [CrossRef]
- Staley, C.; Kaiser, T.; Vaughn, B.P.; Graiziger, C.; Hamilton, M.J.; Kabage, A.J.; Khoruts, A.; Sadowsky, M.J. Durable Long-Term Bacterial Engraftment Following Encapsulated Fecal Microbiota Transplantation To Treat Clostridium Difficile Infection. mBio 2019, 10, e01586-19. [Google Scholar] [CrossRef] [PubMed]
- Moayyedi, P.; Surette, M.G.; Kim, P.T.; Libertucci, J.; Wolfe, M.; Onischi, C.; Armstrong, D.; Marshall, J.K.; Kassam, Z.; Reinisch, W.; et al. Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology 2015, 149, 102–109.e6. [Google Scholar] [CrossRef]
- Paramsothy, S.; Kamm, M.A.; Kaakoush, N.O.; Walsh, A.J.; van den Bogaerde, J.; Samuel, D.; Leong, R.W.L.; Connor, S.; Ng, W.; Paramsothy, R.; et al. Multidonor Intensive Faecal Microbiota Transplantation for Active Ulcerative Colitis: A Randomised Placebo-Controlled Trial. Lancet 2017, 389, 1218–1228. [Google Scholar] [CrossRef] [PubMed]
- Costello, S.P.; Hughes, P.A.; Waters, O.; Bryant, R.V.; Vincent, A.D.; Blatchford, P.; Katsikeros, R.; Makanyanga, J.; Campaniello, M.A.; Mavrangelos, C.; et al. Effect of Fecal Microbiota Transplantation on 8-Week Remission in Patients With Ulcerative Colitis: A Randomized Clinical Trial. JAMA J. Am. Med. Assoc. 2019, 321, 156–164. [Google Scholar] [CrossRef]
- Haifer, C.; Paramsothy, S.; Kaakoush, N.O.; Saikal, A.; Ghaly, S.; Yang, T.; Luu, L.D.W.; Borody, T.J.; Leong, R.W. Lyophilised Oral Faecal Microbiota Transplantation for Ulcerative Colitis (LOTUS): A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Gastroenterol. Hepatol. 2022, 7, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-W.; Phelps, E.; Ganapini, V.; Khan, N.; Ouyang, F.; Xu, H.; Khanna, S.; Tariq, R.; Friedman-Moraco, R.J.; Woodworth, M.H.; et al. Fecal Microbiota Transplantation for the Treatment of Recurrent and Severe Clostridium Difficile Infection in Solid Organ Transplant Recipients: A Multicenter Experience. Am. J. Transpl. 2019, 19, 501–511. [Google Scholar] [CrossRef]
- Fehily, S.R.; Basnayake, C.; Wright, E.K.; Kamm, M.A. Fecal Microbiota Transplantation Therapy in Crohn’s Disease: Systematic Review. J. Gastroenterol. Hepatol. 2021, 36, 2672–2686. [Google Scholar] [CrossRef]
- Sokol, H.; Landman, C.; Seksik, P.; Berard, L.; Montil, M.; Nion-Larmurier, I.; Bourrier, A.; Le Gall, G.; Lalande, V.; De Rougemont, A.; et al. Fecal Microbiota Transplantation to Maintain Remission in Crohn’s Disease: A Pilot Randomized Controlled Study. Microbiome 2020, 8, 12. [Google Scholar] [CrossRef]
- Kong, L.; Lloyd-Price, J.; Vatanen, T.; Seksik, P.; Beaugerie, L.; Simon, T.; Vlamakis, H.; Sokol, H.; Xavier, R.J. Linking Strain Engraftment in Fecal Microbiota Transplantation With Maintenance of Remission in Crohn’s Disease. Gastroenterology 2020, 159, 2193–2202.e5. [Google Scholar] [CrossRef]
- Pigneur, B.; Sokol, H. Fecal Microbiota Transplantation in Inflammatory Bowel Disease: The Quest for the Holy Grail. Mucosal. Immunol. 2016, 9, 1360–1365. [Google Scholar] [CrossRef]
- Danne, C.; Rolhion, N.; Sokol, H. Recipient Factors in Faecal Microbiota Transplantation: One Stool Does Not Fit All. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Vermeire, S.; Joossens, M.; Verbeke, K.; Wang, J.; Machiels, K.; Sabino, J.; Ferrante, M.; Van Assche, G.; Rutgeerts, P.; Raes, J. Donor Species Richness Determines Faecal Microbiota Transplantation Success in Inflammatory Bowel Disease. J. Crohns. Colitis. 2016, 10, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Mara, K.; Pardi, D.S.; Khanna, S. Long-Term Safety of Fecal Microbiota Transplantation for Recurrent Clostridioides Difficile Infection. Gastroenterology 2021, 160, 1961–1969.e3. [Google Scholar] [CrossRef] [PubMed]
