Supplementation with Flaxseed Oil Rich in Alpha-Linolenic Acid Improves Verbal Fluency in Healthy Older Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Inclusion and Exclusion Criteria
2.3.1. Eligibility Criteria
- MMSE score of 27 and higher.
- CDR score of 0.
- Age: 65 or older, 80 or younger (at the time of registration).
- Right-handedness.
2.3.2. Exclusion Criteria
- Participants with a history of severe cranial nerve disease or internal diseases.
- Participants with severe visual or hearing impairment.
- Extremely obese/thin (BMI less than 17 kg/m2 and more than 30 kg/m2).
- Participants who have continuously consumed foods containing horse-mackerel type of fish.
- Frequent fish consumers (3 or more servings/week as a staple food).
- Participants with psychosis or psychiatric symptoms that make it difficult for them to participate in the study.
- Other subjects the principal investigator considered difficult to participate in the study.
2.4. Randomization
2.5. Experimental Food and Study Settings
2.6. Subject Background Information Collections
2.7. Assessment of Cognitive Function
2.7.1. Mini-Mental State Examination
2.7.2. Montreal Cognitive Assessment
2.7.3. Frontal Assessment Battery at Bedside
2.7.4. Stroop Test
2.7.5. Digit Cancellation Task
2.7.6. Wechsler Adult Intelligence Scale
Coding
Symbol Search
Digit Span
Arithmetic
Block Design
Matrix Reasoning
Visual Puzzles
2.8. Wechsler Memory Scale-Revised
2.9. Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ministry of Health. Future Aging of the Population. Available online: https://www.mhlw.go.jp/file/04-Houdouhappyou-12304500-Roukenkyoku-Ninchishougyakutaiboushitaisakusuishinshitsu/02_1.pdf (accessed on 14 March 2023).
- Asfia, S.; Bucholc, J.; McCaffrey, N.; Mihalopoulos, C.; Muldowney, A.; Engel, L. Understanding the Quality of Life Impacts of Providing Informal Care to People with Dementia: A Systematic Review of Qualitative Studies. J. Alzheimers Dis. 2022, 88, 1293–1309. [Google Scholar] [CrossRef] [PubMed]
- Isham, L.; Hewison, A.; Bradbury-Jones, C. When Older People Are Violent or Abusive Toward Their Family Caregiver: A Review of Mixed-Methods Research. Trauma Violence Abuse 2019, 20, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.; Livingston, G. Mental health/psychiatric issues in elder abuse and neglect. Clin. Geriatr. Med. 2014, 30, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Samieri, C.; Morris, M.C.; Bennett, D.A.; Berr, C.; Amouyel, P.; Dartigues, J.F.; Tzourio, C.; Chasman, D.I.; Grodstein, F. Fish Intake, Genetic Predisposition to Alzheimer Disease, and Decline in Global Cognition and Memory in 5 Cohorts of Older Persons. Am. J. Epidemiol. 2018, 187, 933–940. [Google Scholar] [CrossRef]
