Mixed Berry Juice and Cellulose Fiber Have Differential Effects on Peripheral Blood Mononuclear Cell Respiration in Overweight Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
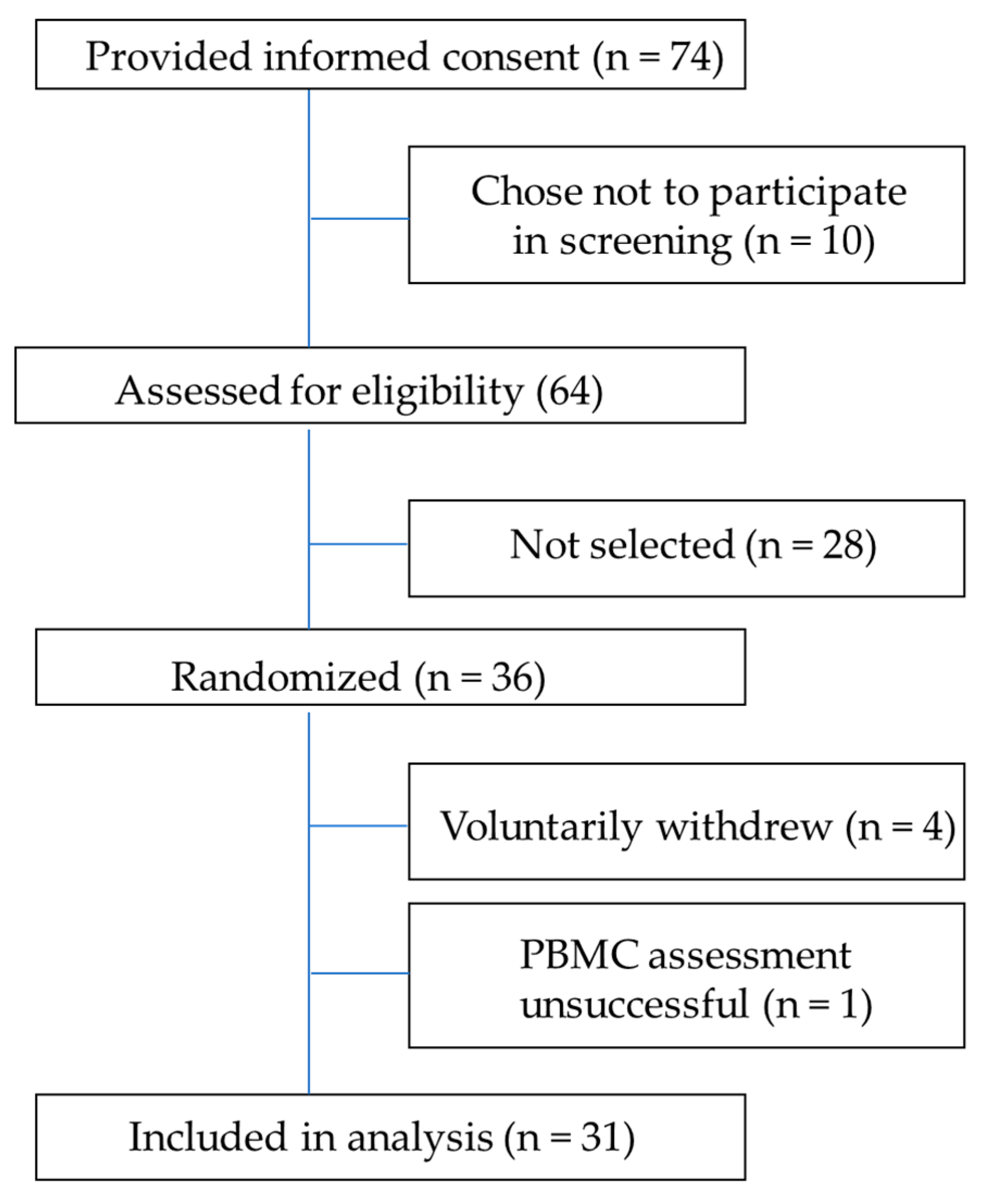
2.2. Study Participants
2.3. Diets and Dietary Treatment Foods
2.4. Identification of Phenolics in Berries and Berry Juice via LC-MS/MS
2.5. Sample Collection and Preparation
2.6. High-Resolution Respirometry
2.7. DNA Extraction and Mitochondrial PCR
2.8. Calculations and Statistics
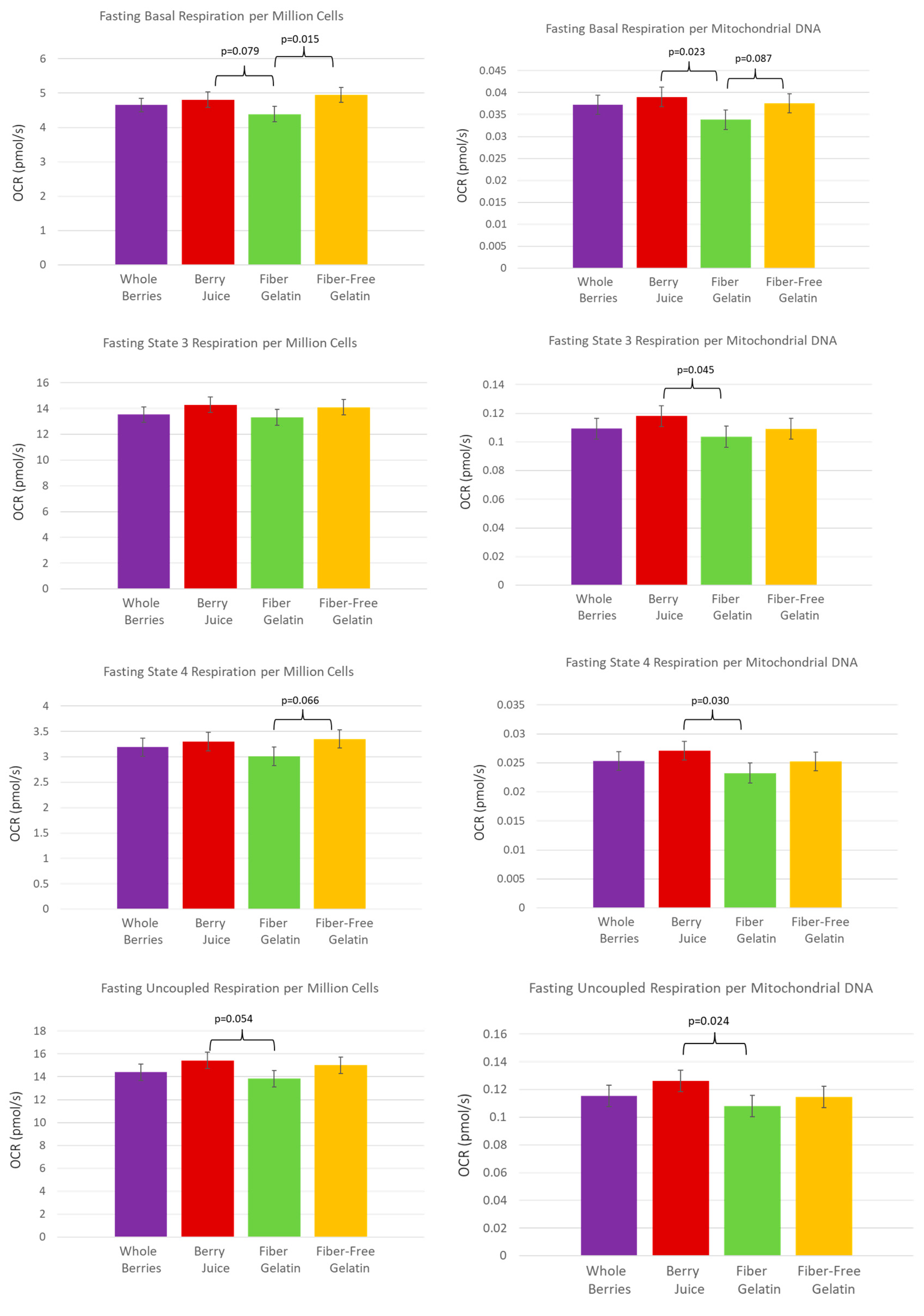
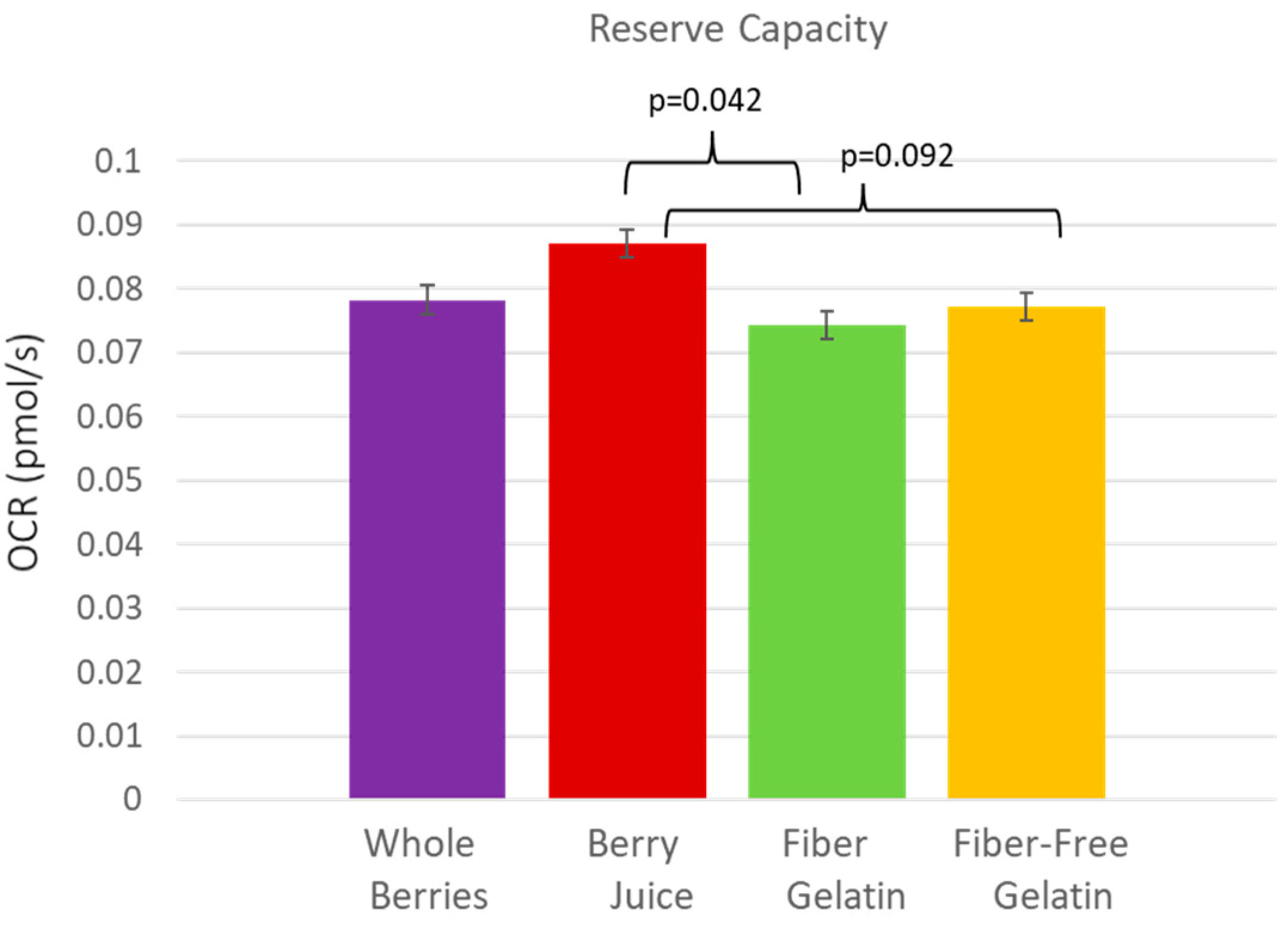
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riordan, J.; Solverson, P. Berry Anthocyanins in Rodent and Human Obesity and Diabetes: A Review of the Evidence. BioMed 2022, 2, 210–237. [Google Scholar] [CrossRef]
- Jayarathne, S.; Stull, A.J.; Park, O.H.; Kim, J.H.; Thompson, L.; Moustaid-Moussa, N. Protective Effects of Anthocyanins in Obesity-Associated Inflammation and Changes in Gut Microbiome. Mol. Nutr. Food Res. 2019, 63, e1900149. [Google Scholar] [CrossRef]
- Solverson, P.M.; Rumpler, W.V.; Leger, J.L.; Redan, B.W.; Ferruzzi, M.G.; Baer, D.J.; Castonguay, T.W.; Novotny, J.A. Blackberry Feeding Increases Fat Oxidation and Improves Insulin Sensitivity in Overweight and Obese Males. Nutrients 2018, 10, 1048. [Google Scholar] [CrossRef]
- Carobbio, S.; Pellegrinelli, V.; Vidal-Puig, A. Adipose Tissue Function and Expandability as Determinants of Lipotoxicity and the Metabolic Syndrome. Adv. Exp. Med. Biol. 2017, 960, 161–196. [Google Scholar] [CrossRef]
- Skates, E.; Overall, J.; DeZego, K.; Wilson, M.; Esposito, D.; Lila, M.A.; Komarnytsky, S. Berries containing anthocyanins with enhanced methylation profiles are more effective at ameliorating high fat diet-induced metabolic damage. Food Chem. Toxicol. 2018, 111, 445–453. [Google Scholar] [CrossRef]
- de Mello, A.H.; Costa, A.B.; Engel, J.D.G.; Rezin, G.T. Mitochondrial dysfunction in obesity. Life Sci. 2018, 192, 26–32. [Google Scholar] [CrossRef]
