Circulating Short-Chain Fatty Acids and Non-Alcoholic Fatty Liver Disease Severity in Patients with Type 2 Diabetes Mellitus
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Sample and Clinical Information Collection
2.3. Measurement of NAFLD
2.4. Measurement of SCFAs
2.5. Statistical Analysis
3. Results
3.1. Demographic and Clinical Data of the Study Population
3.2. The Composition of Serum SCFAs in T2D Subjects with Different NAFLD Severity
3.3. Circulating SCFA Levels and the NAFLD Severity in T2D Subjects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pappachan, J.M.; Antonio, F.A.; Edavalath, M.; Mukherjee, A. Non-alcoholic fatty liver disease: A diabetologist’s perspective. Endocrine 2014, 45, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Doulberis, M.; Kotronis, G.; Gialamprinou, D.; Kountouras, J.; Katsinelos, P. Non-alcoholic fatty liver disease: An update with special focus on the role of gut microbiota. Metab. Clin. Exp. 2017, 71, 182–197. [Google Scholar] [CrossRef] [PubMed]
- Yeh, M.L.; Huang, C.F.; Huang, C.I.; Holmes, J.A.; Hsieh, M.H.; Tsai, Y.S.; Liang, P.C.; Tsai, P.C.; Hsieh, M.Y.; Lin, Z.Y.; et al. Hepatitis B-related outcomes following direct-acting antiviral therapy in Taiwanese patients with chronic HBV/HCV co-infection. J. Hepatol. 2020, 73, 62–71. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C: Final update of the series. J. Hepatol. 2020, 73, 1170–1218. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Tiniakos, D.G.; Vos, M.B.; Brunt, E.M. Nonalcoholic fatty liver disease: Pathology and pathogenesis. Annu. Rev. Pathol. 2010, 5, 145–171. [Google Scholar] [CrossRef] [PubMed]
- Araujo, A.R.; Rosso, N.; Bedogni, G.; Tiribelli, C.; Bellentani, S. Global epidemiology of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: What we need in the future. Liver Int. Off. J. Int. Assoc. Study Liver 2018, 38 (Suppl. 1), 47–51. [Google Scholar] [CrossRef]
- Bhatt, H.B.; Smith, R.J. Fatty liver disease in diabetes mellitus. Hepatobiliary Surg. Nutr. 2015, 4, 101–108. [Google Scholar] [CrossRef]