- Marcella, C.; Cui, B.; Kelly, C.R.; Ianiro, G.; Cammarota, G.; Zhang, F. Systematic Review: The Global Incidence of Faecal Microbiota Transplantation-Related Adverse Events from 2000 to 2020. Aliment. Pharm. 2021, 53, 33–42. [Google Scholar] [CrossRef]
- Qazi, T.; Amaratunga, T.; Barnes, E.L.; Fischer, M.; Kassam, Z.; Allegretti, J.R. The Risk of Inflammatory Bowel Disease Flares after Fecal Microbiota Transplantation: Systematic Review and Meta-Analysis. Gut Microbes. 2017, 8, 574–588. [Google Scholar] [CrossRef]
- Kassam, Z.; Dubois, N.; Ramakrishna, B.; Ling, K.; Qazi, T.; Smith, M.; Kelly, C.R.; Fischer, M.; Allegretti, J.R.; Budree, S.; et al. Donor Screening for Fecal Microbiota Transplantation. N. Engl. J. Med. 2019, 381, 2070–2072. [Google Scholar] [CrossRef]
- DeFilipp, Z.; Bloom, P.P.; Torres Soto, M.; Mansour, M.K.; Sater, M.R.A.; Huntley, M.H.; Turbett, S.; Chung, R.T.; Chen, Y.-B.; Hohmann, E.L. Drug-Resistant E. Coli Bacteremia Transmitted by Fecal Microbiota Transplant. N. Engl. J. Med. 2019, 381, 2043–2050. [Google Scholar] [CrossRef]
- Zellmer, C.; Sater, M.R.A.; Huntley, M.H.; Osman, M.; Olesen, S.W.; Ramakrishna, B. Shiga Toxin-Producing Escherichia Coli Transmission via Fecal Microbiota Transplant. Clin. Infect. Dis. 2021, 72, e876–e880. [Google Scholar] [CrossRef]
- Drewes, J.L.; Corona, A.; Sanchez, U.; Fan, Y.; Hourigan, S.K.; Weidner, M.; Sidhu, S.D.; Simner, P.J.; Wang, H.; Timp, W.; et al. Transmission and Clearance of Potential Procarcinogenic Bacteria during Fecal Microbiota Transplantation for Recurrent Clostridioides Difficile. JCI Insight 2019, 4, e130848. [Google Scholar] [CrossRef] [PubMed]
- Nooij, S.; Ducarmon, Q.R.; Laros, J.F.J.; Zwittink, R.D.; Norman, J.M.; Smits, W.K.; Verspaget, H.W.; Keller, J.J.; Terveer, E.M.; Kuijper, E.J.; et al. Fecal Microbiota Transplantation Influences Procarcinogenic Escherichia Coli in Recipient Recurrent Clostridioides Difficile Patients. Gastroenterology 2021, 161, 1218–1228.e5. [Google Scholar] [CrossRef] [PubMed]
- Alang, N.; Kelly, C.R. Weight Gain after Fecal Microbiota Transplantation. Open Forum. Infect. Dis. 2015, 2, ofv004. [Google Scholar] [CrossRef] [PubMed]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut Microbiota from Twins Discordant for Obesity Modulate Metabolism in Mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef]
- Basson, A.R.; Zhou, Y.; Seo, B.; Rodriguez-Palacios, A.; Cominelli, F. Autologous Fecal Microbiota Transplantation for the Treatment of Inflammatory Bowel Disease. Transl. Res. 2020, 226, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rinott, E.; Youngster, I.; Yaskolka Meir, A.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Knights, D.; Tuohy, K.; Fava, F.; Scholz, M.U.; et al. Effects of Diet-Modulated Autologous Fecal Microbiota Transplantation on Weight Regain. Gastroenterology 2021, 160, 158–173.e10. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Lewis, J.D.; Chen, E.Z.; Baldassano, R.N.; Otley, A.R.; Griffiths, A.M.; Lee, D.; Bittinger, K.; Bailey, A.; Friedman, E.S.; Hoffmann, C.; et al. Inflammation, Antibiotics, and Diet as Environmental Stressors of the Gut Microbiome in Pediatric Crohn’s Disease. Cell Host. Microbe. 2015, 18, 489–500. [Google Scholar] [CrossRef]