- Putti, R.; Sica, R.; Migliaccio, V.; Lionetti, L. Diet impact on mitochondrial bioenergetics and dynamics. Front. Physiol. 2015, 6, 109. [Google Scholar] [CrossRef]
- Zorzano, A.; Liesa, M.; Palacin, M. Role of mitochondrial dynamics proteins in the pathophysiology of obesity and type 2 diabetes. Int. J. Biochem. Cell Biol. 2009, 41, 1846–1854. [Google Scholar] [CrossRef]
- Marciniak, C.; Marechal, X.; Montaigne, D.; Neviere, R.; Lancel, S. Cardiac contractile function and mitochondrial respiration in diabetes-related mouse models. Cardiovasc. Diabetol. 2014, 13, 118. [Google Scholar] [CrossRef]
- Heinonen, S.; Buzkova, J.; Muniandy, M.; Kaksonen, R.; Ollikainen, M.; Ismail, K.; Hakkarainen, A.; Lundbom, J.; Lundbom, N.; Vuolteenaho, K.; et al. Impaired Mitochondrial Biogenesis in Adipose Tissue in Acquired Obesity. Diabetes 2015, 64, 3135–3145. [Google Scholar] [CrossRef]
- Rong, J.X.; Qiu, Y.; Hansen, M.K.; Zhu, L.; Zhang, V.; Xie, M.; Okamoto, Y.; Mattie, M.D.; Higashiyama, H.; Asano, S.; et al. Adipose mitochondrial biogenesis is suppressed in db/db and high-fat diet-fed mice and improved by rosiglitazone. Diabetes 2007, 56, 1751–1760. [Google Scholar] [CrossRef]
- Tyrrell, D.J.; Bharadwaj, M.S.; Van Horn, C.G.; Kritchevsky, S.B.; Nicklas, B.J.; Molina, A.J. Respirometric Profiling of Muscle Mitochondria and Blood Cells Are Associated with Differences in Gait Speed among Community-Dwelling Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 1394–1399. [Google Scholar] [CrossRef]
- Yin, X.; Lanza, I.R.; Swain, J.M.; Sarr, M.G.; Nair, K.S.; Jensen, M.D. Adipocyte mitochondrial function is reduced in human obesity independent of fat cell size. J. Clin. Endocrinol. Metab. 2014, 99, E209–E216. [Google Scholar] [CrossRef]
- Tyrrell, D.J.; Bharadwaj, M.S.; Van Horn, C.G.; Marsh, A.P.; Nicklas, B.J.; Molina, A.J. Blood-cell bioenergetics are associated with physical function and inflammation in overweight/obese older adults. Exp. Gerontol. 2015, 70, 84–91. [Google Scholar] [CrossRef]
