Analysis of Feeding Behavior Characteristics in the Cu/Zn Superoxide Dismutase 1 (SOD1) SOD1G93A Mice Model for Amyotrophic Lateral Sclerosis (ALS)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Video Recording of Opening and Closing Mouth Movements in ALS Model Mice
2.3. Development of an AI Model for Detecting Opening and Closing Mouth Movements in Mice
2.4. Observation of Feeding Behavior and Body Weight Measurement of ALS Model Mice
2.5. Electrophysiological Investigation of MesV in ALS Mice Model
2.6. Statistical Analysis
3. Results
3.1. AI Model for Opening and Closing Mouth Movement in Mice
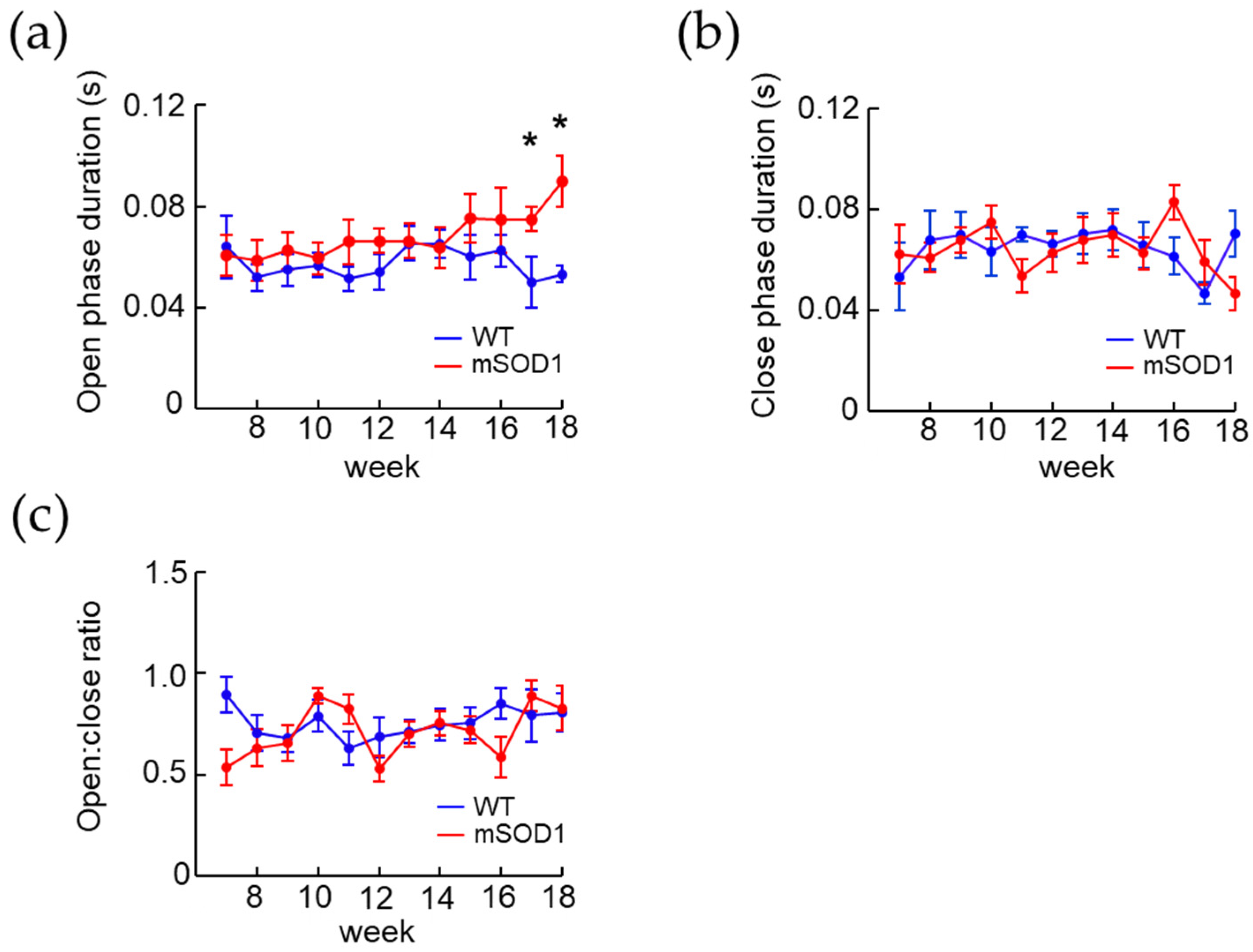
3.2. Changes in Body Weight and Feeding Behavior in the ALS Mice Model over Time
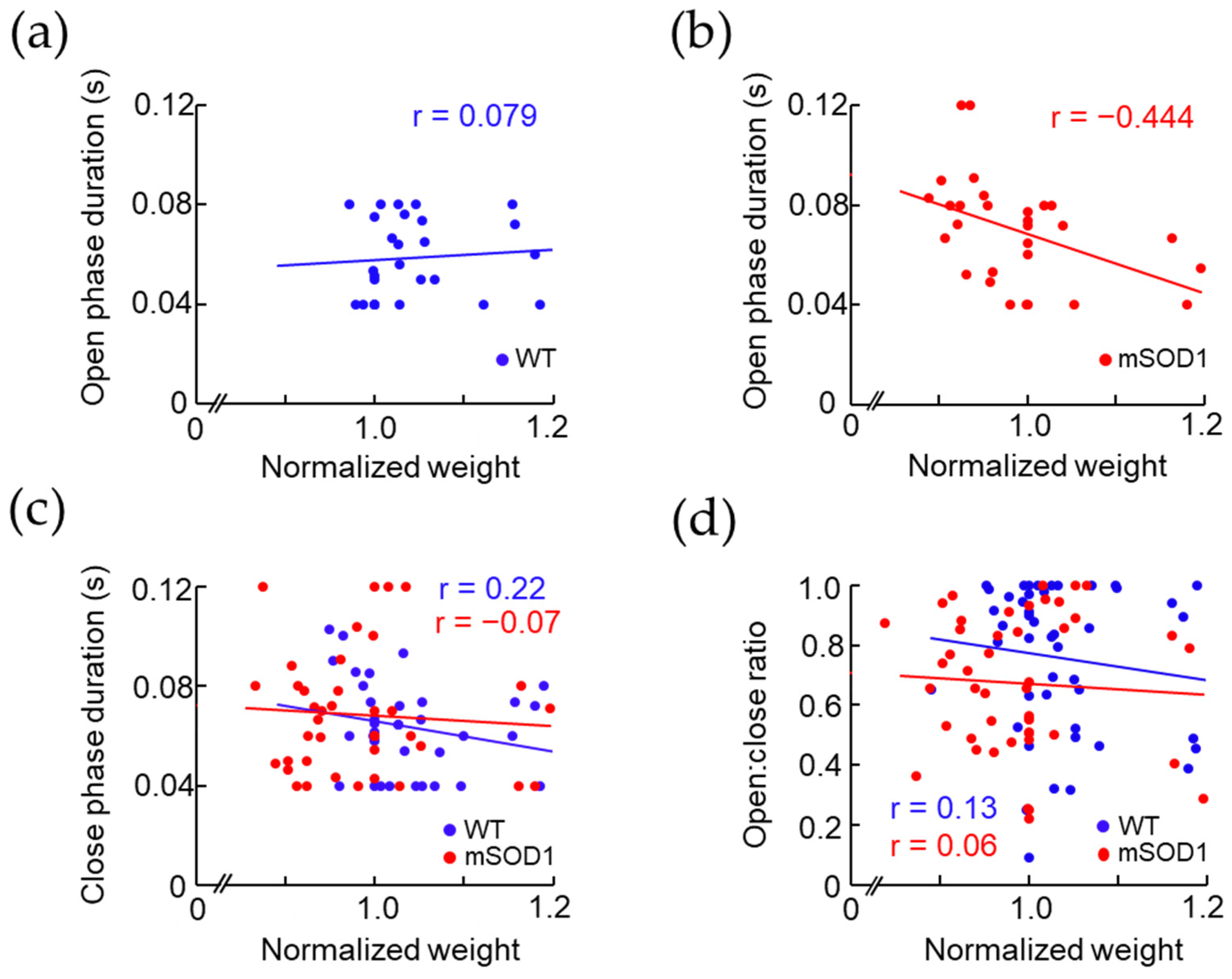
3.3. Correlation between the Open and Close Phase Duration and Body Weight in ALS Mice Model after 12 Weeks of Age
3.4. Electrophysiological Characteristic Modulation of MesV in 12-Week-Old ALS Mice Model
4. Discussion
4.1. Mastication Movement Analysis of ALS Model Mice Using AI
4.2. Relationship between Modulation of Masticatory Movement and Body Weight in ALS Mice Model
4.3. Changes in MesV Properties in Mature ALS Model Mice
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Chiò, A.; Logroscino, G.; Hardiman, O.; Swingler, R.; Mitchell, D.; Beghi, E.; Traynor, B.G.; Eurals Consortium. Prognostic factors in ALS: A critical review. Amyotroph. Lateral Scler. 2009, 10, 310–323. [Google Scholar] [CrossRef]
- Hillel, A.D.; Miller, R. Bulbar amyotrophic lateral sclerosis: Patterns of progression and clinical management. Head Neck 1989, 11, 51–59. [Google Scholar] [CrossRef]
- Mitchell, J.D.; Callagher, P.; Gardham, J.; Mitchell, C.; Dixon, M.; Addison-Jones, R.; Bennett, W.; O’Brien, M.R. Timelines in the diagnostic evaluation of people with suspected amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND)—A 20-year review: Can we do better? Amyotroph. Lateral Scler. 2010, 11, 537–541. [Google Scholar] [CrossRef]