- Targher, G.; Lonardo, A.; Byrne, C.D. Nonalcoholic fatty liver disease and chronic vascular complications of diabetes mellitus. Nat. Rev. Endocrinol. 2018, 14, 99–114. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Vigliotti, C.; Witjes, J.; Le, P.; Holleboom, A.G.; Verheij, J.; Nieuwdorp, M.; Clement, K. Gut microbiota and human NAFLD: Disentangling microbial signatures from metabolic disorders. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 279–297. [Google Scholar] [CrossRef]
- Chen, J.; Vitetta, L. Gut Microbiota Metabolites in NAFLD Pathogenesis and Therapeutic Implications. Int. J. Mol. Sci. 2020, 21, 5214. [Google Scholar] [CrossRef]
- Cummings, J.H.; Pomare, E.W.; Branch, W.J.; Naylor, C.P.; Macfarlane, G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987, 28, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Rios-Covian, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de Los Reyes-Gavilan, C.G.; Salazar, N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Chakraborti, C.K. New-found link between microbiota and obesity. World J. Gastrointest. Pathophysiol. 2015, 6, 110–119. [Google Scholar] [CrossRef]
- Mandaliya, D.K.; Seshadri, S. Short Chain Fatty Acids, pancreatic dysfunction and type 2 diabetes. Pancreatol. Off. J. Int. Assoc. Pancreatol. 2019, 19, 280–284. [Google Scholar] [CrossRef]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Viardot, A.; Psichas, A.; Morrison, D.J.; Murphy, K.G.; Zac-Varghese, S.E.; MacDougall, K.; Preston, T.; Tedford, C.; Finlayson, G.S.; et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 2015, 64, 1744–1754. [Google Scholar] [CrossRef]
- Canfora, E.E.; Meex, R.C.R.; Venema, K.; Blaak, E.E. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 2019, 15, 261–273. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Fowler, K.J.; Hamilton, G.; Cui, J.Y.; Sy, E.Z.; Balanay, M.; Hooker, J.C.; Szeverenyi, N.; Sirlin, C.B. Liver fat imaging-a clinical overview of ultrasound, CT, and MR imaging. Br. J. Radiol. 2018, 91, 20170959. [Google Scholar] [CrossRef] [PubMed]