- Tyrrell, D.J.; Bharadwaj, M.S.; Jorgensen, M.J.; Register, T.C.; Molina, A.J. Blood cell respirometry is associated with skeletal and cardiac muscle bioenergetics: Implications for a minimally invasive biomarker of mitochondrial health. Redox Biol. 2016, 10, 65–77. [Google Scholar] [CrossRef]
- Lahteenmaki, E.I.; Koski, M.; Koskela, I.; Lehtonen, E.; Kankaanpaa, A.; Kainulainen, H.; Walker, S.; Lehti, M. Resistance exercise with different workloads have distinct effects on cellular respiration of peripheral blood mononuclear cells. Physiol. Rep. 2022, 10, e15394. [Google Scholar] [CrossRef]
- Sommer, N.; Theine, F.F.; Pak, O.; Tello, K.; Richter, M.; Gall, H.; Wilhelm, J.; Savai, R.; Weissmann, N.; Seeger, W.; et al. Mitochondrial Respiration in Peripheral Blood Mononuclear Cells Negatively Correlates with Disease Severity in Pulmonary Arterial Hypertension. J. Clin. Med. 2022, 11, 4132. [Google Scholar] [CrossRef]
- Herpich, C.; Franz, K.; Klaus, S.; Muller-Werdan, U.; Ost, M.; Norman, K. Age-related fatigue is associated with reduced mitochondrial function in peripheral blood mononuclear cells. Exp. Gerontol. 2021, 144, 111177. [Google Scholar] [CrossRef]
- Solverson, P.M.; Henderson, T.R.; Debelo, H.; Ferruzzi, M.G.; Baer, D.J.; Novotny, J.A. An Anthocyanin-Rich Mixed-Berry Intervention May Improve Insulin Sensitivity in a Randomized Trial of Overweight and Obese Adults. Nutrients 2019, 11, 2876. [Google Scholar] [CrossRef]
- Furrer, A.; Cladis, D.P.; Kurilich, A.; Manoharan, R.; Ferruzzi, M.G. Changes in phenolic content of commercial potato varieties through industrial processing and fresh preparation. Food Chem. 2017, 218, 47–55. [Google Scholar] [CrossRef]
- Song, B.J.; Sapper, T.N.; Burtch, C.E.; Brimmer, K.; Goldschmidt, M.; Ferruzzi, M.G. Photo- and thermodegradation of anthocyanins from grape and purple sweet potato in model beverage systems. J. Agric. Food Chem. 2013, 61, 1364–1372. [Google Scholar] [CrossRef]
- Mengist, M.F.; Burtch, H.; Debelo, H.; Pottorff, M.; Bostan, H.; Nunn, C.; Corbin, S.; Kay, C.D.; Bassil, N.; Hummer, K.; et al. Development of a genetic framework to improve the efficiency of bioactive delivery from blueberry. Sci. Rep. 2020, 10, 17311. [Google Scholar] [CrossRef]