- Petrov, D.; Mansfield, C.; Moussy, A.; Hermine, O. ALS clinical trials review: 20 years of failure. Are We Any Closer to Registering a New Treatment? Front. Aging Neurosci. 2017, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Sawada, H. Clinical efficacy of edaravone for the treatment of amyotrophic lateral sclerosis. Expert Opin. Pharmacother. 2017, 18, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ichinomiya, T.; Savchenko, P.; Wang, D.; Sawada, A.; Li, X.; Duong, T.; Li, W.; Bonds, J.A.; Kim, E.J.; et al. Subpial delivery of adeno-associated virus 9-synapsin-caveolin-1 (AAV9-SynCav1) preserves motor neuron and neuromuscular junction morphology, motor function, delays disease onset, and extends survival in hSOD1G93A mice. Theranostics 2022, 12, 5389–5403. [Google Scholar] [CrossRef] [PubMed]
- Kawai, S.; Tsukuda, M.; Mochimatsu, I.; Enomoto, H.; Kagesato, Y.; Hirose, H.; Kuroiwa, Y.; Suzuki, Y. A study of the early stage of dysphagia in amyotrophic lateral sclerosis. Dysphagia 2003, 18, 1–8. [Google Scholar] [CrossRef]
- Jani, M.P.; Gore, G.B. Swallowing characteristics in amyotrophic lateral sclerosis’. NeuroRehabilitation 2016, 39, 273–276. [Google Scholar] [CrossRef]
- Rosen, D.R.; Siddique, T.; Patterson, D.; Figlewicz, D.A.; Sapp, P.; Hentati, A.; Donaldson, D.; Goto, J.; O’Regan, J.P.; Deng, H.X. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 1993, 362, 59–62. [Google Scholar] [CrossRef]
- Lutz, C. Mouse models of ALS: Past, present and future. Brain Res. 2018, 1693, 1–10. [Google Scholar] [CrossRef]
- Gurney, M.E.; Pu, H.; Chiu, A.Y.; Dal Canto, M.C.; Polchow, C.Y.; Alexander, D.D.; Caliendo, J.; Hentati, A.; Kwon, Y.W.; Deng, H.X. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science 1994, 264, 1772–1775. [Google Scholar] [CrossRef] [PubMed]
- Weydt, P.; Hong, S.Y.; Kliot, M.; Möller, T. Assessing disease onset and progression in the SOD1 mouse model of ALS. NeuroReport 2003, 14, 1051–1054. [Google Scholar] [CrossRef] [PubMed]
- Kieran, D.; Kalmar, B.; Dick, J.R.; Riddoch-Contreras, J.; Burnstock, G.; Greensmith, L. Treatment with arimoclomol, a coinducer of heat shock proteins, delays disease progression in ALS mice. Nat. Med. 2004, 10, 402–405. [Google Scholar] [CrossRef] [PubMed]
