Consumption of Solnul™ Resistant Potato Starch Produces a Prebiotic Effect in a Randomized, Placebo-Controlled Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Investigational Product
2.2. Study Design and Participant Selection
2.3. Safety
2.4. Microbiome Analysis
2.5. Self-Reported Bristol Stool Chart Scoring
2.6. Statistical Analysis
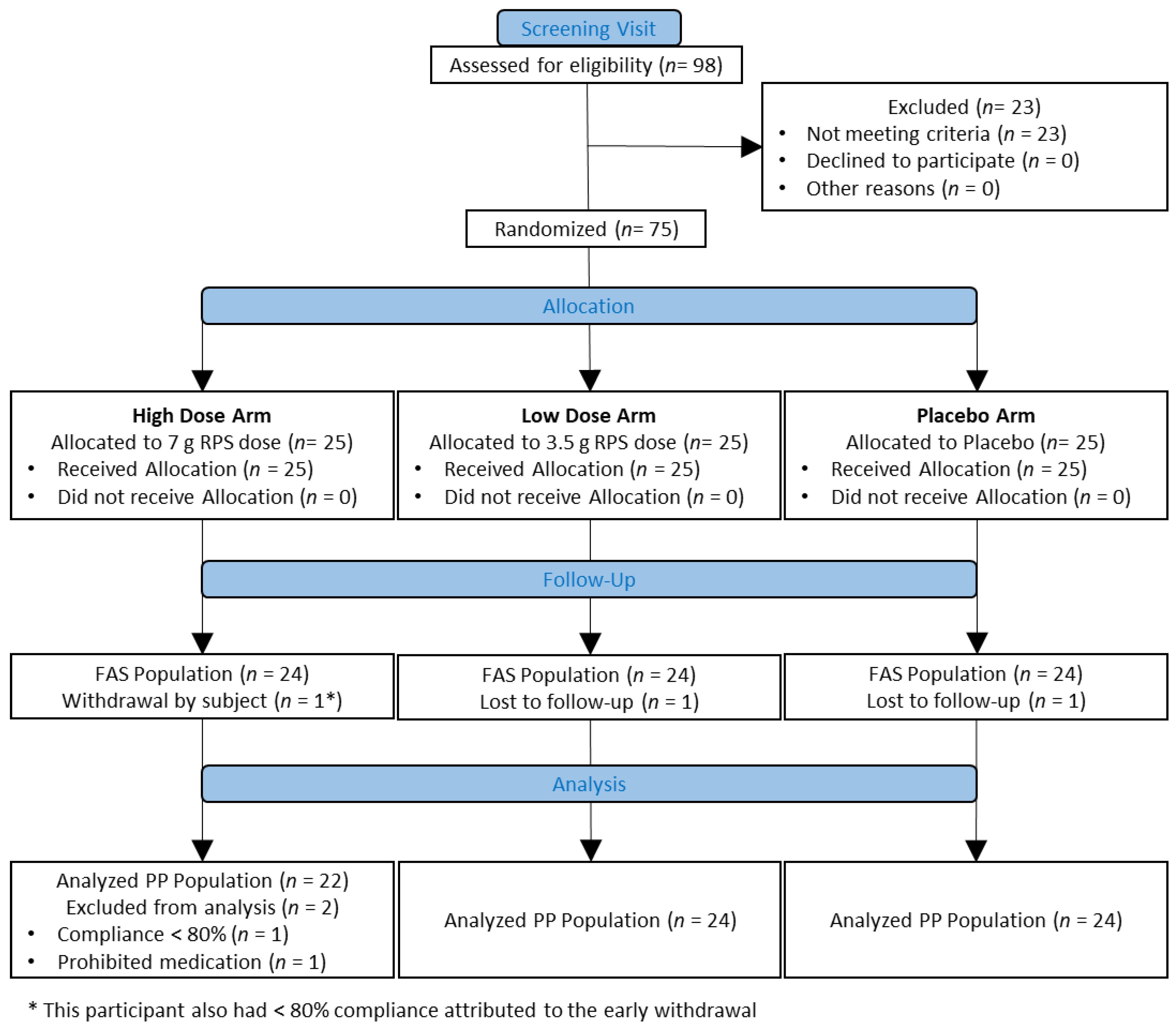
3. Results
3.1. Participant Characteristics
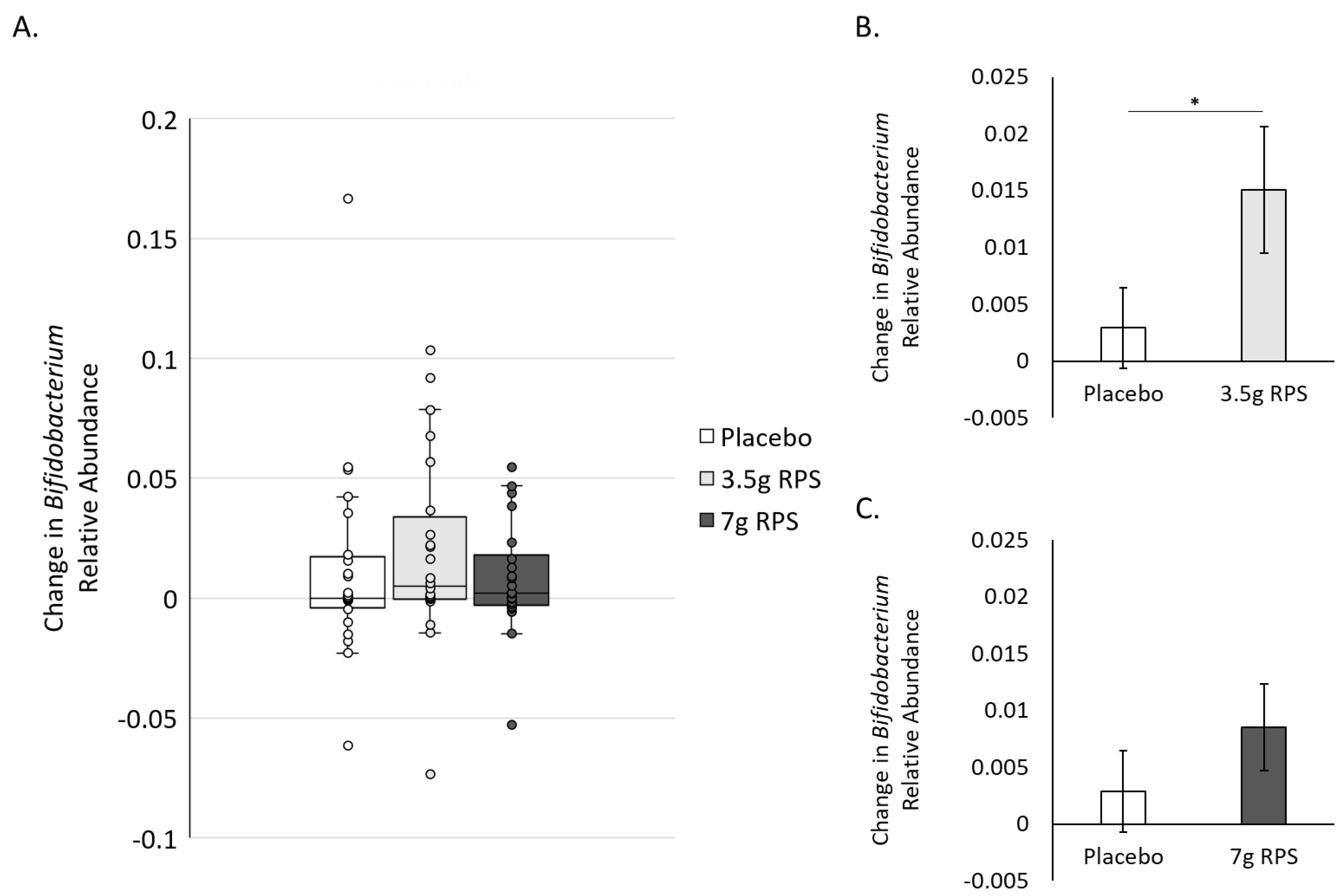
3.2. RPS Supplementation Increases Relative Abundance of Bifidobacterium
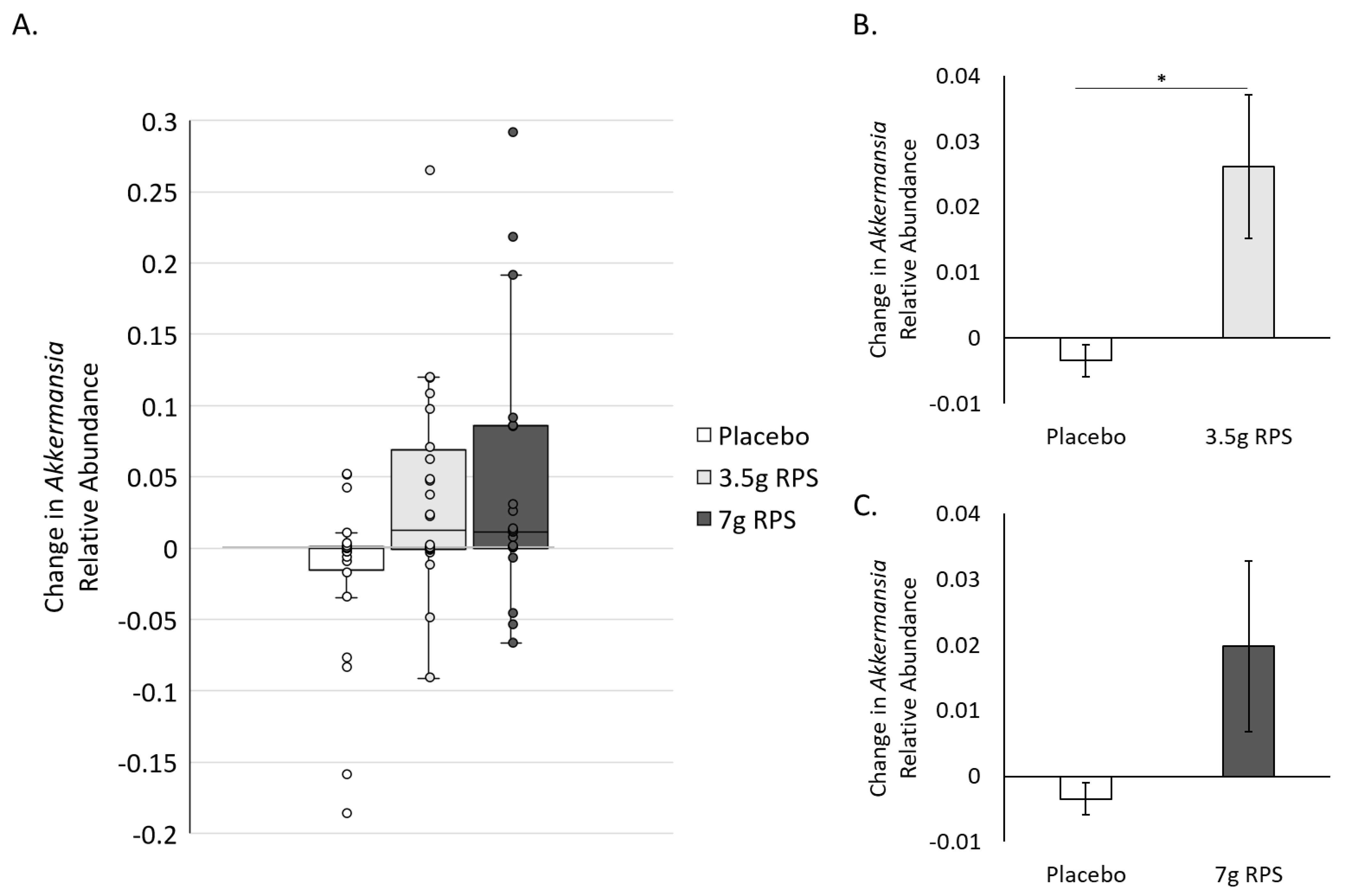
3.3. RPS Supplementation Increases Relative Abundance of Akkermansia
3.4. RPS Supplementation Improves Constipation- and Diarrhea-Associated Bowel Movements