- Fasching, M.; Fontana-Ayoub, M.; Gnaiger, E. Mitochondrial respiration medium-MiR06. Mitochondr. Physiol. Netw. 2016, 14.13, 1–4. [Google Scholar]
- Boyle, K.E.; Zheng, D.; Anderson, E.J.; Neufer, P.D.; Houmard, J.A. Mitochondrial lipid oxidation is impaired in cultured myotubes from obese humans. Int. J. Obes. 2012, 36, 1025–1031. [Google Scholar] [CrossRef]
- Chance, B.; Williams, G.R. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J. Biol. Chem. 1955, 217, 409–427. [Google Scholar] [CrossRef]
- Gnaiger, E. Mitochondrial Pathways and Respiratory Control; Oroboros Instruments Corp: Innsbruck, Austria, 2014. [Google Scholar]
- Rooney, J.P.; Ryde, I.T.; Sanders, L.H.; Howlett, E.H.; Colton, M.D.; Germ, K.E.; Mayer, G.D.; Greenamyre, J.T.; Meyer, J.N. PCR based determination of mitochondrial DNA copy number in multiple species. Methods Mol. Biol. 2015, 1241, 23–38. [Google Scholar] [CrossRef]
- Venegas, V.; Halberg, M.C. Measurement of mitochondrial DNA copy number. Methods Mol. Biol. 2012, 837, 327–335. [Google Scholar] [CrossRef]
- Bouwens, M.; Afman, L.A.; Muller, M. Fasting induces changes in peripheral blood mononuclear cell gene expression profiles related to increases in fatty acid beta-oxidation: Functional role of peroxisome proliferator activated receptor alpha in human peripheral blood mononuclear cells. Am. J. Clin. Nutr. 2007, 86, 1515–1523. [Google Scholar] [CrossRef]
- Giampieri, F.; Alvarez-Suarez, J.M.; Mazzoni, L.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Gonzalez-Paramas, A.M.; Santos-Buelga, C.; Quiles, J.L.; Bompadre, S.; Mezzetti, B.; et al. An anthocyanin-rich strawberry extract protects against oxidative stress damage and improves mitochondrial functionality in human dermal fibroblasts exposed to an oxidizing agent. Food Funct. 2014, 5, 1939–1948. [Google Scholar] [CrossRef]