- Pigneur, B.; Lepage, P.; Mondot, S.; Schmitz, J.; Goulet, O.; Doré, J.; Ruemmele, F.M. Mucosal Healing and Bacterial Composition in Response to Enteral Nutrition Vs Steroid-Based Induction Therapy-A Randomised Prospective Clinical Trial in Children With Crohn’s Disease. J. Crohns. Colitis. 2019, 13, 846–855. [Google Scholar] [CrossRef]
- Dziechciarz, P.; Horvath, A.; Shamir, R.; Szajewska, H. Meta-Analysis: Enteral Nutrition in Active Crohn’s Disease in Children. Aliment. Pharm. 2007, 26, 795–806. [Google Scholar] [CrossRef]
- Swaminath, A.; Feathers, A.; Ananthakrishnan, A.N.; Falzon, L.; Li Ferry, S. Systematic Review with Meta-Analysis: Enteral Nutrition Therapy for the Induction of Remission in Paediatric Crohn’s Disease. Aliment. Pharm. 2017, 46, 645–656. [Google Scholar] [CrossRef]
- Borrelli, O.; Cordischi, L.; Cirulli, M.; Paganelli, M.; Labalestra, V.; Uccini, S.; Russo, P.M.; Cucchiara, S. Polymeric Diet Alone Versus Corticosteroids in the Treatment of Active Pediatric Crohn’s Disease: A Randomized Controlled Open-Label Trial. Clin. Gastroenterol. Hepatol. 2006, 4, 744–753. [Google Scholar] [CrossRef]
- Miele, E.; Shamir, R.; Aloi, M.; Assa, A.; Braegger, C.; Bronsky, J.; de Ridder, L.; Escher, J.C.; Hojsak, I.; Kolacek, S.; et al. Nutrition in Pediatric Inflammatory Bowel Disease: A Position Paper on Behalf of the Porto Inflammatory Bowel Disease Group of the European Society of Pediatric Gastroenterology, Hepatology and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 687–708. [Google Scholar] [CrossRef]
- van Rheenen, P.F.; Aloi, M.; Assa, A.; Bronsky, J.; Escher, J.C.; Fagerberg, U.L.; Gasparetto, M.; Gerasimidis, K.; Griffiths, A.; Henderson, P.; et al. The Medical Management of Paediatric Crohn’s Disease: An ECCO-ESPGHAN Guideline Update. J. Crohn. Colitis. 2020, 15, 171–194. [Google Scholar] [CrossRef]
- Diederen, K.; Li, J.V.; Donachie, G.E.; de Meij, T.G.; de Waart, D.R.; Hakvoort, T.B.M.; Kindermann, A.; Wagner, J.; Auyeung, V.; te Velde, A.A.; et al. Exclusive Enteral Nutrition Mediates Gut Microbial and Metabolic Changes That Are Associated with Remission in Children with Crohn’s Disease. Sci. Rep. 2020, 10, 18879. [Google Scholar] [CrossRef]
- Schwerd, T.; Frivolt, K.; Clavel, T.; Lagkouvardos, I.; Katona, G.; Mayr, D.; Uhlig, H.H.; Haller, D.; Koletzko, S.; Bufler, P. Exclusive Enteral Nutrition in Active Pediatric Crohn Disease: Effects on Intestinal Microbiota and Immune Regulation. J. Allergy Clin. Immunol. 2016, 138, 592–596. [Google Scholar] [CrossRef]
- Kaakoush, N.O.; Day, A.S.; Leach, S.T.; Lemberg, D.A.; Nielsen, S.; Mitchell, H.M. Effect of Exclusive Enteral Nutrition on the Microbiota of Children with Newly Diagnosed Crohn’s Disease. Clin. Transl. Gastroenterol. 2015, 6, e71. [Google Scholar] [CrossRef] [PubMed]
- Quince, C.; Ijaz, U.Z.; Loman, N.; Eren, A.M.; Saulnier, D.; Russell, J.; Haig, S.J.; Calus, S.T.; Quick, J.; Barclay, A.; et al. Extensive Modulation of the Fecal Metagenome in Children With Crohn’s Disease During Exclusive Enteral Nutrition. Am. J. Gastroenterol. 2015, 110, 1718–1729; quiz 1730. [Google Scholar] [CrossRef] [PubMed]