- Boursier, J.; Diehl, A.M. Implication of gut microbiota in nonalcoholic fatty liver disease. PLoS Pathog. 2015, 11, e1004559. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.S.; Chambers, E.S.; Morrison, D.J.; Frost, G. The role of short chain fatty acids in appetite regulation and energy homeostasis. Int. J. Obes. 2015, 39, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Yin, Y.; Li, Z.; Zhang, W. Gut Microbiota-Derived Components and Metabolites in the Progression of Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients 2019, 11, 1712. [Google Scholar] [CrossRef] [PubMed]
- Boursier, J.; Diehl, A.M. Nonalcoholic Fatty Liver Disease and the Gut Microbiome. Clin. Liver Dis. 2016, 20, 263–275. [Google Scholar] [CrossRef]
- The Italian Association for the Study of the Liver. AISF position paper on nonalcoholic fatty liver disease (NAFLD): Updates and future directions. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2017, 49, 471–483. [Google Scholar] [CrossRef]
- Heimann, E.; Nyman, M.; Palbrink, A.K.; Lindkvist-Petersson, K.; Degerman, E. Branched short-chain fatty acids modulate glucose and lipid metabolism in primary adipocytes. Adipocyte 2016, 5, 359–368. [Google Scholar] [CrossRef]
- Pakiet, A.; Wilczynski, M.; Rostkowska, O.; Korczynska, J.; Jablonska, P.; Kaska, L.; Proczko-Stepaniak, M.; Sobczak, E.; Stepnowski, P.; Magkos, F.; et al. The Effect of One Anastomosis Gastric Bypass on Branched-Chain Fatty Acid and Branched-Chain Amino Acid Metabolism in Subjects with Morbid Obesity. Obes. Surg. 2020, 30, 304–312. [Google Scholar] [CrossRef]
- Aragones, G.; Colom-Pellicer, M.; Aguilar, C.; Guiu-Jurado, E.; Martinez, S.; Sabench, F.; Antonio Porras, J.; Riesco, D.; Del Castillo, D.; Richart, C.; et al. Circulating microbiota-derived metabolites: A “liquid biopsy? Int. J. Obes. 2020, 44, 875–885. [Google Scholar] [CrossRef]
- Su, X.; Magkos, F.; Zhou, D.; Eagon, J.C.; Fabbrini, E.; Okunade, A.L.; Klein, S. Adipose tissue monomethyl branched-chain fatty acids and insulin sensitivity: Effects of obesity and weight loss. Obesity 2015, 23, 329–334. [Google Scholar] [CrossRef]