- de Sales, N.F.F.; da Costa, L.S.; Carneiro, T.I.A.; Minuzzo, D.A.; Oliveira, F.L.; Cabral, L.M.C.; Torres, A.G.; El-Bacha, T. Anthocyanin-Rich Grape Pomace Extract (Vitis vinifera L.) from Wine Industry Affects Mitochondrial Bioenergetics and Glucose Metabolism in Human Hepatocarcinoma HepG2 Cells. Molecules 2018, 23, 611. [Google Scholar] [CrossRef]
- Solverson, P.; Albaugh, G.P.; Harrison, D.J.; Luthria, D.L.; Baer, D.J.; Novotny, J.A. High-dose administration of purified cyanidin 3-glucose or a blackberry extract causes improved mitochondrial function but reduced content in 3T3-L1 adipocytes. Food Front. 2022, 3, 276–284. [Google Scholar] [CrossRef]
- Tang, X.; Shen, T.; Jiang, X.; Xia, M.; Sun, X.; Guo, H.; Ling, W. Purified anthocyanins from bilberry and black currant attenuate hepatic mitochondrial dysfunction and steatohepatitis in mice with methionine and choline deficiency. J. Agric. Food Chem. 2015, 63, 552–561. [Google Scholar] [CrossRef]
- Skemiene, K.; Rakauskaite, G.; Trumbeckaite, S.; Liobikas, J.; Brown, G.C.; Borutaite, V. Anthocyanins block ischemia-induced apoptosis in the perfused heart and support mitochondrial respiration potentially by reducing cytosolic cytochrome c. Int. J. Biochem. Cell Biol. 2013, 45, 23–29. [Google Scholar] [CrossRef]
- Neves, D.; Valentao, P.; Bernardo, J.; Oliveira, M.C.; Ferreira, M.G.; Pereira, D.M.; Andrade, P.B.; Videira, R.A. A new insight on elderberry anthocyanins bioactivity: Modulation of mitochondrial redox chain functionality and cell redox state. J. Funct. Foods 2019, 56, 145–155. [Google Scholar] [CrossRef]
- D’Amico, D.; Andreux, P.A.; Valdes, P.; Singh, A.; Rinsch, C.; Auwerx, J. Impact of the Natural Compound Urolithin A on Health, Disease, and Aging. Trends Mol. Med. 2021, 27, 687–699. [Google Scholar] [CrossRef]
- Singh, A.; D’Amico, D.; Andreux, P.A.; Dunngalvin, G.; Kern, T.; Blanco-Bose, W.; Auwerx, J.; Aebischer, P.; Rinsch, C. Direct supplementation with Urolithin A overcomes limitations of dietary exposure and gut microbiome variability in healthy adults to achieve consistent levels across the population. Eur. J. Clin. Nutr. 2022, 76, 297–308. [Google Scholar] [CrossRef]