- Ylilauri, M.P.T.; Hantunen, S.; Lönnroos, E.; Salonen, J.T.; Tuomainen, T.P.; Virtanen, J.K. Associations of dairy, meat, and fish intakes with risk of incident dementia and with cognitive performance: The Kuopio Ischaemic Heart Disease Risk Factor Study (KIHD). Eur. J. Nutr. 2022, 61, 2531–2542. [Google Scholar] [CrossRef] [PubMed]
- Boespflug, E.L.; McNamara, R.K.; Eliassen, J.C.; Schidler, M.D.; Krikorian, R. Fish Oil Supplementation Increases Event-Related Posterior Cingulate Activation in Older Adults with Subjective Memory Impairment. J. Nutr. Health Aging 2016, 20, 161–169. [Google Scholar] [CrossRef]
- Brenna, J.T.; Salem, N., Jr.; Sinclair, A.J.; Cunnane, S.C. α-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot. Essent. Fatty Acids 2009, 80, 85–91. [Google Scholar] [CrossRef]
- Blondeau, N.; Lipsky, R.H.; Bourourou, M.; Duncan, M.W.; Gorelick, P.B.; Marini, A.M. Alpha-linolenic acid: An omega-3 fatty acid with neuroprotective properties-ready for use in the stroke clinic? Biomed. Res. Int. 2015, 2015, 519830. [Google Scholar] [CrossRef]
- Hadjighassem, M.; Kamalidehghan, B.; Shekarriz, N.; Baseerat, A.; Molavi, N.; Mehrpour, M.; Joghataei, M.T.; Tondar, M.; Ahmadipour, F.; Meng, G.Y. Oral consumption of α-linolenic acid increases serum BDNF levels in healthy adult humans. Nutr. J. 2015, 14, 20. [Google Scholar] [CrossRef]
- Burdge, G.C.; Jones, A.E.; Wootton, S.A. Eicosapentaenoic and docosapentaenoic acids are the principal products of α-linolenic acid metabolism in young men. Br. J. Nutr. 2002, 88, 355–363. [Google Scholar] [CrossRef]
- Burdge, G.C.; Wootton, S.A. Conversion of alpha-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br. J. Nutr. 2002, 88, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Burdge, G.C. α-linolenic acid interconversion is sufficient as a source of longer chain ω-3 polyunsaturated fatty acids in humans: An opinion. Lipids 2022, 57, 267–287. [Google Scholar] [CrossRef]
- Hashimoto, M.; Matsuzaki, K.; Hossain, S.; Ito, T.; Wakatsuki, H.; Tanabe, Y.; Ohno, M.; Kato, S.; Yamashita, K.; Shido, O. Perilla Seed Oil Enhances Cognitive Function and Mental Health in Healthy Elderly Japanese Individuals by Enhancing the Biological Antioxidant Potential. Foods 2021, 10, 1130. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Chen, M.; Chowdhury, R.; Wu, J.H.; Sun, Q.; Campos, H.; Mozaffarian, D.; Hu, F.B. α-Linolenic acid and risk of cardiovascular disease: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2012, 96, 1262–1273. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health. National Institute of Health and Nutrition (Japan). The National Health and Nutrition Survey in Japan. 2019. Available online: https://www.nibiohn.go.jp/eiken/kenkounippon21/download_files/eiyouchousa/2019.pdf (accessed on 14 March 2023).