- Tiihonen, K.; Ouwehand, A.C.; Rautonen, N. Effect of overweight on gastrointestinal microbiology and immunology: Correlation with blood biomarkers. Br. J. Nutr. 2010, 103, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Granado-Serrano, A.B.; Martin-Gari, M.; Sanchez, V.; Riart Solans, M.; Berdun, R.; Ludwig, I.A.; Rubio, L.; Vilaprinyo, E.; Portero-Otin, M.; Serrano, J.C.E. Faecal bacterial and short-chain fatty acids signature in hypercholesterolemia. Sci. Rep. 2019, 9, 1772. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, H.E.; Teterina, A.; Comelli, E.M.; Taibi, A.; Arendt, B.M.; Fischer, S.E.; Lou, W.; Allard, J.P. Nonalcoholic fatty liver disease is associated with dysbiosis independent of body mass index and insulin resistance. Sci. Rep. 2018, 8, 1466. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, M.A.G.; Canfora, E.E.; Blaak, E.E. Faecal microbial metabolites of proteolytic and saccharolytic fermentation in relation to degree of insulin resistance in adult individuals. Benef. Microbes 2021, 12, 259–266. [Google Scholar] [CrossRef]
- Rahman, M.N.; Diantini, A.; Fattah, M.; Barliana, M.I.; Wijaya, A. A highly sensitive, simple, and fast gas chromatography-mass spectrometry method for the quantification of serum short-chain fatty acids and their potential features in central obesity. Anal. Bioanal. Chem. 2021, 413, 6837–6844. [Google Scholar] [CrossRef]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef]
- den Besten, G.; Bleeker, A.; Gerding, A.; van Eunen, K.; Havinga, R.; van Dijk, T.H.; Oosterveer, M.H.; Jonker, J.W.; Groen, A.K.; Reijngoud, D.J.; et al. Short-Chain Fatty Acids Protect Against High-Fat Diet-Induced Obesity via a PPARgamma-Dependent Switch from Lipogenesis to Fat Oxidation. Diabetes 2015, 64, 2398–2408. [Google Scholar] [CrossRef]
- Ran-Ressler, R.R.; Khailova, L.; Arganbright, K.M.; Adkins-Rieck, C.K.; Jouni, Z.E.; Koren, O.; Ley, R.E.; Brenna, J.T.; Dvorak, B. Branched chain fatty acids reduce the incidence of necrotizing enterocolitis and alter gastrointestinal microbial ecology in a neonatal rat model. PLoS ONE 2011, 6, e29032. [Google Scholar] [CrossRef]