- Wooley, C.M.; Sher, R.B.; Kale, A.; Frankel, W.N.; Cox, G.A.; Seburn, K.L. Gait analysis detects early changes in transgenic SOD1(G93A) mice. Muscle Nerve 2005, 32, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Stam, N.C.; Nithianantharajah, J.; Howard, M.L.; Atkin, J.D.; Cheema, S.S.; Hannan, A.J. Sex-specific behavioural effects of environmental enrichment in a transgenic mouse model of amyotrophic lateral sclerosis. Eur. J. Neurosci. 2008, 28, 717–723. [Google Scholar] [CrossRef]
- Smittkamp, S.E.; Brown, J.W.; Stanford, J.A. Time-course and characterization of orolingual motor deficits in B6SJL-Tg(SOD1-G93A)1Gur/J mice. Neuroscience 2008, 151, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Kasarskis, E.J.; Neville, H.E. Management of ALS: Nutritional care. Neurology 1996, 47 (Suppl. 2), S118–S120. [Google Scholar] [CrossRef] [PubMed]
- Bruestle, D.A.; Cutler, R.G.; Telljohann, R.S.; Mattson, M.P. Decline in daily running distance presages disease onset in a mouse model of ALS. NeuroMolecular Med. 2009, 11, 58–62. [Google Scholar] [CrossRef]
- Dell, A.I.; Bender, J.A.; Branson, K.; Couzin, I.D.; de Polavieja, G.G.; Noldus, L.P.J.J.; Pérez-Escudero, A.; Perona, P.; Straw, A.D.; Wikelski, M.; et al. Automated image-based tracking and its application in ecology. Trends Ecol. Evol. 2014, 29, 417–428. [Google Scholar] [CrossRef]
- Camomilla, V.; Bergamini, E.; Fantozzi, S.; Vannozzi, G. Trends supporting the in-field use of wearable inertial sensors for sport performance evaluation: A systematic review. Sensors 2018, 18, 873. [Google Scholar] [CrossRef]
- Moriuchi, E.; Hamanaka, R.; Koga, Y.; Fujishita, A.; Yoshimi, T.; Yasuda, G.; Kohara, H.; Yoshida, N. Development and evaluation of a jaw-tracking system for mice: Reconstruction of three-dimensional movement trajectories on an arbitrary point on the mandible. Biomed. Eng. OnLine 2019, 18, 59. [Google Scholar] [CrossRef] [PubMed]
- Utsumi, D.; Nakamura, A.; Matsuo, K.; Zeredo, J.L.; Koga, Y.; Yoshida, N. Motor coordination of masseter and temporalis muscle during mastication in mice. J. Stomat. Occ. Med. 2010, 3, 187–194. [Google Scholar] [CrossRef]
- Lever, T.E.; Gorsek, A.; Cox, K.T.; O’Brien, K.F.; Capra, N.F.; Hough, M.S.; Murashov, A.K. An animal model of oral dysphagia in amyotrophic lateral sclerosis. Dysphagia 2009, 24, 180–195. [Google Scholar] [CrossRef]