3.5. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Englyst, H.N.; Kingman, S.M.; Hudson, G.J.; Cummings, J.H. Measurement of resistant starch in vitro and in vivo. Br. J. Nutr. 1996, 75, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Sajilata, M.; Singhal, R.S.; Kulkarni, P.R. Resistant Starch? A Review. Compr. Rev. Food Sci. Food Saf. 2006, 5, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Patterson, M.A.; Maiya, M.; Stewart, M.L. Resistant Starch Content in Foods Commonly Consumed in the United States: A Narrative Review. J. Acad. Nutr. Diet. 2020, 120, 230–244. [Google Scholar] [CrossRef]
- Deehan, E.C.; Yang, C.; Perez-Muñoz, M.E.; Nguyen, N.K.; Cheng, C.C.; Triador, L.; Zhang, Z.; Bakal, J.A.; Walter, J. Precision Microbiome Modulation with Discrete Dietary Fiber Structures Directs Short-Chain Fatty Acid Production. Cell Host Microbe 2020, 27, 389–404.e6. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.H.; Englyst, H.N. Measurement of starch fermentation in the human large intestine. Can. J. Physiol. Pharmacol. 1991, 69, 121–129. [Google Scholar] [CrossRef]
- Landon, S.; Colyer, C.G.B.; Salman, H. The Resistant Starch Report. Food Australia Supplement. 2012. Available online: https://www.ingredion.com/content/dam/ingredion/pdf-downloads/apac/APAC_2013_Hi-maize_The_Resistant_Starch_Report.pdf (accessed on 27 February 2023).