- Ministry of Health. Dietary Reference Intakes for Japanese. 2020. Available online: https://www.mhlw.go.jp/content/10900000/000862500.pdf (accessed on 14 March 2023).
- Nicholls, M.E.; Chapman, H.L.; Loetscher, T.; Grimshaw, G.M. The relationship between hand preference, hand performance, and general cognitive ability. J. Int. Neuropsychol. Soc. 2010, 16, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Sinn, N.; Milte, C.M.; Street, S.J.; Buckley, J.D.; Coates, A.M.; Petkov, J.; Howe, P.R. Effects of n-3 fatty acids, EPA v. DHA, on depressive symptoms, quality of life, memory and executive function in older adults with mild cognitive impairment: A 6-month randomised controlled trial. Br. J. Nutr. 2012, 107, 1682–1693. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Takachi, R.; Ishihara, J.; Ishii, Y.; Sasazuki, S.; Sawada, N.; Shinozawa, Y.; Tanaka, J.; Kato, E.; Kitamura, K.; et al. Validity of Short and Long Self-Administered Food Frequency Questionnaires in Ranking Dietary Intake in Middle-Aged and Elderly Japanese in the Japan Public Health Center-Based Prospective Study for the Next Generation (JPHC-NEXT) Protocol Area. J. Epidemiol. 2016, 26, 420–432. [Google Scholar] [CrossRef]
- Garrow, J.S.; Webster, J. Quetelet’s index (W/H2) as a measure of fatness. Int. J. Obes. 1985, 9, 147–153. [Google Scholar]
- Nuttall, F.Q. Body Mass Index: Obesity, BMI, and Health: A Critical Review. Nutr. Today 2015, 50, 117–128. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Crum, R.M.; Anthony, J.C.; Bassett, S.S.; Folstein, M.F. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA 1993, 269, 2386–2391. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Slachevsky, A.; Litvan, I.; Pillon, B. The FAB: A Frontal Assessment Battery at bedside. Neurology 2000, 55, 1621–1626. [Google Scholar] [CrossRef] [PubMed]
- Benton, A.L.; deS Hamsher, K. Multilingual Aphasia Examination: Manual of Lnstructions; AJA Associates: Iowa City, IA, USA, 1989. [Google Scholar]
- Chayer, C.; Freedman, M. Frontal lobe functions. Curr. Neurol. Neurosci. Rep. 2001, 1, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Ito, E. Neuropsychological Studies of Verbal Fluency Tests; Graduate School of Environmental Studies, Nagoya University: Nagoya, Japan, 2006; p. 150. [Google Scholar]