- Mori, Y.; Oichi, T.; Enomoto-Iwamoto, M.; Saito, T. Automatic detection of medial and lateral compartments from histological sections of mouse knee joints using the single-shot multibox detector algorithm. Cartilage 2022, 13, 19476035221074009. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, A.T.; Claridge-Chang, A. The surveillance state of behavioral automation. Curr. Opin. Neurobiol. 2012, 22, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.J.; Perona, P. Toward a science of computational ethology. Neuron 2014, 84, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Gao, J.; Zhao, D. A review of the application of deep learning in medical image classification and segmentation. Ann. Transl. Med. 2020, 8, 713. [Google Scholar] [CrossRef]
- Liu, W.; Anguelov, D.; Erhan, D.; Szegedy, C.; Reed, S.; Fu, C.Y.; Berg, A.C. SSD: Single shot MultiBox detector. In Computer Vision—ECCV; Springer: Cham, Switzerland, 2016; pp. 21–37. [Google Scholar] [CrossRef]
- ‘Krizhevsky, A.; Sutskever, I.; Hinton, G.E. ImageNet classification with deep convolutional neural networks. Commun. ACM 2017, 60, 84–90. [Google Scholar] [CrossRef]
- Simonyan, K.; Zisserman, A. Very deep convolutional networks for large-scale image recognition. arXiv 2015, arXiv:1409.1556. [Google Scholar]
- Wang, Y.K.; Syu, H.Y.; Chen, Y.H.; Chung, C.S.; Tseng, Y.S.; Ho, S.Y.; Huang, C.W.; Wu, I.C.; Wang, H.C. Endoscopic images by a single-shot multibox detector for the identification of early cancerous lesions in the esophagus: A pilot study. Cancers 2021, 13, 321. [Google Scholar] [CrossRef]
- Zhang, Z.; Coyle, J.L.; Sejdić, E. Automatic hyoid bone detection in fluoroscopic images using deep learning. Sci. Rep. 2018, 8, 12310. [Google Scholar] [CrossRef] [PubMed]
- Komuro, A.; Morimoto, T.; Iwata, K.; Inoue, T.; Masuda, Y.; Kato, T.; Hidaka, O. Putative feed-forward control of jaw-closing muscle activity during rhythmic jaw movements in the anesthetized rabbit. J. Neurophysiol. 2001, 86, 2834–2844. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, S.; Kinoshita, K.; Muroi, Y.; Ishii, T. The effects of bilateral lesions of the mesencephalic trigeminal sensory nucleus on nocturnal feeding and related behaviors in mice. Life Sci. 2013, 93, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Mameli, O.; Stanzani, S.; Russo, A.; Pellitteri, R.; Manca, P.; De Riu, P.L.; Caria, M.A. Involvement of trigeminal mesencephalic nucleus in kinetic encoding of whisker movements. Brain Res. Bull. 2014, 102, 37–45. [Google Scholar] [CrossRef]