- Bird, A.R.; Conlon, M.A.; Christophersen, C.T.; Topping, D.L. Resistant starch, large bowel fermentation and a broader perspective of prebiotics and probiotics. Benef. Microbes 2010, 1, 423–431. [Google Scholar] [CrossRef]
- Miketinas, D.C.; Shankar, K.; Maiya, M.; Patterson, M.A. Usual Dietary Intake of Resistant Starch in US Adults from NHANES 2015–2016. J. Nutr. 2020, 150, 2738–2747. [Google Scholar] [CrossRef]
- Baghurst, P.A.; Baghurst, K.I.; Record, S.J. Dietary fibre, non-starch polysaccharides and resistant starch: A review. Food Aust. 1996, 48, S3–S35. [Google Scholar]
- Cummings, J.H.; Beatty, E.R.; Kingman, S.M.; Bingham, S.A.; Englyst, H.N. Digestion and physiological properties of resistant starch in the human large bowel. Br. J. Nutr. 1996, 75, 733–747. [Google Scholar] [CrossRef]
- Maki, K.C.; Sanders, L.; Reeves, M.S.; Kaden, V.N.; Rains, T.M.; Cartwright, Y. Beneficial effects of resistant starch on laxation in healthy adults. Int. J. Food Sci. Nutr. 2009, 60, 296–305. [Google Scholar] [CrossRef]
- Khosroshahi, H.T.; Vaziri, N.D.; Abedi, B.; Asl, B.H.; Ghojazadeh, M.; Jing, W.; Vatankhah, A.M. Effect of high amylose resistant starch (HAM-RS2) supplementation on biomarkers of inflammation and oxidative stress in hemodialysis patients: A randomized clinical trial. Hemodial. Int. 2018, 22, 492–500. [Google Scholar] [CrossRef]
- Cassettari, V.M.G.; Machado, N.C.; Lourenção, P.L.T.D.A.; Carvalho, M.A.; Ortolan, E.V.P. Combinations of laxatives and green banana biomass on the treatment of functional constipation in children and adolescents: A randomized study. J. Pediatr. (Rio J.) 2019, 95, 27–33. [Google Scholar] [CrossRef]
- Alfa, M.J.; Strang, D.; Tappia, P.S.; Olson, N.; DeGagne, P.; Bray, D.; Murray, B.-L.; Hiebert, B. A Randomized Placebo Controlled Clinical Trial to Determine the Impact of Digestion Resistant Starch MSPrebiotic® on Glucose, Insulin, and Insulin Resistance in Elderly and Mid-Age Adults. Front. Med. 2018, 4, 260. [Google Scholar] [CrossRef]
- Bodinham, C.L.; Smith, L.; Wright, J.; Frost, G.S.; Robertson, M.D. Dietary Fibre Improves First-phase Insulin Secretion in Overweight Individuals. PLoS ONE 2012, 7, e40834. [Google Scholar] [CrossRef] [PubMed]
- Gargari, B.P.; Namazi, N.; Khalili, M.; Sarmadi, B.; Jafarabadi, M.A.; Dehghan, P. Is there any place for resistant starch, as alimentary prebiotic, for patients with type 2 diabetes? Complement. Ther. Med. 2015, 23, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Karimi, P.; Farhangi, M.A.; Sarmadi, B.; Gargari, B.P.; Javid, A.Z.; Pouraghaei, M.; Dehghan, P. The Therapeutic Potential of Resistant Starch in Modulation of Insulin Resistance, Endotoxemia, Oxidative Stress and Antioxidant Biomarkers in Women with Type 2 Diabetes: A Randomized Controlled Clinical Trial. Ann. Nutr. Metab. 2015, 68, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.M.; Beyl, R.A.; Marlatt, K.L.; Martin, C.K.; Aryana, K.J.; Marco, M.L.; Martin, R.J.; Keenan, M.J.; Ravussin, E. Effect of 12 wk of resistant starch supplementation on cardiometabolic risk factors in adults with prediabetes: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 492–501. [Google Scholar] [CrossRef]
- Robertson, M.D.; Bickerton, A.S.; Dennis, A.L.; Vidal, H.; Frayn, K.N. Insulin-sensitizing effects of dietary resistant starch and effects on skeletal muscle and adipose tissue metabolism. Am. J. Clin. Nutr. 2005, 82, 559–567. [Google Scholar] [CrossRef]
- Maki, K.C.; Pelkman, C.L.; Finocchiaro, E.T.; Kelley, K.M.; Lawless, A.L.; Schild, A.L.; Rains, T.M. Resistant Starch from High-Amylose Maize Increases Insulin Sensitivity in Overweight and Obese Men. J. Nutr. 2012, 142, 717–723. [Google Scholar] [CrossRef]
- Johnston, K.L.; Thomas, E.; Bell, J.D.; Frost, G.S.; Robertson, M.D. Resistant starch improves insulin sensitivity in metabolic syndrome. Diabet. Med. 2010, 27, 391–397. [Google Scholar] [CrossRef]