- Nobili, V.; Putignani, L.; Mosca, A.; Del Chierico, F.; Vernocchi, P.; Alisi, A.; Stronati, L.; Cucchiara, S.; Toscano, M.; Drago, L. Bifidobacteria and lactobacilli in the gut microbiome of children with non-alcoholic fatty liver disease: Which strains act as health players? Arch. Med. Sci. AMS 2018, 14, 81–87. [Google Scholar] [CrossRef]
- Hu, D.; Yang, W.; Mao, P.; Cheng, M. Combined Amelioration of Prebiotic Resveratrol and Probiotic Bifidobacteria on Obesity and Nonalcoholic Fatty Liver Disease. Nutr. Cancer 2021, 73, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.V.; Frassetto, A.; Kowalik, E.J., Jr.; Nawrocki, A.R.; Lu, M.M.; Kosinski, J.R.; Hubert, J.A.; Szeto, D.; Yao, X.; Forrest, G.; et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS ONE 2012, 7, e35240. [Google Scholar] [CrossRef]
- Razavi, A.C.; Potts, K.S.; Kelly, T.N.; Bazzano, L.A. Sex, gut microbiome, and cardiovascular disease risk. Biol. Sex Differ. 2019, 10, 29. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Pouteau, E.; Rochat, F.; Jann, A.; Meirim, I.; Sanchez-Garcia, J.L.; Ornstein, K.; German, B.; Ballevre, O. Chicory increases acetate turnover, but not propionate and butyrate peripheral turnovers in rats. Br. J. Nutr. 2008, 99, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Montandon, S.A.; Jornayvaz, F.R. Effects of Antidiabetic Drugs on Gut Microbiota Composition. Genes 2017, 8, 250. [Google Scholar] [CrossRef] [PubMed]
- Brunkwall, L.; Orho-Melander, M. The gut microbiome as a target for prevention and treatment of hyperglycaemia in type 2 diabetes: From current human evidence to future possibilities. Diabetologia 2017, 60, 943–951. [Google Scholar] [CrossRef]
| All Participants | No or Mild NAFLD | Moderate or Severe NAFLD | p Value | |
|---|---|---|---|---|
| Number | 259 | 142 | 117 | |
| Age, years | 61.4 ± 10.6 | 63.6 ± 10.9 | 58.8 ± 9.5 | 0.03 |
| (25.4–88.1) | (35.8–88.2) | (25.8–82.5) | ||
| Sex, % | 0.76 | |||
| Male | 58.3 | 59.2 | 57.3 | |
| Female | 41.7 | 40.8 | 42.7 | |
| Habit of smoking, % | 27.9 | 28.4 | 27.4 | 0.85 |
| Alcohol drinking, % | 21.7 | 18.4 | 25.6 | 0.16 |