- Ahn, I.S.; Kim, J.H.; Kim, S.; Chung, J.W.; Kim, H.; Kang, H.S.; Kim, D.K. Impairment of instrumental activities of daily living in patients with mild cognitive impairment. Psychiatry Investig. 2009, 6, 180–184. [Google Scholar] [CrossRef]
- Hisano, S. Relationship between frontal assessment battery scores and activities of daily living/instrumental activities of daily living ability in older adults. J. Phys. Ther. Sci. 2018, 30, 1237–1240. [Google Scholar] [CrossRef] [PubMed]
- Ong, M.; Pek, K.; Tan, C.N.; Chew, J.; Lim, J.P.; Yew, S.; Yeo, A.; Lim, W.S. Social Frailty and Executive Function: Association with Geriatric Syndromes, Life Space and Quality of Life in Healthy Community-Dwelling Older Adults. J. Frailty Aging 2022, 11, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Alfano, D.P.; Grummisch, J.A.; Gordon, J.L.; Hadjistavropoulos, T. A Neuropsychological Approach to Mild Cognitive Impairment. Arch. Clin. Neuropsychol. 2022, 37, 873–890. [Google Scholar] [CrossRef]
- Cardoso, C.; Afonso, C.; Bandarra, N.M. Dietary DHA and health: Cognitive function ageing. Nutr. Res. Rev. 2016, 29, 281–294. [Google Scholar] [CrossRef]
- Pereira, A.H.; Gonçalves, A.B.; Holz, M.; Gonçalves, H.A.; Kochhann, R.; Joanette, Y.; Zimmermann, N.; Fonseca, R.P. Influence of age and education on the processing of clustering and switching in verbal fluency tasks. Dement. Neuropsychol. 2018, 12, 360–367. [Google Scholar] [CrossRef]
- Troyer, A.K.; Moscovitch, M.; Winocur, G. Clustering and switching as two components of verbal fluency: Evidence from younger and older healthy adults. Neuropsychology 1997, 11, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Beatty, W.W.; Testa, J.A.; English, S.; Winn, P. Influences of clustering and switching on the verbal fluency performance of patients with alzheimer’s disease. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 1997, 4, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Couëdelo, L.; Buaud, B.; Abrous, H.; Chamekh-Coelho, I.; Majou, D.; Boué-Vaysse, C. Effect of increased levels of dietary α-linolenic acid on the n-3 PUFA bioavailability and oxidative stress in rat. Br. J. Nutr. 2022, 127, 1320–1333. [Google Scholar] [CrossRef]
- Rapoport, S.I.; Rao, J.S.; Igarashi, M. Brain metabolism of nutritionally essential polyunsaturated fatty acids depends on both the diet and the liver. Prostaglandins Leukot. Essent. Fatty Acids 2007, 77, 251–261. [Google Scholar] [CrossRef] [PubMed]
| Cognitive Functions | Task |
|---|---|