- Dainty, S.A.; Klingel, S.L.; E Pilkey, S.; McDonald, E.; McKeown, B.; Emes, M.J.; Duncan, A.M. Resistant Starch Bagels Reduce Fasting and Postprandial Insulin in Adults at Risk of Type 2 Diabetes. J. Nutr. 2016, 146, 2252–2259. [Google Scholar] [CrossRef]
- Gower, B.A.; Bergman, R.; Stefanovski, D.; Darnell, B.; Ovalle, F.; Fisher, G.; Sweatt, S.K.; Resuehr, H.S.; Pelkman, C. Baseline insulin sensitivity affects response to high-amylose maize resistant starch in women: A randomized, controlled trial. Nutr. Metab. 2016, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Sardá, F.A.H.; Giuntini, E.B.; Gomez, M.L.P.; Lui, M.C.Y.; Negrini, J.A.; Tadini, C.C.; Lajolo, F.M.; Menezes, E.W. Impact of resistant starch from unripe banana flour on hunger, satiety, and glucose homeostasis in healthy volunteers. J. Funct. Foods 2016, 24, 63–74. [Google Scholar] [CrossRef]
- Menezes, E.W.; Dan, M.C.T.; Cardenette, G.H.L.; Goñi, I.; Bello-Pérez, L.A.; Lajolo, F.M. In Vitro Colonic Fermentation and Glycemic Response of Different Kinds of Unripe Banana Flour. Plant Foods Hum. Nutr. 2010, 65, 379–385. [Google Scholar] [CrossRef]
- Blé-Castillo, J.L.; Aparicio-Trápala, M.A.; Francisco-Luria, M.U.; Córdova-Uscanga, R.; Rodríguez-Hernández, A.; Méndez, J.D.; Díaz-Zagoya, J.C. Effects of Native Banana Starch Supplementation on Body Weight and Insulin Sensitivity in Obese Type 2 Diabetics. Int. J. Environ. Res. Public Health 2010, 7, 1953–1962. [Google Scholar] [CrossRef]
- Drake, A.M.; Coughlan, M.T.; Christophersen, C.T.; Snelson, M. Resistant Starch as a Dietary Intervention to Limit the Progression of Diabetic Kidney Disease. Nutrients 2022, 14, 4547. [Google Scholar] [CrossRef] [PubMed]
- Shamloo, M.; Mollard, R.; Wang, H.; Kingra, K.; Tangri, N.; MacKay, D. A randomized double-blind cross-over trial to study the effects of resistant starch prebiotic in chronic kidney disease (ReSPECKD). Trials 2022, 23, 72. [Google Scholar] [CrossRef]
- Baxter, N.T.; Schmidt, A.W.; Venkataraman, A.; Kim, K.S.; Waldron, C.; Schmidt, T.M. Dynamics of Human Gut Microbiota and Short-Chain Fatty Acids in Response to Dietary Interventions with Three Fermentable Fibers. Mbio 2019, 10, e02566-18. [Google Scholar] [CrossRef]
- Alfa, M.J.; Strang, D.; Tappia, P.S.; Graham, M.; Van Domselaar, G.; Forbes, J.D.; Laminman, V.; Olson, N.; DeGagne, P.; Bray, D.; et al. A randomized trial to determine the impact of a digestion resistant starch composition on the gut microbiome in older and mid-age adults. Clin. Nutr. 2017, 37, 797–807. [Google Scholar] [CrossRef]
- Kemp, J.A.; Dos Santos, H.F.; de Jesus, H.E.; Esgalhado, M.; de Paiva, B.R.; Azevedo, R.; Stenvinkel, P.; Bergman, P.; Lindholm, B.; Ribeiro-Alves, M.; et al. Resistant Starch Type-2 Supplementation Does Not Decrease Trimethylamine N-Oxide (TMAO) Plasma Level in Hemodialysis Patients. J. Am. Nutr. Assoc. 2022, 41, 788–795. [Google Scholar] [CrossRef]
- Fong, J.V.N.; Miketinas, D.; Moore, L.W.; Nguyen, D.T.; Graviss, E.A.; Ajami, N.; Patterson, M.A. Precision Nutrition Model Predicts Glucose Control of Overweight Females Following the Consumption of Potatoes High in Resistant Starch. Nutrients 2022, 14, 268. [Google Scholar] [CrossRef] [PubMed]
- Laffin, M.R.; Tayebi Khosroshahi, H.; Park, H.; Laffin, L.J.; Madsen, K.; Kafil, H.S.; Abedi, B.; Shiralizadeh, S.; Vaziri, N.D. Amylose resistant starch (HAM-RS2) supplementation increases the proportion of Faecalibacterium bacteria in end-stage renal disease patients: Microbial analysis from a randomized placebo-controlled trial. Hemodial. Int. 2019, 23, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Venkataraman, A.; Sieber, J.R.; Schmidt, A.W.; Waldron, C.; Theis, K.R.; Schmidt, T.M. Variable responses of human microbiomes to dietary supplementation with resistant starch. Microbiome 2016, 4, 33. [Google Scholar] [CrossRef]