- Ghiboub, M.; Penny, S.; Verburgt, C.M.; Boneh, R.S.; Wine, E.; Cohen, A.; Dunn, K.A.; Pinto, D.M.; Benninga, M.A.; de Jonge, W.J.; et al. Metabolome Changes With Diet-Induced Remission in Pediatric Crohn’s Disease. Gastroenterology 2022, 163, 922–936.e15. [Google Scholar] [CrossRef] [PubMed]
- Frivolt, K.; Schwerd, T.; Werkstetter, K.J.; Schwarzer, A.; Schatz, S.B.; Bufler, P.; Koletzko, S. Repeated Exclusive Enteral Nutrition in the Treatment of Paediatric Crohn’s Disease: Predictors of Efficacy and Outcome. Aliment. Pharmacol. Ther. 2014, 39, 1398–1407. [Google Scholar] [CrossRef]
- Gerasimidis, K.; Bertz, M.; Hanske, L.; Junick, J.; Biskou, O.; Aguilera, M.; Garrick, V.; Russell, R.K.; Blaut, M.; McGrogan, P.; et al. Decline in Presumptively Protective Gut Bacterial Species and Metabolites Are Paradoxically Associated with Disease Improvement in Pediatric Crohn’s Disease during Enteral Nutrition. Inflamm. Bowel Dis 2014, 20, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Leach, S.T.; Mitchell, H.M.; Eng, W.R.; Zhang, L.; Day, A.S. Sustained Modulation of Intestinal Bacteria by Exclusive Enteral Nutrition Used to Treat Children with Crohn’s Disease. Aliment. Pharm. 2008, 28, 724–733. [Google Scholar] [CrossRef]
- Keller, J.J.; Ooijevaar, R.E.; Hvas, C.L.; Terveer, E.M.; Lieberknecht, S.C.; Högenauer, C.; Arkkila, P.; Sokol, H.; Gridnyev, O.; Mégraud, F.; et al. A Standardised Model for Stool Banking for Faecal Microbiota Transplantation: A Consensus Report from a Multidisciplinary UEG Working Group. United Eur. Gastroenterol. J. 2021, 9, 229–247. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G.; Tilg, H.; Rajilic-Stojanovic, M.; Kump, P.; Satokari, R.; Sokol, H.; Arkkila, P.; Pintus, C.; Hart, A.; et al. European Consensus Conference on Faecal Microbiota Transplantation in Clinical Practice. Gut 2017, 66, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Levine, A.; Walters, T.D.; Focht, G.; Otley, A.; López, V.N.; Koletzko, S.; Baldassano, R.; Mack, D.; Hyams, J.; et al. Which PCDAI Version Best Reflects Intestinal Inflammation in Pediatric Crohn Disease? J. Pediatr. Gastroenterol. Nutr. 2017, 64, 254–260. [Google Scholar] [CrossRef]
- Reitmeier, S.; Kiessling, S.; Neuhaus, K.; Haller, D. Comparing Circadian Rhythmicity in the Human Gut Microbiome. STAR Protoc. 2020, 1, 100148. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Bonk, F.; Popp, D.; Harms, H.; Centler, F. PCR-Based Quantification of Taxa-Specific Abundances in Microbial Communities: Quantifying and Avoiding Common Pitfalls. J. Microbiol. Methods 2018, 153, 139–147. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A Versatile Open Source Tool for Metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Murali, A.; Bhargava, A.; Wright, E.S. IDTAXA: A Novel Approach for Accurate Taxonomic Classification of Microbiome Sequences. Microbiome 2018, 6, 140. [Google Scholar] [CrossRef] [PubMed]
- Yarza, P.; Richter, M.; Peplies, J.; Euzeby, J.; Amann, R.; Schleifer, K.-H.; Ludwig, W.; Glöckner, F.O.; Rosselló-Móra, R. The All-Species Living Tree Project: A 16S RRNA-Based Phylogenetic Tree of All Sequenced Type Strains. Syst. Appl. Microbiol. 2008, 31, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Ernst, F.G.M.; Shetty, S.A.; Borman, T.; Lahti, L.; Cao, Y.; Olson, N.D.; Waldron, L.; Ramos, M.; Bravo, H.C.; Kancherla, J.; et al. Mia: Microbiome Analysis; Bioconductor version: Release (3.16). 2023. Available online: https://github.com/microbiome/mia (accessed on 14 February 2023).