- Venugopal, S.; Hsiao, C.F.; Sonoda, T.; Wiedau-Pazos, M.; Chandler, S.H. Homeostatic dysregulation in membrane properties of masticatory motoneurons compared with oculomotor neurons in a mouse model for amyotrophic lateral sclerosis. J. Neurosci. 2015, 35, 707–720. [Google Scholar] [CrossRef]
- Seki, S.; Yamamoto, T.; Quinn, K.; Spigelman, I.; Pantazis, A.; Olcese, R.; Wiedau-Pazos, M.; Chandler, S.H.; Venugopal, S. Circuit-specific early impairment of proprioceptive sensory neurons in the SOD1G93A mouse model for ALS. J. Neurosci. 2019, 39, 8798–8815. [Google Scholar] [CrossRef]
- Rocha, M.C.; Pousinha, P.A.; Correia, A.M.; Sebastião, A.M.; Ribeiro, J.A. Early changes of neuromuscular transmission in the SOD1(G93A) mice model of ALS start long before motor symptoms onset. PLoS ONE 2013, 8, e73846. [Google Scholar] [CrossRef]
- Kida, K.; Tsuji, T.; Tanaka, S.; Kogo, M. Zinc deficiency with reduced mastication impairs spatial memory in young adult mice. Physiol. Behav. 2015, 52, 231–237. [Google Scholar] [CrossRef]
- Okayasu, I.; Yamada, Y.; Kohno, S.; Yoshida, N. New Animal Model for Studying Mastication in Oral Motor Disorders. J. Dent. Res. 2003, 82, 318–321. [Google Scholar] [CrossRef]
- Kim, Y.-A.; Lee, S.-S.; Yoo, J.; Kim, E.-M.; Nam, M.S.; Kim, K.K. Effects of Gouda Cheese and Allium Hookeri on Thermogenesis in Mice. Food Sci. Nutr. 2021, 9, 1232–1239. [Google Scholar] [CrossRef]
- Lee, S.-S.; Kim, Y.-A.; Eun, B.; Yoo, J.; Kim, E.-M.; Nam, M.S.; Kim, K.K. Betaine, a Component of Lycium Chinense, Enhances Muscular Endurance of Mice and Myogenesis of Myoblasts. Food Sci. Nutr. 2021, 9, 5083–5091. [Google Scholar] [CrossRef] [PubMed]
- Ushimura, A.; Tsuji, T.; Tanaka, S.; Kogo, M.; Yamamoto, T. Neuropeptide-Y modulates eating patterns and masticatory muscle activity in rats. Behav. Brain Res. 2015, 278, 520–526. [Google Scholar] [CrossRef]