- Hanes, D.; Nowinski, B.; Lamb, J.J.; Larson, I.A.; McDonald, D.; Knight, R.; Song, S.J.; Patno, N. The gastrointestinal and microbiome impact of a resistant starch blend from potato, banana, and apple fibers: A randomized clinical trial using smart caps. Front. Nutr. 2022, 9, 987216. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.J.; Heaton, K.W. Stool Form Scale as a Useful Guide to Intestinal Transit Time. Scand. J. Gastroenterol. 1997, 32, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Tukey, J. Exploratory Data Analysis, 1st ed.; Addison-Wesley Pub Co.: Reading, MA, USA, 1977; pp. 29–38. [Google Scholar]
- Timmer, C.; Davids, M.; Nieuwdorp, M.; Levels, J.; Langendonk, J.; Breederveld, M.; Mozafari, N.A.; Langeveld, M. Differences in faecal microbiome composition between adult patients with UCD and PKU and healthy control subjects. Mol. Genet. Metab. Rep. 2021, 29, 100794. [Google Scholar] [CrossRef]
- Caverly, L.J.; Lu, J.; Carmody, L.A.; Kalikin, L.M.; Shedden, K.; Opron, K.; Azar, M.; Cahalan, S.; Foster, B.; VanDevanter, D.R.; et al. Measures of Cystic Fibrosis Airway Microbiota during Periods of Clinical Stability. Ann. Am. Thorac. Soc. 2019, 16, 1534–1542. [Google Scholar] [CrossRef]
- Castaño-Rodríguez, N.; Underwood, A.; Merif, J.; Riordan, S.M.; Rawlinson, W.D.; Mitchell, H.M.; Kaakoush, N.O. Gut Microbiome Analysis Identifies Potential Etiological Factors in Acute Gastroenteritis. Infect. Immun. 2018, 86, e00060-18. [Google Scholar] [CrossRef]
- DeMartino, P.; Cockburn, D.W. Resistant starch: Impact on the gut microbiome and health. Curr. Opin. Biotechnol. 2019, 61, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Toscano, M.; De Grandi, R.; Stronati, L.; De Vecchi, E.; Drago, L. Effect of Lactobacillus rhamnosus HN001 and Bifidobacterium longum BB536 on the healthy gut microbiota composition at phyla and species level: A preliminary study. World J. Gastroenterol. 2017, 23, 2696–2704. [Google Scholar] [CrossRef]
- Hibberd, A.; Yde, C.; Ziegler, M.; Honoré, A.; Saarinen, M.; Lahtinen, S.; Stahl, B.; Jensen, H.; Stenman, L. Probiotic or synbiotic alters the gut microbiota and metabolism in a randomised controlled trial of weight management in overweight adults. Benef. Microbes 2019, 10, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.J.; Patno, N. Short-Term Tolerability, Safety, and Gut Microbial Composition Responses to a Multi-Strain Probiotic Supplement: An Open-Label Study in Healthy Adults. Integr. Med. 2021, 20, 24–34. [Google Scholar]
- Dizman, N.; Hsu, J.; Bergerot, P.G.; Gillece, J.D.; Folkerts, M.; Reining, L.; Trent, J.; Highlander, S.K.; Pal, S.K. Randomized trial assessing impact of probiotic supplementation on gut microbiome and clinical outcome from targeted therapy in metastatic renal cell carcinoma. Cancer Med. 2020, 10, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-H.; Yen, H.-R.; Lu, W.-L.; Ho, H.-H.; Lin, W.-Y.; Kuo, Y.-W.; Huang, Y.-Y.; Tsai, S.-Y.; Lin, H.-C. Adjuvant Probiotics of Lactobacillus salivarius subsp. salicinius AP-32, L. johnsonii MH-68, and Bifidobacterium animalis subsp. lactis CP-9 Attenuate Glycemic Levels and Inflammatory Cytokines in Patients with Type 1 Diabetes Mellitus. Front. Endocrinol. 2022, 13, 754401. [Google Scholar] [CrossRef]
- Maier, T.V.; Lucio, M.; Lee, L.H.; VerBerkmoes, N.C.; Brislawn, C.J.; Bernhardt, J.; Lamendella, R.; McDermott, J.E.; Bergeron, N.; Heinzmann, S.S.; et al. Impact of Dietary Resistant Starch on the Human Gut Microbiome, Metaproteome, and Metabolome. mBio 2017, 8, e01343-17. [Google Scholar] [CrossRef]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2016, 23, 107–113. [Google Scholar] [CrossRef]
- Cani, P.D.; Depommier, C.; Derrien, M.; Everard, A.; de Vos, W.M. Akkermansia muciniphila: Paradigm for next-generation beneficial microorganisms. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 625–637. [Google Scholar] [CrossRef]
- Costa, E.S.; França, C.N.; Fonseca, F.A.H.; Kato, J.T.; Bianco, H.T.; Freitas, T.T.; Fonseca, H.A.R.; Neto, A.M.F.; Izar, M.C. Beneficial effects of green banana biomass consumption in patients with pre-diabetes and type 2 diabetes: A randomised controlled trial. Br. J. Nutr. 2019, 121, 1365–1375. [Google Scholar] [CrossRef]