| Global cognitive status | Mini-Mental State Examination |
| Montreal Cognitive Assessment | |
| Frontal Assessment Battery at bedside | |
| Executive function | Stroop Test |
| Attention | Digit Cancellation Task |
| Intelligence | Wechsler Adult Intelligence Scale |
| Processing speed | Coding |
| Symbol Search | |
| Working memory | Digit Span |
| Arithmetic | |
| Perceptual reasoning | Block Design |
| Matrix Reasoning | |
| Visual Puzzles | |
| Memory | Wechsler Memory Scale Revised |
| Short-term memory | Verbal Memory: logical memory and verbal-paired associate |
| Visual Memory: figural memory, visual-paired associates and visual reproduction | |
| General Memory: verbal and visual memory | |
| Attention and concentration | Mental control: digit span and visual memory span |
| Control | ALA | ||||
|---|---|---|---|---|---|
| Means | SD | Means | SD | p-Value | |
| Number of subjects | 30 | 30 | |||
| Gender, % female | 53.3 | 46.7 | |||
| Age(years) | 72.1 | 4.3 | 71.9 | 3.7 | 0.411 |
| Height(m) | 1.6 | 0.1 | 1.6 | 0.1 | 0.813 |
| Body weight(kg) | 57.0 | 9.3 | 58.9 | 7.5 | 0.400 |
| BMI(kg/m2) | 22.4 | 2.5 | 23.1 | 2.1 | 0.273 |
| ω-3 PUFA intake(g/day) | |||||
| pre-intervention | 2.16 | 0.72 | 2.35 | 0.87 | 0.354 |
| post-intervention | 2.50 | 0.91 | 2.54 | 1.00 | 0.852 |
| ω-6 PUFA intake(g/day) | |||||
| pre-intervention | 10.80 | 3.15 | 10.34 | 3.33 | 0.587 |
| post-intervention | 11.49 | 3.76 | 11.23 | 4.06 | 0.803 |
| Baseline | Week 6 | Week 12 | Week 6—Baseline | Week 12—Baseline | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | ALA | p-Value | Control | ALA | Control | ALA | Control | ALA | p-Value | Control | ALA | p-Value | |
| MMSE | |||||||||||||
| Total score | 29.27 (0.83) | 28.77 (1.01) | 0.04 | - | − | 28.33 (1.90) | 27.97 (1.97) | − | − | − | −0.93 (2.13) | −0.80 (2.01) | 0.084 |
| MoCA | |||||||||||||
| Total score | 24.47 (1.72) | 23.40 (1.85) | 0.024 | - | − | 26.17 (1.95) | 25.03 (2.53) | − | − | − | 1.70 (1.88) | 1.63 (2.65) | 0.911 |
| FAB | |||||||||||||
| Similarities | 2.57 (0.50) | 2.33 (0.48) | 0.071 | 2.73 (0.45) | 2.43 (0.57) | 2.80 (0.41) | 2.57 (0.50) | 0.17 (0.38) | 0.10 (0.48) | 0.553 | 0.23 (0.43) | 0.23 (0.57) | 1.000 |
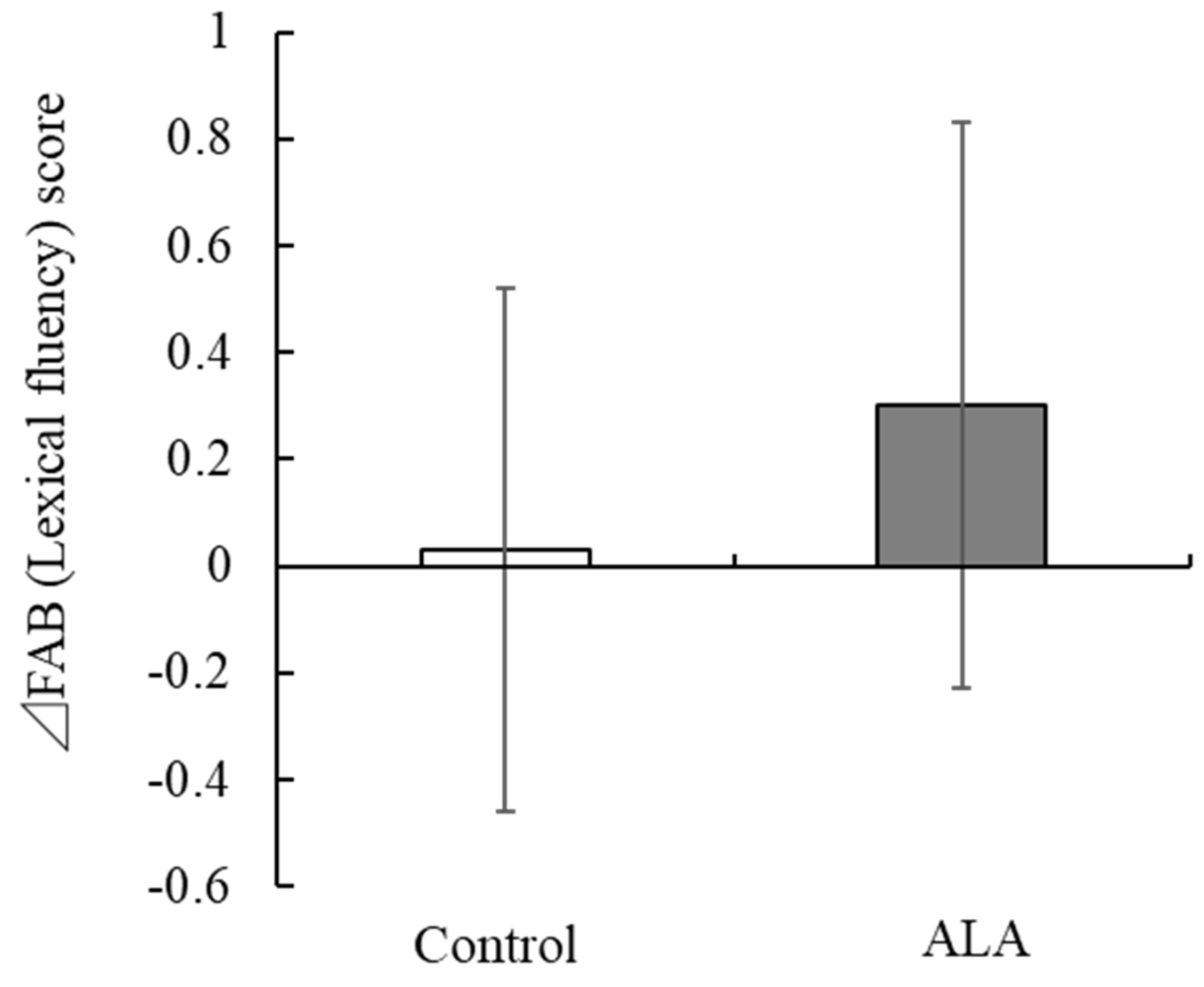
| Lexical fluency | 2.80 (0.41) | 2.50 (0.63) | 0.032 | 2.70 (0.53) | 2.60 (0.56) | 2.83 (0.38) | 2.80 (0.41) | −0.10 (0.55) | 0.10 (0.61) | 0.186 | 0.03 (0.49) | 0.30 (0.53) | 0.049 |
| Motor series | 2.37 (0.85) | 2.40 (0.89) | 0.883 | 2.60 (0.77) | 2.73 (0.64) | 2.47 (0.86) | 2.90 (0.40) | 0.23 (0.86) | 0.33 (0.99) | 0.678 | 0.10 (1.03) | 0.50 (1.04) | 0.140 |