- Dixon, P. VEGAN, a Package of R Functions for Community Ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Mallick, H.; Rahnavard, A.; McIver, L.J.; Ma, S.; Zhang, Y.; Nguyen, L.H.; Tickle, T.L.; Weingart, G.; Ren, B.; Schwager, E.H.; et al. Multivariable Association Discovery in Population-Scale Meta-Omics Studies. PLoS Comput. Biol. 2021, 17, e1009442. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.; Griffiths, A.; Markowitz, J.; Wilson, D.C.; Turner, D.; Russell, R.K.; Fell, J.; Ruemmele, F.M.; Walters, T.; Sherlock, M.; et al. Pediatric Modification of the Montreal Classification for Inflammatory Bowel Disease: The Paris Classification. Inflamm. Bowel Dis. 2011, 17, 1314–1321. [Google Scholar] [CrossRef]
- Ricciuto, A.; Aardoom, M.; Orlanski-Meyer, E.; Navon, D.; Carman, N.; Aloi, M.; Bronsky, J.; Däbritz, J.; Dubinsky, M.; Hussey, S.; et al. Predicting Outcomes in Pediatric Crohn’s Disease for Management Optimization: Systematic Review and Consensus Statements From the Pediatric Inflammatory Bowel Disease–Ahead Program. Gastroenterology 2021, 160, 403–436.e26. [Google Scholar] [CrossRef] [PubMed]
- Atarashi, K.; Tanoue, T.; Oshima, K.; Suda, W.; Nagano, Y.; Nishikawa, H.; Fukuda, S.; Saito, T.; Narushima, S.; Hase, K.; et al. T Reg Induction by a Rationally Selected Mixture of Clostridia Strains from the Human Microbiota. Nature 2013, 500, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermúdez-Humarán, L.G.; Gratadoux, J.-J.; Blugeon, S.; Bridonneau, C.; Furet, J.-P.; Corthier, G.; et al. Faecalibacterium Prausnitzii Is an Anti-Inflammatory Commensal Bacterium Identified by Gut Microbiota Analysis of Crohn Disease Patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef] [PubMed]
- Day, A.S.; Whitten, K.E.; Sidler, M.; Lemberg, D.A. Systematic Review: Nutritional Therapy in Paediatric Crohn’s Disease. Aliment. Pharm. 2008, 27, 293–307. [Google Scholar] [CrossRef]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vázquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The Treatment-Naive Microbiome in New-Onset Crohn’s Disease. Cell Host. Microbe. 2014, 15, 382–392. [Google Scholar] [CrossRef]
- Pascal, V.; Pozuelo, M.; Borruel, N.; Casellas, F.; Campos, D.; Santiago, A.; Martinez, X.; Varela, E.; Sarrabayrouse, G.; Machiels, K.; et al. A Microbial Signature for Crohn’s Disease. Gut 2017, 66, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Morgan, X.C.; Tickle, T.L.; Sokol, H.; Gevers, D.; Devaney, K.L.; Ward, D.V.; Reyes, J.A.; Shah, S.A.; LeLeiko, N.; Snapper, S.B.; et al. Dysfunction of the Intestinal Microbiome in Inflammatory Bowel Disease and Treatment. Genome. Biol. 2012, 13, R79. [Google Scholar] [CrossRef] [PubMed]
- Franzosa, E.A.; Sirota-Madi, A.; Avila-Pacheco, J.; Fornelos, N.; Haiser, H.J.; Reinker, S.; Vatanen, T.; Hall, A.B.; Mallick, H.; McIver, L.J.; et al. Gut Microbiome Structure and Metabolic Activity in Inflammatory Bowel Disease. Nat. Microbiol. 2019, 4, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Targeting Immune Cell Circuits and Trafficking in Inflammatory Bowel Disease. Nat. Immunol. 2019, 20, 970–979. [Google Scholar] [CrossRef]