| Hypertension, % | 60.6 | 63.4 | 57.3 | 0.31 |
| Gout, % | 11.6 | 13.4 | 9.4 | 0.32 |
| Hyperlipidemia, % | 80.7 | 78.9 | 82.9 | 0.41 |
| DM duration, years | 10.2 ± 8.3 | 10.9 ± 8.7 | 7.5 ± 7.2 | 0.002 |
| BMI, kg/m2 | 26.6 ± 4.4 | 25.7 ± 4.0 | 28.9 ± 4.5 | <0.001 |
| BMI ≥ 27 kg/m2 | 46.3 | 33.3 | 62.1 | <0.001 |
| Medication | ||||
| Sulfonylurea (%) | 40.5 | 39.4 | 41.9 | 0.69 |
| DPP4 inhibitor (%) | 65.6 | 63.4 | 68.4 | 0.40 |
| Metformin (%) | 74.9 | 69.0 | 82.1 | 0.02 |
| Thiazolidinediones (%) | 27.4 | 29.6 | 24.8 | 0.39 |
| Insulin (%) | 12.7 | 14.1 | 11.1 | 0.47 |
| Statin (%) | 45.9 | 45.8 | 46.2 | 0.95 |
| Short-chain fatty acid | Median (25th, 75th percentile) | |||
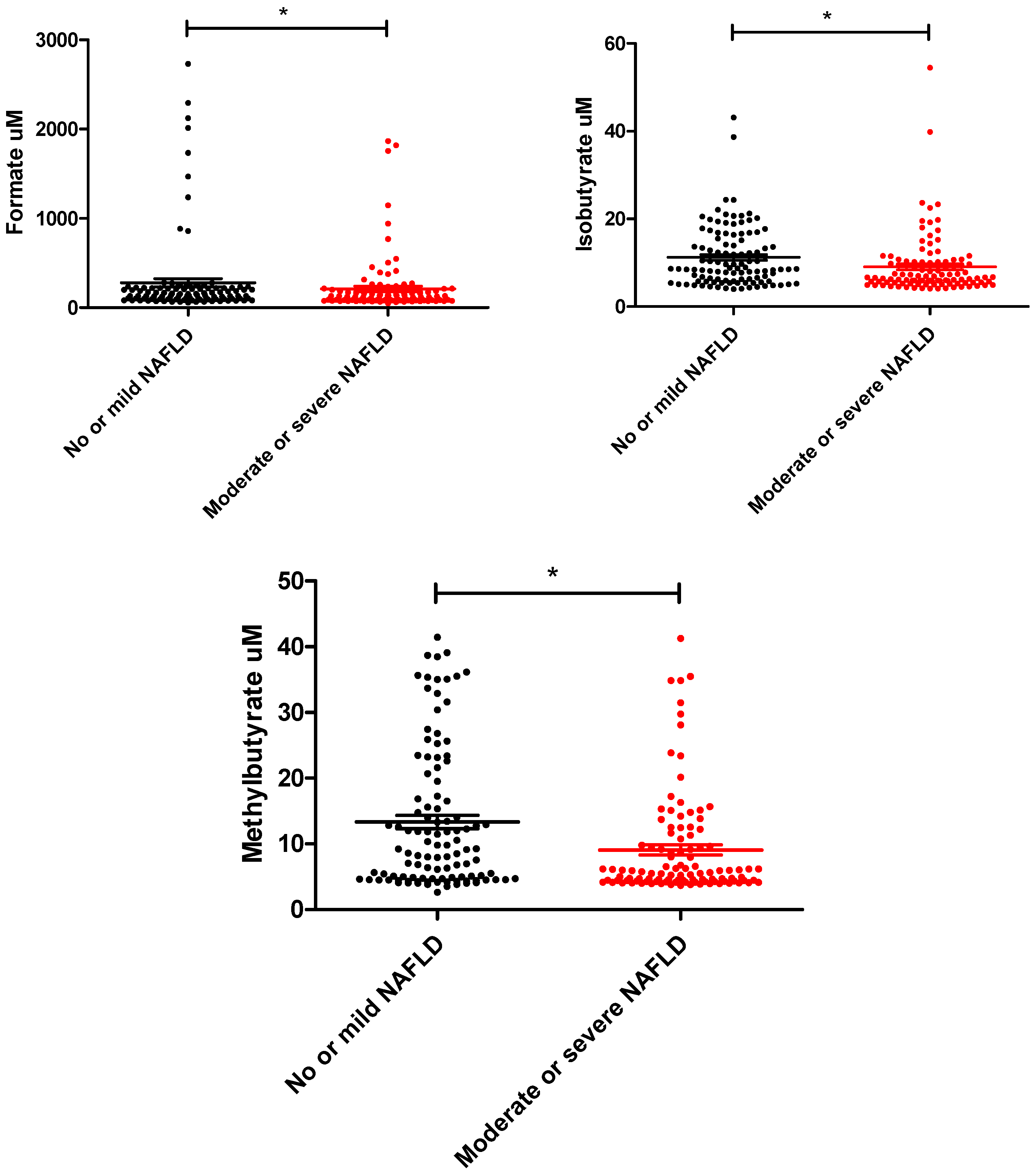
| Formate | 125.0 (89.7,214.4) | 141.8 (97.5, 218.5) | 105.7 (82.6, 171.8) | 0.01 |
| Acetate | 97.4 (74.7, 133.6) | 89.2 (71.7, 125.7) | 97.1 (74.0, 137.6) | 0.33 |
| Propionate | 15.4 (11.7, 21.4) | 15.0 (11.5, 21.7) | 14.5 (10.8, 20.7) | 0.49 |
| Butyrate | 8.1 (6.1, 9.8) | 7.9 (5.4, 9.7) | 8.1 (7.1, 9.3) | 0.43 |
| Isobutyrate | 7.6 (5.4, 12.5) | 8.6 (5.8, 15.4) | 6.6 (5.4, 10.2) | 0.003 |
| Methylbutyrate | 6.2 (4.5, 13.5) | 9.2 (4.7, 18.9) | 5.6 (4.3, 10.9) | 0.001 |
| Valerate | 2.7 (1.7, 5.0) | 2.8 (1.6, 5.7) | 2.5 (1.6, 4.6) | 0.33 |
| Isovalerate | 17.8 (4.0, 24.9) | 8.1 (3.3, 22.9) | 18.9 (4.9, 22.9) | 0.04 |
| Methylvalerate | 1.4 (0.7, 3.3) | 1.6 (0.7, 3.7) | 1.5 (0.8, 2.6) | 0.45 |
| Laboratory parameters | Mean ± SD or median (25th, 75th percentile) | |||
| Hb (g/dL) | 13.7 ± 1.7 | 13.2 ± 1.8 | 14.3 ± 1.7 | <0.001 |
| UA (mg/dL) | 5.9 ± 1.6 | 6.0 ± 1.7 | 6.0 ± 1.6 | 0.93 |
| GOT (U/L) | 30.0 ± 14.5 | 29.7 ± 15.3 | 38.8 ± 20.9 | <0.001 |
| GPT (U/L) | 32.8 ± 23.8 | 31.9 ± 26.0 | 48.7 ± 34.5 | <0.001 |
| Creatinine (mg/dL) | 1.0 ± 0.5 | 1.2 ± 0.7 | 0.9 ± 0.3 | <0.001 |
| Cholesterol (mg/dL) | 170.4 ± 40.6 | 171.8 ± 47.4 | 174.7 ± 40.5 | 0.60 |
| Triglyceride (mg/dL) | 120.0 (85.7, 179.3) | 93 (65, 140) | 133 (100, 186) | <0.001 |
| HDL (mg/dL) | 45.2 ± 17.4 | 44.2 ± 12.5 | 44.5 ± 26.6 | 0.92 |
| LDL (mg/dL) | 96.3 ± 33.3 | 98.3 ± 38.8 | 98.5 ± 32.9 | 0.96 |