- Wedekind, K.J.; Mansfield, H.R.; Montgomery, L. Enumeration and isolation of cellulolytic and hemicellulolytic bacteria from human feces. Appl. Environ. Microbiol. 1988, 54, 1530–1535. [Google Scholar] [CrossRef]
- Chassard, C.; Delmas, E.; Robert, C.; Bernalier-Donadille, A. The cellulose-degrading microbial community of the human gut varies according to the presence or absence of methanogens. FEMS Microbiol. Ecol. 2010, 74, 205–213. [Google Scholar] [CrossRef]
- Holloway, W.D.; Tasman-Jones, C.; Lee, S.P. Digestion of certain fractions of dietary fiber in humans. Am. J. Clin. Nutr. 1978, 31, 927–930. [Google Scholar] [CrossRef]
- Slavin, J.L.; Brauer, P.M.; Marlett, J.A. Neutral detergent fiber, hemicellulose and cellulose digestibility in human subjects. J. Nutr. 1981, 111, 287–297. [Google Scholar] [CrossRef]
- Kelsay, J.L.; Goering, H.K.; Behall, K.M.; Prather, E.S. Effect of fiber from fruits and vegetables on metabolic responses of human subjects: Fiber intakes, fecal excretions, and apparent digestibilities. Am. J. Clin. Nutr. 1981, 34, 1849–1852. [Google Scholar] [CrossRef]
- Joshi, S.; Agte, V. Digestibility of dietary fiber components in vegetarian men. Plant Foods Hum. Nutr. 1995, 48, 39–44. [Google Scholar] [CrossRef]
- Bach Knudsen, K.E.; Serena, A.; Canibe, N.; Juntunen, K.S. New insight into butyrate metabolism. Proc. Nutr. Soc. 2003, 62, 81–86. [Google Scholar] [CrossRef]
- Lappi, J.; Mykkanen, H.; Bach Knudsen, K.E.; Kirjavainen, P.; Katina, K.; Pihlajamaki, J.; Poutanen, K.; Kolehmainen, M. Postprandial glucose metabolism and SCFA after consuming wholegrain rye bread and wheat bread enriched with bioprocessed rye bran in individuals with mild gastrointestinal symptoms. Nutr. J. 2014, 13, 104. [Google Scholar] [CrossRef]
- Priebe, M.G.; Wang, H.; Weening, D.; Schepers, M.; Preston, T.; Vonk, R.J. Factors related to colonic fermentation of nondigestible carbohydrates of a previous evening meal increase tissue glucose uptake and moderate glucose-associated inflammation. Am. J. Clin. Nutr. 2010, 91, 90–97. [Google Scholar] [CrossRef]