- Sorbara, M.T.; Pamer, E.G. Microbiome-Based Therapeutics. Nat. Rev. Microbiol. 2022, 20, 365–380. [Google Scholar] [CrossRef]
- Buffie, C.G.; Pamer, E.G. Microbiota-Mediated Colonization Resistance against Intestinal Pathogens. Nat. Rev. Immunol. 2013, 13, 790–801. [Google Scholar] [CrossRef]
- Axelrad, J.E.; Olén, O.; Askling, J.; Lebwohl, B.; Khalili, H.; Sachs, M.C.; Ludvigsson, J.F. Gastrointestinal Infection Increases Odds of Inflammatory Bowel Disease in a Nationwide Case-Control Study. Clin. Gastroenterol. Hepatol. 2019, 17, 1311–1322.e7. [Google Scholar] [CrossRef]
- Singh, H.; Nugent, Z.; Yu, B.N.; Lix, L.M.; Targownik, L.E.; Bernstein, C.N. Higher Incidence of Clostridium Difficile Infection Among Individuals With Inflammatory Bowel Disease. Gastroenterology 2017, 153, 430–438.e2. [Google Scholar] [CrossRef]
- Irving, P.M.; de Lusignan, S.; Tang, D.; Nijher, M.; Barrett, K. Risk of Common Infections in People with Inflammatory Bowel Disease in Primary Care: A Population-Based Cohort Study. BMJ Open Gastroenterol. 2021, 8, e000573. [Google Scholar] [CrossRef]
- Nguyen, G.C.; Leung, W.; Weizman, A.V. Increased Risk of Vancomycin-Resistant Enterococcus (VRE) Infection among Patients Hospitalized for Inflammatory Bowel Disease in the United States. Inflamm. Bowel Dis. 2011, 17, 1338–1342. [Google Scholar] [CrossRef]
- Boumaza, A.; Ben Azzouz, E.; Arrindell, J.; Lepidi, H.; Mezouar, S.; Desnues, B. Whipple’s Disease and Tropheryma Whipplei Infections: From Bench to Bedside. Lancet Infect. Dis. 2022, 22, e280–e291. [Google Scholar] [CrossRef] [PubMed]
- Keita, A.K.; Raoult, D.; Fenollar, F. Tropheryma Whipplei as a Commensal Bacterium. Future Microbiol. 2013, 8, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Fenollar, F.; Trani, M.; Davoust, B.; Salle, B.; Birg, M.-L.; Rolain, J.-M.; Raoult, D. Prevalence of Asymptomatic Tropheryma Whipplei Carriage among Humans and Nonhuman Primates. J. Infect. Dis 2008, 197, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Raoult, D.; Fenollar, F.; Rolain, J.M.; Minodier, P.; Bosdure, E.; Li, W.; Garnier, J.M.; Richet, H. Tropheryma Whipplei in Children with Gastroenteritis. Emerg. Infect. Dis. 2010, 16, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Bulut, K.; Markova, A.; Canbay, A.E.; Schmidt, W.E.; Kahraman, A. Whipple’s Disease—A Rare and Challenging Complication in a Patient with Crohn’s Disease. Z Gastroenterol. 2022, 60, 598–601. [Google Scholar] [CrossRef] [PubMed]
- Mousa, O.Y.; Mousa, Y.S.; Nimri, S.M. Granulomas in Small Bowel Crohn’s Masking Whipple’s Disease. Am. J. Gastroenterol. 2017, 112, S1322. [Google Scholar] [CrossRef]
- Klochan, C.; Anderson, T.A.; Rose, D.; Dimitrov, R.K.; Johnson, R.M. Nearly Fatal Case of Whipple’s Disease in a Patient Mistakenly on Anti-TNF Therapy. ACG Case Rep. J 2013, 1, 25–28. [Google Scholar] [CrossRef]