- Del Negro, C.A.; Chandler, S.H. Physiological and theoretical analysis of K+ currents controlling discharge in neonatal rat mesencephalic trigeminal neurons. J. Neurophysiol. 1997, 77, 537–553. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Enomoto, A.; Tanaka, S.; Hsiao, C.F.; Nykamp, D.Q.; Izhikevich, E.; Chandler, S.H. Persistent sodium currents in mesencephalic v neurons participate in burst generation and control of membrane excitability. J. Neurophysiol. 2005, 93, 2710–2722. [Google Scholar] [CrossRef]
- Xing, J.-L.; Hu, S.-J.; Yang, J. Electrophysiological features of neurons in the mesencephalic trigeminal nuclei. Neurosignals 2014, 22, 79–91. [Google Scholar] [CrossRef]
- Tanaka, S.; Wu, N.; Hsaio, C.F.; Turman, J., Jr.; Chandler, S.H. Development of inward rectification and control of membrane excitability in mesencephalic v neurons. J. Neurophysiol. 2003, 89, 1288–1298. [Google Scholar] [CrossRef]
- Tanaka, S.; Chandler, S.H. Serotonergic modulation of persistent sodium currents and membrane excitability via cyclic AMP-protein kinase A cascade in mesencephalic V neurons. J. Neurosci. Res. 2006, 83, 1362–1372. [Google Scholar] [CrossRef]
- Wu, N.; Hsiao, C.F.; Chandler, S.H. Membrane resonance and subthreshold membrane oscillations in mesencephalic V neurons: Participants in burst generation. J. Neurosci. 2001, 21, 3729–37239. [Google Scholar] [CrossRef]
- Yoshida, S.; Oka, H. Membrane properties of dissociated trigeminal mesencephalic neurons of the adult rat. Neurosci. Res. 1998, 30, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Mathis, M.W.; Mathis, A. Deep learning tools for the measurement of animal behavior in neuroscience. Curr. Opin. Neurobiol. 2020, 60, 1–11. [Google Scholar] [CrossRef]
- Hollis, J.H. The effect of mastication on food intake, satiety and body weight. Physiol. Behav. 2018, 193, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, S.; Seki, S.; Terman, D.H.; Pantazis, A.; Olcese, R.; Wiedau-Pazos, M.; Chandler, S.H. Resurgent Na+ current offers noise modulation in bursting neurons. PLOS Comput. Biol. 2019, 15, e1007154. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.Q.; Wei, Q.; Wu, Z.Y. Sensory nerve disturbance in amyotrophic lateral sclerosis. Life Sci. 2018, 203, 242–245. [Google Scholar] [CrossRef] [PubMed]