- Nilsson, A.C.; Ostman, E.M.; Knudsen, K.E.; Holst, J.J.; Bjorck, I.M. A cereal-based evening meal rich in indigestible carbohydrates increases plasma butyrate the next morning. J. Nutr. 2010, 140, 1932–1936. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Canfora, E.E.; van der Beek, C.M.; Jocken, J.W.E.; Goossens, G.H.; Holst, J.J.; Olde Damink, S.W.M.; Lenaerts, K.; Dejong, C.H.C.; Blaak, E.E. Colonic infusions of short-chain fatty acid mixtures promote energy metabolism in overweight/obese men: A randomized crossover trial. Sci. Rep. 2017, 7, 2360. [Google Scholar] [CrossRef]

| Gelatin Control | Whole Berries | Berry Juice | Gelatin + Fiber | |
|---|---|---|---|---|
| Daily dose (g) | 523 | 523 | 502 | 523 |
| Total sugar (g) | 37.6 | 37.6 | 37.4 | 37.6 |
| Glucose (g) | 19.6 | 19.6 | 19.6 | 19.6 |
| Fructose (g) | 18.0 | 18.0 | 17.8 | 18.0 |
| Soluble fiber (g) | 0 | 0 | 0 | 0 |
| Insoluble fiber (g) | 0 | 9.5 | 0 | 9.5 |
| Anthocyanins (mg) | 0 | 143.5 | 101.1 | 0 |
| Flavonols (mg) | 0 | 15.3 | 6.4 | 0 |
| Flavan-3-ols (mg) | 0 | 2.9 | 0.7 | 0 |
| Phenolic acids (mg) | 0 | 38.2 | 6.1 | 0 |
| Females (n = 16) | Males (n = 15) | |||
|---|---|---|---|---|
| Overweight (n = 6) | Obese (n = 10) | Overweight (n = 8) | Obese (n = 7) | |
| Weight, kg | 67.9 ± 3.1 | 89.8 ± 10.6 | 82.7 ± 11.0 | 100.5 ± 8.0 |
| BMI, kg/m2 | 26.6 ± 0.7 | 33.7 ± 2.9 | 26.9 ± 1.3 | 32.2 ± 1.3 |
| Age, y | 59.3 ± 9.4 | 60.2 ± 8.1 | 60.0 ± 13.3 | 62.1 ± 10.3 |
| mg per 100 g Fresh Weight | ||
|---|---|---|
| Whole Berries | Berry Juice | |
| Anthocyanins | ||
| Cyanidin-3-Gal | 1.70 ± 0.08 | 3.19 ± 0.05 |
| Cyanidin-3-Glu | 14.05 ± 0.96 | 6.57 ± 0.20 |
| Cyanidin-3-Ara | 0.906 ± 0.168 | 2.01 ± 0.05 |
| Delphinidin-3-Gal | 0.874 ± 0.103 | 0.101 ± 0.002 |
| Delphinidin-3-Glu | ND | trace |
| Delphinidin-3-Ara | 0.830 ± 0.081 | 0.053 ± 0.002 |
| Malvidin-3-Gal | 2.54 ± 0.15 | 1.05 ± 0.06 |
| Malvidin-3-Glu | 0.500 ± 0.060 | 0.012 ± 0.003 |
| Malvidin-3-Ara | 3.05 ± 0.17 | 0.805 ± 0.017 |
| Peonidin-3-Gal | 0.793 ± 0.102 | 3.43 ± 0.10 |
| Peonidin-3-Glu | 0.070 ± 0.012 | 0.17 ± 0.02 |