- Liu, C.K.; Seo, J.; Pravodelov, V.; Frazier, S.; Guy, M.; Concilio, K.; Lau-Ng, R.; Brandeis, G.; Watson, J.; van der Velde, J.; et al. Pilot Study of Autologous Fecal Microbiota Transplants in Nursing Home Residents: Feasibility and Safety. Contemp. Clin. Trials Commun. 2022, 27, 100906. [Google Scholar] [CrossRef]
- Taur, Y.; Coyte, K.; Schluter, J.; Robilotti, E.; Figueroa, C.; Gjonbalaj, M.; Littmann, E.R.; Ling, L.; Miller, L.; Gyaltshen, Y.; et al. Reconstitution of the Gut Microbiota of Antibiotic-Treated Patients by Autologous Fecal Microbiota Transplant. Sci. Transl. Med. 2018, 10, eaap9489. [Google Scholar] [CrossRef]
- Jones, C.M.A.; Connors, J.; Dunn, K.A.; Bielawski, J.P.; Comeau, A.M.; Langille, M.G.I.; Van Limbergen, J. Bacterial Taxa and Functions Are Predictive of Sustained Remission Following Exclusive Enteral Nutrition in Pediatric Crohn’s Disease. Inflamm. Bowel Dis. 2020, 26, 1026–1037. [Google Scholar] [CrossRef]
- Dunn, K.A.; Moore-Connors, J.; MacIntyre, B.; Stadnyk, A.W.; Thomas, N.A.; Noble, A.; Mahdi, G.; Rashid, M.; Otley, A.R.; Bielawski, J.P.; et al. Early Changes in Microbial Community Structure Are Associated with Sustained Remission After Nutritional Treatment of Pediatric Crohn’s Disease. Inflamm. Bowel Dis. 2016, 22, 2853–2862. [Google Scholar] [CrossRef] [PubMed]
- Paramsothy, S.; Paramsothy, R.; Rubin, D.T.; Kamm, M.A.; Kaakoush, N.O.; Mitchell, H.M.; Castano-Rodriguez, N. Faecal Microbiota Transplantation for Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. J. Crohn. Colitis. 2017, 11, 1180–1199. [Google Scholar] [CrossRef] [PubMed]
- Kump, P.; Wurm, P.; Gröchenig, H.P.; Wenzl, H.; Petritsch, W.; Halwachs, B.; Wagner, M.; Stadlbauer, V.; Eherer, A.; Hoffmann, K.M.; et al. The Taxonomic Composition of the Donor Intestinal Microbiota Is a Major Factor Influencing the Efficacy of Faecal Microbiota Transplantation in Therapy Refractory Ulcerative Colitis. Aliment. Pharm. 2018, 47, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Sanchis-Artero, L.; Martínez-Blanch, J.F.; Manresa-Vera, S.; Cortés-Castell, E.; Valls-Gandia, M.; Iborra, M.; Paredes-Arquiola, J.M.; Boscá-Watts, M.; Huguet, J.M.; Gil-Borrás, R.; et al. Evaluation of Changes in Intestinal Microbiota in Crohn’s Disease Patients after Anti-TNF Alpha Treatment. Sci. Rep. 2021, 11, 10016. [Google Scholar] [CrossRef] [PubMed]
- Charbonneau, M.R.; Isabella, V.M.; Li, N.; Kurtz, C.B. Developing a New Class of Engineered Live Bacterial Therapeutics to Treat Human Diseases. Nat. Commun. 2020, 11, 1738. [Google Scholar] [CrossRef]

| Pat. | Age at Diagnosis (Years) | Age at Study Inclusion (Years) | Newly Diagnosed | Symptoms at Presentation | Paris Classification * | Disease Activity (wPCDAI) | |
|---|---|---|---|---|---|---|---|
| Disease Location | Disease Behavior | ||||||
| FMT-1 | 16.5 | 16.5 | Yes | Diarrhea, reduced daily activity | L2, L4a | B1, G0 | moderate (47.5) |
| FMT-2 | 15.2 | 15.2 | Yes | Abdominal pain, diarrhea, involuntary weight loss | L4ab | B1, G0 | moderate (45) |