| Conflicting instructions | 2.83 (0.59) | 2.73 (0.78) | 0.58 | 2.80 (0.66) | 2.90 (0.55) | 2.83 (0.59) | 2.77 (0.77) | −0.03 (0.89) | 0.17 (0.99) | 0.413 | 0.00 (0.26) | 0.03 (1.07) | 0.869 |
| Go–No Go | 2.67 (0.55) | 2.40 (0.93) | 0.182 | 2.43 (0.82) | 2.50 (0.68) | 2.53 (0.73) | 2.17 (0.99) | −0.23 (0.86) | 0.10 (0.88) | 0.144 | −0.13 (0.94) | −0.23 (1.22) | 0.723 |
| Prehension behavior | 3.00 (0.00) | 3.00 (0.00) | - | 3.00 (0.00) | 3.00 (0.00) | 3.00 (0.00) | 3.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | − | 0.00 (0.00) | 0.00 (0.00) | − |
| Total score | 16.23 (1.48) | 15.37 (1.25) | 0.017 | 16.27 (1.60) | 16.17 (1.18) | 16.47 (1.28) | 16.20 (1.54) | 0.03 (1.81) | 0.80 (1.30) | 0.064 | 0.23 (1.45) | 0.83 (1.66) | 0.142 |
| JART | |||||||||||||
| Total number of correct answers | 21.47 (3.41) | 18.80 (5.44) | 0.027 | 21.67 (3.78) | 19.57 (5.35) | 22.33 (3.41) | 20.50 (4.77) | 0.20 (1.03) | 0.77 (1.50) | 0.094 | 0.87 (1.43) | 1.70 (2.12) | 0.080 |
| Full scale IQ | 112.87 (7.00) | 107.47 (11.17) | 0.029 | 113.30 (7.67) | 109.00 (11.01) | 114.67 (6.81) | 110.93 (9.72) | 0.43 (2.06) | 1.53 (3.00) | 0.104 | 1.80 (2.80) | 3.47 (4.34) | 0.082 |
| Verbal IQ | 114.77 (8.04) | 108.90 (12.38) | 0.034 | 115.40 (8.68) | 110.57 (12.22) | 116.87 (7.85) | 112.70 (10.88) | 0.63 (2.37) | 1.67 (3.46) | 0.182 | 2.10 (3.16) | 3.80 (4.66) | 0.103 |
| Performance IQ | 108.80 (5.22) | 104.97 (8.15) | 0.034 | 109.27 (5.58) | 106.13 (7.93) | 110.23 (5.00) | 107.50 (7.02) | 0.47 (1.57) | 1.17 (2.23) | 0.165 | 1.43 (2.08) | 2.53 (3.17) | 0.117 |
| WMSR | |||||||||||||
| Verbal memory | 59.60 (15.22) | 53.43 (12.53) | 0.092 | 68.80 (14.50) | 58.07 (11.46) | 75.20 (17.55) | 64.73 (11.63) | 10.97 (14.84) | 7.93 (12.99) | 0.403 | 15.60 (12.91) | 11.30 (9.77) | 0.151 |
| Visual memory | 55.53 (6.62) | 51.33 (9.64) | 0.054 | 57.63 (6.71) | 54.63 (7.88) | 60.23 (4.79) | 56.63 (6.78) | 9.20 (9.93) | 4.63 (11.10) | 0.098 | 4.70 (6.80) | 5.30 (6.75) | 0.733 |
| General memory | 115.47 (17.83) | 104.77 (18.62) | 0.027 | 126.43 (16.44) | 112.70 (15.56) | 135.43 (19.65) | 121.37 (15.60) | 2.10 (8.81) | 3.30 (5.61) | 0.532 | 19.97 (16.00) | 16.60 (12.49) | 0.367 |
| Attention/Concentration | 59.13 (7.01) | 57.83 (7.40) | 0.488 | 60.70 (8.63) | 57.73 (6.34) | 60.97 (6.64) | 57.60 (7.26) | 1.57 (7.17) | −0.10 (5.38) | 0.313 | 1.83 (6.72) | −0.23 (6.17) | 0.220 |
| GDS | |||||||||||||
| Total score | 2.83 (2.72) | 1.57 (2.94) | 0.089 | 2.27 (2.52) | 1.73 (3.07) | 2.37 (3.18) | 1.73 (3.31) | −0.57 (2.92) | 0.17 (1.51) | 0.227 | −0.47 (3.61) | 0.17 (2.56) | 0.436 |