| Peonidin-3-Ara | 0.588 ± 0.075 | 2.53 ± 0.06 |
| Petunidin-3-Gal | 0.920 ± 0.039 | 0.159 ± 0.003 |
| Petunidin-3-Glu | 0.340 ± 0.011 | 0.016 ± 0.004 |
| Petunidin-3-Ara | 0.076 ± 0.066 | 0.057 ± 0.002 |
| Total Anthocyanins | 27.2 ± 1.9 | 20.1 ± 0.4 |
| Flavan-3-ols | ||
| Catechin | 0.226 ± 0.026 | 0.079 ± 0.003 |
| Epicatechin | 0.330 ± 0.016 | 0.039 ± 0.001 |
| Total Flavan-3-ols | 0.556 ± 0.041 | 0.118 ± 0.004 |
| Flavonols | ||
| Quercetin-3-Gal | 2.01 ± 0.64 | 1.00 ± 0.01 |
| Quercetin-3-Glu | 0.448 ± 0.001 | 0.088 ± 0.001 |
| Kaempferol-3-Glu | 0.466 ± 0.042 | 0.186 ± 0.003 |
| Total Flavonols | 2.93 ± 0.67 | 1.27 ± 0.01 |
| Phenolic Acids | ||
| Chlorogenic acid | 7.09 ± 0.27 | 1.08 ± 0.02 |
| Caffeic acid | 0.211 ± 0.013 | 0.032 ± 0.002 |
| Gallic acid | ND | 0.116 ± 0.004 |
| Total Phenolic acids | 7.30 ± 0.28 | 1.23 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solverson, P.; Albaugh, G.P.; Debelo, H.A.; Ferruzzi, M.G.; Baer, D.J.; Novotny, J.A. Mixed Berry Juice and Cellulose Fiber Have Differential Effects on Peripheral Blood Mononuclear Cell Respiration in Overweight Adults. Nutrients 2023, 15, 1709. https://doi.org/10.3390/nu15071709
Solverson P, Albaugh GP, Debelo HA, Ferruzzi MG, Baer DJ, Novotny JA. Mixed Berry Juice and Cellulose Fiber Have Differential Effects on Peripheral Blood Mononuclear Cell Respiration in Overweight Adults. Nutrients. 2023; 15(7):1709. https://doi.org/10.3390/nu15071709
Chicago/Turabian StyleSolverson, Patrick, George P. Albaugh, Hawi A. Debelo, Mario G. Ferruzzi, David J. Baer, and Janet A. Novotny. 2023. "Mixed Berry Juice and Cellulose Fiber Have Differential Effects on Peripheral Blood Mononuclear Cell Respiration in Overweight Adults" Nutrients 15, no. 7: 1709. https://doi.org/10.3390/nu15071709
APA StyleSolverson, P., Albaugh, G. P., Debelo, H. A., Ferruzzi, M. G., Baer, D. J., & Novotny, J. A. (2023). Mixed Berry Juice and Cellulose Fiber Have Differential Effects on Peripheral Blood Mononuclear Cell Respiration in Overweight Adults. Nutrients, 15(7), 1709. https://doi.org/10.3390/nu15071709