- Johnson, A.J.; Vangay, P.; Al-Ghalith, G.A.; Hillmann, B.M.; Ward, T.L.; Shields-Cutler, R.R.; Kim, A.D.; Shmagel, A.K.; Syed, A.N.; Walter, J.; et al. Daily Sampling Reveals Personalized Diet-Microbiome Associations in Humans. Cell Host Microbe 2019, 25, 789–802.e5. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; Vaughan, E.E.; Plugge, C.M.; de Vos, W.M. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int. J. Syst. Evol. Microbiol. 2004, 54, 1469–1476. [Google Scholar] [CrossRef]
- Biruete, A.; Cross, T.-W.L.; Allen, J.M.; Kistler, B.M.; de Loor, H.; Evenepoel, P.; Fahey, G.C.; Bauer, L.; Swanson, K.S.; Wilund, K.R. Effect of Dietary Inulin Supplementation on the Gut Microbiota Composition and Derived Metabolites of Individuals Undergoing Hemodialysis: A Pilot Study. J. Ren. Nutr. 2021, 31, 512–522. [Google Scholar] [CrossRef]
- Karakan, T.; Tuohy, K.M.; Solingen, G.J.-V. Low-Dose Lactulose as a Prebiotic for Improved Gut Health and Enhanced Mineral Absorption. Front. Nutr. 2021, 8, 672925. [Google Scholar] [CrossRef]
- McRorie, J.W., Jr.; McKeown, N.M. Understanding the Physics of Functional Fibers in the Gastrointestinal Tract: An Evidence-Based Approach to Resolving Enduring Misconceptions about Insoluble and Soluble Fiber. J. Acad. Nutr. Diet. 2017, 117, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Oka, P.; Parr, H.; Barberio, B.; Black, C.J.; Savarino, E.V.; Ford, A.C. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 908–917. [Google Scholar] [CrossRef]
- Reimer, R.A.; Soto-Vaca, A.; Nicolucci, A.C.; Mayengbam, S.; Park, H.; Madsen, K.L.; Menon, R.; Vaughan, E.E. Effect of chicory inulin-type fructan–containing snack bars on the human gut microbiota in low dietary fiber consumers in a randomized crossover trial. Am. J. Clin. Nutr. 2020, 111, 1286–1296. [Google Scholar] [CrossRef] [PubMed]
| 7 g RPS Dose | 3.5 g RPS Dose | Placebo | Total | |
|---|---|---|---|---|
| Number of Participants | 24 | 24 | 24 | 72 |
| Age (Year) # | ||||
| Mean (SD) | 36.6 (15.85) | 38.5 (14.63) | 39.1 (16.50) | 38.1 (15.49) |
| Median | 27.5 | 41.5 | 37.5 | 35.0 |
| Min, Max | 19, 64 | 20, 66 | 19, 66 | 19, 66 |
| Sex | ||||
| Male | 9 (37.5%) | 6 (25.0%) | 7 (29.2%) | 22 (30.6%) |
| Female | 15 (62.5%) | 18 (75.0%) | 17 (70.8%) | 50 (69.4%) |
| Race | ||||
| Indigenous | 0 (0.0%) | 0 (0.0%) | 1 (4.2%) | 1 (1.4%) |
| Asian | 3 (12.5%) | 3 (12.5%) | 0 (0.0%) | 6 (8.3%) |
| Black or African Canadian | 1 (4.2%) | 0 (0.0%) | 0 (0.0%) | 1 (1.4%) |
| White | 19 (79.2%) | 21 (87.5%) | 23 (95.8%) | 63 (87.5%) |
| Other | 1 (4.2%) | 0 (0.0%) | 0 (0.0%) | 1 (1.4%) |
| Ethnicity | ||||
| Hispanic or Latino | 0 (0.0%) | 0 (0.0%) | 2 (8.3%) | 2 (2.8%) |
| Not Hispanic or Latino | 24 (100%) | 24 (100%) | 22 (91.7%) | 70 (97.2%) |
| Height (cm) | ||||
| Mean (SD) | 168.73 (8.919) | 165.60 (7.351) | 167.87 (9.682) | 167.40 (8.684) |
| Median | 167.05 | 165.10 | 166.25 | 166.20 |
| Min, Max | 151.5, 186.9 | 153.5, 186.5 | 151.3, 184.5 | 151.3, 186.9 |
| Weight (kg) | ||||
| Mean (SD) | 69.12 (13.836) | 73.40 (12.281) | 71.20 (13.811) | 71.24 (13.257) |
| Median | 65.20 | 74.60 | 71.00 | 71.40 |
| Min, Max | 47.4, 102.8 | 54.1, 98.2 | 47.0, 105.8 | 47.0, 105.8 |
| BMI (kg/m2) | ||||
| Mean (SD) | 24.10 (3.420) | 26.78 (4.291) | 25.14 (3.735) | 25.34 (3.938) |
| Median | 23.25 | 26.90 | 24.30 | 24.80 |
| Min, Max | 18.8, 31.8 | 19.6, 33.8 | 19.8, 33.3 | 18.8, 33.8 |
| Name | Events | Day | |
|---|---|---|---|
| Visit 1 | Screening | Inclusion/exclusion criteria reviewed, informed consent obtained, medical history and exam, vital signs and blood safety sample taken, fecal collection kits and diaries provided | −30 to −14 days |
| Visit 2 | Baseline | Inclusion/exclusion criteria, dietary supplements and medications, and medical history reviewed, pregnancy test (females only), fecal sample and diaries collected, randomization | Day 0 |