| HbA1C (%) | 7.0 (6.5, 8.0) | 6.8 (6.4, 7.5) | 7.0 (6.5, 8.0) | 0.01 |
| Moderate to Severe NAFLD | Crude OR (95%Cl) | p-Value | Adjusted OR (95%Cl) | p-Value | Adjusted OR (95%Cl) | p-Value |
|---|---|---|---|---|---|---|
| Clinical characteristics | ||||||
| Age, years | 0.97 (0.95–0.99) | 0.01 | 0.97 (0.93–1.01) | 0.16 | 0.97 (0.93–1.01) | 0.17 |
| Sex (female vs. male) | 1.08 (0.66–1.77) | 0.76 | - | - | - | - |
| BMI ≥ 27 kg/m2 | 1.20 (1.12–1.28) | <0.001 | 2.35 (1.09–5.07) | 0.03 | 2.30 (1.06–4.97) | 0.03 |
| Habit of smoking (yes vs. no) | 0.95 (0.55–1.64) | 0.85 | 1.15 (0.44–3.02) | 0.78 | 1.12 (0.43–2.94) | 0.81 |
| Alcohol drinking (yes vs. no) | 1.53 (0.84–2.76) | 0.16 | 1.07 (0.38–3.07) | 0.89 | 1.12 (0.39–3.21) | 0.83 |
| T2D duration, years | 0.94 (0.91–0.98) | 0.004 | 0.99 (0.95–1.04) | 0.67 | 0.99 (0.95–1.04) | 0.68 |
| Hypertension (yes vs. no) | 0.77 (0.45–1.28) | 0.32 | 0.80 (0.38–1.68) | 0.55 | 0.84 (0.39–1.77) | 0.64 |
| Gout (yes vs. no) | 0.67 (0.31–1.48) | 0.32 | 1.10 (0.32–3.84) | 0.88 | 1.05 (0.30–3.73) | 0.94 |
| Hyperlipidemia (yes vs. no) | 1.30 (0.69–2.43) | 0.41 | 1.46 (0.54–3.95) | 0.46 | 1.51 (0.56–4.10) | 0.42 |
| Laboratory data | ||||||
| UA (mg/dL) | 0.99 (0.86–1.15) | 0.93 | - | - | - | - |
| Hb (g/dL) | 1.44 (1.24–1.68) | <0.001 | 1.16 (0.90–1.51) | 0.26 | 1.16 (0.89–1.50) | 0.26 |
| GOT (U/L) | 1.03 (1.01–1.05) | <0.001 | 1.01 (0.97–1.06) | 0.55 | 1.01 (0.97–1.06) | 0.58 |
| GPT (U/L) | 1.02 (1.01–1.03) | <0.001 | 1.00 (0.97–1.03) | 0.94 | 1.00 (0.97–1.03) | 0.96 |
| Cholesterol (mg/dL) | 1.00 (0.99–1.01) | 0.60 | - | - | - | - |
| Log (Triglyceride) | 1.01 (1.00–1.01) | <0.001 | 4.05 (0.72–22.93) | 0.11 | 4.21 (0.74–23.92) | 0.10 |
| HDL (mg/dL) | 1.00 (0.99–1.01) | 0.92 | - | - | - | - |
| LDL (mg/dL) | 1.00 (0.99–1.01) | 0.96 | - | - | - | - |
| HbA1C (%) | 1.12 (0.96–1.31) | 0.15 | - | - | - | - |
| Medication | ||||||
| Sulfonylurea (yes vs. no) | 1.11 (0.67–1.82) | 0.69 | - | - | - | - |
| DPP4 inhibitor (yes vs. no) | 1.25 (0.74–2.10) | 0.40 | - | - | - | - |
| Metformin (yes vs. no) | 2.05 (1.14–3.71) | 0.02 | 2.27 (0.79–6.48) | 0.13 | 2.27 (0.80–6.47) | 0.13 |