- McCombe, P.A.; Wray, N.R.; Henderson, R.D. Extra-motor abnormalities in amyotrophic lateral sclerosis: Another layer of heterogeneity. Expert Rev. Neurother. 2017, 17, 561–577. [Google Scholar] [CrossRef] [PubMed]




| WT | mSOD1 | |
|---|---|---|
| mouse | C57BL/6JJmsSLc | B6SJL-Tg (SOD1G93A)1Gur/J |
| Behavioral physiological Experiments | 10 | 9 |
| Electrophysiological Experiments | 5 | 5 |
| Total | 15 | 14 |
| WT (n = 9) | mSOD1 (n = 15) | |
|---|---|---|
| RMP, mV | −52.5 ± 4.5 | −84.8 ± 5.7 * |
| Rin, MΩ | 26.2 ± 5.8 | 21.3 ± 6.0 |
| Cm, pF | 82.1 ± 9.4 | 105.4 ± 9.6 |
| WT (n = 9) | mSOD1 (n = 15) | |
|---|---|---|
| AP | ||
| Spike height, mV | 79.1 ± 3.1 | 72.6 ± 2.4 |
| Slope, mV/ms | −0.017 ± 0.006 | −0.014 ± 0.007 |
| Half width, ms | 0.26 ± 0.04 | 0.24 ± 0.08 |
| AHP | ||
| AHP peak, mV | −8.8 ± 1.3 | 8.3 ± 0.5 |
| duration, s | 0.03 ± 0.01 | 0.04 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitaoka, Y.; Seki, S.; Kawata, S.; Nishiura, A.; Kawamura, K.; Hiraoka, S.-i.; Kogo, M.; Tanaka, S. Analysis of Feeding Behavior Characteristics in the Cu/Zn Superoxide Dismutase 1 (SOD1) SOD1G93A Mice Model for Amyotrophic Lateral Sclerosis (ALS). Nutrients 2023, 15, 1651. https://doi.org/10.3390/nu15071651
Kitaoka Y, Seki S, Kawata S, Nishiura A, Kawamura K, Hiraoka S-i, Kogo M, Tanaka S. Analysis of Feeding Behavior Characteristics in the Cu/Zn Superoxide Dismutase 1 (SOD1) SOD1G93A Mice Model for Amyotrophic Lateral Sclerosis (ALS). Nutrients. 2023; 15(7):1651. https://doi.org/10.3390/nu15071651
Chicago/Turabian StyleKitaoka, Yoshihiro, Soju Seki, Sou Kawata, Akira Nishiura, Kohei Kawamura, Shin-ichiro Hiraoka, Mikihiko Kogo, and Susumu Tanaka. 2023. "Analysis of Feeding Behavior Characteristics in the Cu/Zn Superoxide Dismutase 1 (SOD1) SOD1G93A Mice Model for Amyotrophic Lateral Sclerosis (ALS)" Nutrients 15, no. 7: 1651. https://doi.org/10.3390/nu15071651
APA StyleKitaoka, Y., Seki, S., Kawata, S., Nishiura, A., Kawamura, K., Hiraoka, S.-i., Kogo, M., & Tanaka, S. (2023). Analysis of Feeding Behavior Characteristics in the Cu/Zn Superoxide Dismutase 1 (SOD1) SOD1G93A Mice Model for Amyotrophic Lateral Sclerosis (ALS). Nutrients, 15(7), 1651. https://doi.org/10.3390/nu15071651