| Visit 3 | Week 1 | Fecal sample and diaries collected, unused study products collected, compliance calculated, and product re-dispensed, review medication and dietary supplement history, new fecal collection kits and diaries dispensed | ~Day 8 (+2 day window) |
| Visit 4 | Week 4 | Fecal sample and diaries collected, unused study products collected and compliance calculated, review medication and dietary supplement history | ~Day 29 (+3 day window) |
| Type 1 | Type 2 | Type 3 | Type 4 | Type 5 | Type 6 | Type 7 | Total | |
|---|---|---|---|---|---|---|---|---|
| Placebo | 12 | 34 | 50 | 190 | 92 | 32 | 15 | 429 |
| 3.5 g RPS | 9 | 27 | 100 | 209 | 53 | 46 | 11 | 455 |
| 7 g RPS | 34 | 49 | 50 | 136 | 109 | 40 | 5 | 423 |
| Type 1 | Not Type 1 | p Value | |
|---|---|---|---|
| Placebo | 3 | 165 | 0.99 |
| 3.5 g RPS | 2 | 166 | |
| Type 1 or 2 | Not Type 1 or 2 | ||
| Placebo | 23 | 145 | 0.0085 |
| 3.5 g RPS | 9 | 159 | |
| Type 7 | Not Type 7 | ||
| Placebo | 6 | 162 | 0.03 |
| 3.5 g RPS | 0 | 168 | |
| Type 6 or 7 | Not Type 6 or 7 | ||
| Placebo | 13 | 155 | 0.35 |
| 3.5 g RPS | 19 | 149 | |
| Type 5, 6 or 7 | Not Type 5, 6 or 7 | ||
| Placebo | 49 | 119 | 0.26 |
| 3.5 g RPS | 39 | 129 |
| Type 1 | Not Type 1 | p Value | |
|---|---|---|---|
| Placebo | 3 | 165 | 0.13 |
| 7 g RPS | 8 | 146 | |
| Type 1 or 2 | Not Type 1 or 2 | ||
| Placebo | 23 | 145 | 0.44 |
| 7 g RPS | 26 | 128 | |
| Type 7 | Not Type 7 | ||
| Placebo | 6 | 162 | 0.51 |
| 7 g RPS | 3 | 151 | |
| Type 6 or 7 | Not Type 6 or 7 | ||
| Placebo | 13 | 155 | 0.26 |
| 7 g RPS | 7 | 147 | |
| Type 5, 6 or 7 | Not Type 5, 6 or 7 | ||
| Placebo | 49 | 119 | 0.026 |
| 7 g RPS | 28 | 126 |
| 7 g RPS Dose | 3.5 g RPS Dose | Placebo | Total | |||||
|---|---|---|---|---|---|---|---|---|
| Participants (n = 25) | Events (n = 14) | Participants (n = 25) | Events (n = 22) | Participants (n = 25) | Events (n = 20) | Participants (n = 72) | Events (n = 56) | |
| Overall | 10 (40.0%) | 14 (100%) | 11 (44.0%) | 22 (100%) | 12 (50.0%) | 20 (100%) | 33 (44.0%) | 56 (100%) |
| Serious | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fatal | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Discontinuation | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Severity | ||||||||
| Mild | 9 (36.0%) | 12 (85.7%) | 6 (24.0%) | 9 (40.9%) | 10 (40.0%) | 15 (75.0%) | 25 (33.3%) | 36 (64.3%) |
| Moderate | 1 (4.0%) | 1 (7.1%) | 8 (32.0%) | 12 (54.5%) | 4 (16.0%) | 5 (25.0%) | 13 (17.3%) | 18 (32.1%) |
| Severe | 1 (4.0%) | 1 (7.1%) | 1 (4.0%) | 1 (4.5%) | 0 | 0 | 2 (2.7%) | 2 (3.6%) |
| Relationship | ||||||||
| Related | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Possibly related | 2 (8.0%) | 2 (14.3%) | 7 (28.0%) | 10 (45.5%) | 2 (8.0%) | 5 (25.0%) | 11 (14.7%) | 17 (30.4%) |
| Not related | 9 (36.0%) | 12 (85.7%) | 7 (28.0%) | 12 (54.5%) | 12 (48.0%) | 15 (75.0%) | 28 (37.3%) | 39 (69.6%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bush, J.R.; Baisley, J.; Harding, S.V.; Alfa, M.J. Consumption of Solnul™ Resistant Potato Starch Produces a Prebiotic Effect in a Randomized, Placebo-Controlled Clinical Trial. Nutrients 2023, 15, 1582. https://doi.org/10.3390/nu15071582
Bush JR, Baisley J, Harding SV, Alfa MJ. Consumption of Solnul™ Resistant Potato Starch Produces a Prebiotic Effect in a Randomized, Placebo-Controlled Clinical Trial. Nutrients. 2023; 15(7):1582. https://doi.org/10.3390/nu15071582
Chicago/Turabian StyleBush, Jason R., Joshua Baisley, Scott V. Harding, and Michelle J. Alfa. 2023. "Consumption of Solnul™ Resistant Potato Starch Produces a Prebiotic Effect in a Randomized, Placebo-Controlled Clinical Trial" Nutrients 15, no. 7: 1582. https://doi.org/10.3390/nu15071582
APA StyleBush, J. R., Baisley, J., Harding, S. V., & Alfa, M. J. (2023). Consumption of Solnul™ Resistant Potato Starch Produces a Prebiotic Effect in a Randomized, Placebo-Controlled Clinical Trial. Nutrients, 15(7), 1582. https://doi.org/10.3390/nu15071582