| Thiazolidinediones (yes vs. no) | 0.79 (0.45–1.36) | 0.39 | - | - | - | - |
| Insulin (yes vs. no) | 0.76 (0.36–1.61) | 0.48 | - | - | - | - |
| Statin (yes vs. no) | 1.02 (0.62–1.66) | 0.95 | - | - | - | - |
| SCFA | ||||||
| Log (Formate) | 0.47 (0.20–1.07) | 0.07 | - | - | ||
| Log (Acetate) | 2.28 (0.54–9.60) | 0.26 | - | - | ||
| Log (Propionate) | 0.79 (0.22–2.79) | 0.71 | - | - | ||
| Log (Butyrate) | 1.66 (0.41–6.66) | 0.48 | - | - | ||
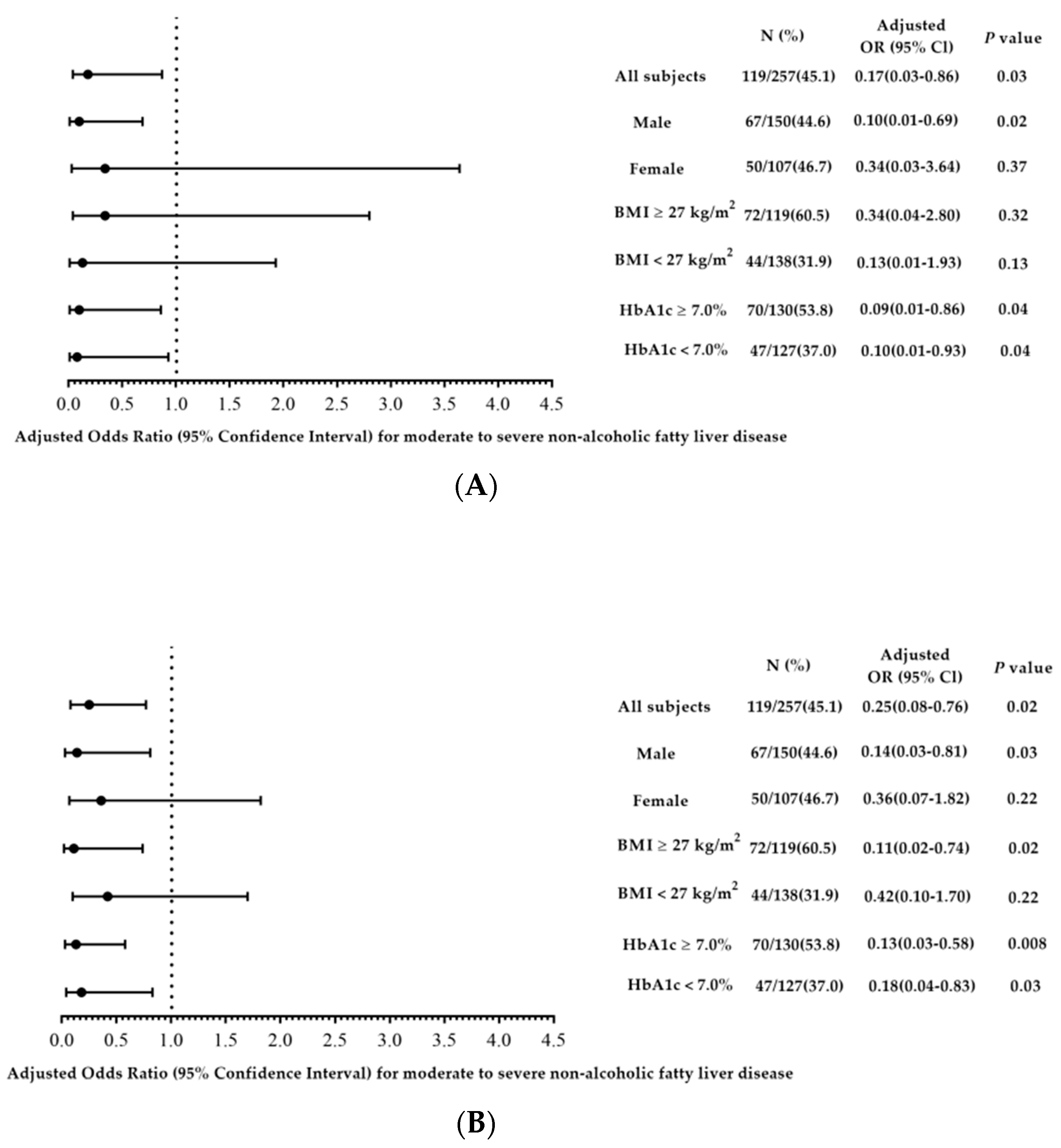
| Log (Isobutyrate) | 0.16 (0.05–0.56) | 0.004 | 0.17 (0.03–0.86) | 0.03 | ||
| Log (Methylbutyrate) | 0.21 (0.08–0.53) | 0.001 | - | - | 0.25 (0.08–0.76) | 0.02 |
| Log (Valerate) | 0.66 (0.31–1.40) | 0.28 | - | - | ||
| Log (Isovalerate) | 2.09 (1.14–3.82) | 0.02 | - | - | ||
| Log (Methylvalerate) | 0.77 (0.40–1.50) | 0.44 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, H.-J.; Hung, W.-C.; Hung, W.-W.; Lee, Y.-J.; Chen, Y.-C.; Lee, C.-Y.; Tsai, Y.-C.; Dai, C.-Y. Circulating Short-Chain Fatty Acids and Non-Alcoholic Fatty Liver Disease Severity in Patients with Type 2 Diabetes Mellitus. Nutrients 2023, 15, 1712. https://doi.org/10.3390/nu15071712
Tsai H-J, Hung W-C, Hung W-W, Lee Y-J, Chen Y-C, Lee C-Y, Tsai Y-C, Dai C-Y. Circulating Short-Chain Fatty Acids and Non-Alcoholic Fatty Liver Disease Severity in Patients with Type 2 Diabetes Mellitus. Nutrients. 2023; 15(7):1712. https://doi.org/10.3390/nu15071712
Chicago/Turabian StyleTsai, Hui-Ju, Wei-Chun Hung, Wei-Wen Hung, Yen-Jung Lee, Yo-Chia Chen, Chun-Ying Lee, Yi-Chun Tsai, and Chia-Yen Dai. 2023. "Circulating Short-Chain Fatty Acids and Non-Alcoholic Fatty Liver Disease Severity in Patients with Type 2 Diabetes Mellitus" Nutrients 15, no. 7: 1712. https://doi.org/10.3390/nu15071712
APA StyleTsai, H.-J., Hung, W.-C., Hung, W.-W., Lee, Y.-J., Chen, Y.-C., Lee, C.-Y., Tsai, Y.-C., & Dai, C.-Y. (2023). Circulating Short-Chain Fatty Acids and Non-Alcoholic Fatty Liver Disease Severity in Patients with Type 2 Diabetes Mellitus. Nutrients, 15(7), 1712. https://doi.org/10.3390/nu15071712