| FMT-3 | 7.8 | 11.3 | No | Bloody diarrhea, abdominal pain, vomiting, poor well-being, involuntary weight loss | L3, L4a | B1p, G1 | severe (72.5) |
| FMT-4 | 9.2 | 15.3 | No | Abdominal pain, diarrhea, poor well-being | L2, L4a | B1, G1 | moderate (52.5) |
| FMT-5 | 12.8 | 12.8 | Yes | Diarrhea, involuntary weight loss, anal abscess, uveitis | L3, L4ab | B3p, G0 | moderate (42.5) |
| FMT-6 | 14.3 | 14.4 | Yes | Abdominal pain | L1, L4a | B1, G1 | mild (40) |
| FMT-7 | 11.3 | 11.3 | Yes | Abdominal pain, vomiting, poor well-being, involuntary weight loss | L3, L4ab | B1, G0 | severe (67.5) |
| Pat. * | EEN Weeks Completed at Time Point of Stool Donation | Stool Weight (g) from Single Donation | Bristol Stool Scale (1–7) | Bacterial Richness (Number of zOTUs at Genus Level Pre-/Post- Shipment) | Shannon-Index (Pre-/Post- Shipment) | Number of Capsules Produced 1 |
|---|---|---|---|---|---|---|
| FMT-1 | 5 | 71 | 2 | 38/39 | 3.3/3.4 | 30 |
| FMT-2 | 4 | 55 | 1 | 47/48 | 3.8/4.1 | 30 |
| 5 | 201 | 2 | 48/49 | 4.0/3.9 | 30 | |
| FMT-3 | 3 | 68 | 6 | 18/16 | 2.6/2.4 | 30 |
| 4 | 37 | 6 | 18/16 | 3.1/2.6 | 21 | |
| FMT-4 | 3 | 240 | 7 | 15/15 | 2.1/2.9 | 10 |
| FMT-5 | 3 | 10 | 6 | 39/40 | 4.0/3.8 | 0 |
| FMT-6 | 7 | 21 | 4 | 30/32 | 3.8/3.5 | 30 |
| FMT-7 | 6 | 100 | 4 | 38/43 | 3.9/4.0 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoelz, H.; Heetmeyer, J.; Tsakmaklis, A.; Hiergeist, A.; Siebert, K.; De Zen, F.; Häcker, D.; Metwaly, A.; Neuhaus, K.; Gessner, A.; et al. Is Autologous Fecal Microbiota Transfer after Exclusive Enteral Nutrition in Pediatric Crohn’s Disease Patients Rational and Feasible? Data from a Feasibility Test. Nutrients 2023, 15, 1742. https://doi.org/10.3390/nu15071742
Hoelz H, Heetmeyer J, Tsakmaklis A, Hiergeist A, Siebert K, De Zen F, Häcker D, Metwaly A, Neuhaus K, Gessner A, et al. Is Autologous Fecal Microbiota Transfer after Exclusive Enteral Nutrition in Pediatric Crohn’s Disease Patients Rational and Feasible? Data from a Feasibility Test. Nutrients. 2023; 15(7):1742. https://doi.org/10.3390/nu15071742
Chicago/Turabian StyleHoelz, Hannes, Jeannine Heetmeyer, Anastasia Tsakmaklis, Andreas Hiergeist, Kolja Siebert, Federica De Zen, Deborah Häcker, Amira Metwaly, Klaus Neuhaus, André Gessner, and et al. 2023. "Is Autologous Fecal Microbiota Transfer after Exclusive Enteral Nutrition in Pediatric Crohn’s Disease Patients Rational and Feasible? Data from a Feasibility Test" Nutrients 15, no. 7: 1742. https://doi.org/10.3390/nu15071742
APA StyleHoelz, H., Heetmeyer, J., Tsakmaklis, A., Hiergeist, A., Siebert, K., De Zen, F., Häcker, D., Metwaly, A., Neuhaus, K., Gessner, A., Vehreschild, M. J. G. T., Haller, D., & Schwerd, T. (2023). Is Autologous Fecal Microbiota Transfer after Exclusive Enteral Nutrition in Pediatric Crohn’s Disease Patients Rational and Feasible? Data from a Feasibility Test. Nutrients, 15(7), 1742. https://doi.org/10.3390/nu15071742