| Stroop | |||||||||||||
| Step1 | 53.93 (8.45) | 50.23 (8.39) | 0.094 | 56.53 (6.48) | 49.87 (9.27) | 57.93 (7.93) | 52.23 (8.24) | 2.60 (6.09) | −0.37 (4.07) | 0.031 | 4.00 (4.39) | 2.00 (4.23) | 0.078 |
| Step2 | 44.73 (10.76) | 42.53 (8.25) | 0.378 | 47.73 (6.81) | 42.90 (8.18) | 47.07 (10.54) | 42.93 (7.32) | 3.00 (8.99) | 0.37 (3.99) | 0.148 | 2.33 (9.24) | 0.40 (4.52) | 0.307 |
| Step3 | 36.47 (5.37) | 32.93 (5.98) | 0.019 | 36.73 (5.64) | 34.17 (5.52) | 37.80 (5.80) | 34.23 (6.25) | 0.27 (2.77) | 1.23 (3.72) | 0.258 | 1.33 (4.31) | 1.30 (3.72) | 0.975 |
| Step4 | 31.60 (4.85) | 26.97 (8.58) | 0.013 | 32.63 (6.08) | 28.37 (6.33) | 33.57 (6.06) | 28.93 (6.45) | 1.03 (3.82) | 1.40 (5.99) | 0.778 | 1.97 (4.61) | 1.97 (4.73) | 1.000 |
| Stroop interference rate | 16.25 (19.07) | 15.07 (10.21) | 0.766 | 15.53 (7.75) | 13.42 (9.70) | 19.55 (14.84) | 17.41 (9.56) | −0.72 (18.04) | −1.65 (8.76) | 0.800 | 3.30 (21.78) | 2.34 (9.26) | 0.826 |
| Reverse− Stroop interference rate | 12.76 (10.18) | 19.39 (17.21) | 0.075 | 10.93 (10.78) | 17.06 (12.53) | 10.31 (14.32) | 15.46 (10.75) | −1.83 (11.45) | −2.33 (17.08) | 0.896 | −2.45 (16.37) | −3.93 (17.29) | 0.736 |
| WAIS-IV | |||||||||||||
| Perceptual reasoning index | 87.43 (11.95) | 82.60 (10.54) | 0.102 | 93.20 (13.49) | 89.43 (11.38) | 95.67 (12.39) | 88.90 (9.03) | 5.77 (8.34) | 6.83 (8.49) | 0.625 | 8.23 (8.28) | 6.30 (7.96) | 0.360 |
| Working memory index | 93.20 (9.07) | 87.20 (9.36) | 0.014 | 94.17 (10.54) | 88.63 (9.11) | 99.17 (11.67) | 90.20 (10.92) | 0.97 (9.86) | 1.43 (6.70) | 0.831 | 5.97 (6.86) | 3.00 (7.00) | 0.103 |
| Processing speed index | 113.13 (10.98) | 106.57 (10.64) | 0.022 | 114.77 (10.26) | 107.53 (11.94) | 116.87 (12.09) | 111.30 (11.36) | 1.63 (6.69) | 0.97 (8.51) | 0.737 | 3.73 (8.25) | 4.73 (8.49) | 0.645 |
| D-CAT | |||||||||||||
| 1 digit total | 277.77 (57.11) | 257.40 (62.39) | 0.192 | 305.00 (57.75) | 279.77 (65.01) | 302.87 (61.07) | 288.23 (60.68) | 27.23 (26.18) | 22.37 (50.04) | 0.639 | 25.10 (39.00) | 30.83 (44.05) | 0.596 |
| 2 digits total | 236.17 (34.49) | 232.77 (42.73) | 0.736 | 236.17 (35.04) | 232.90 (44.38) | 242.97 (35.10) | 232.70 (46.83) | 0.00 (25.29) | 0.13 (24.39) | 0.983 | 6.80 (24.07) | −0.07 (26.73) | 0.300 |
| 3 digits total | 181.20 (30.72) | 176.77 (35.82) | 0.609 | 188.23 (36.20) | 173.40 (31.43) | 191.77 (37.61) | 180.03 (37.84) | 7.03 (17.84) | −3.37 (23.73) | 0.060 | 10.57 (25.78) | 3.27 (29.99) | 0.316 |
| 1 digit miss | 0.02 (0.04) | 0.02 (0.03) | 0.723 | 0.02 (0.04) | 0.03 (0.04) | 0.03 (0.05) | 0.03 (0.05) | 0.00 (0.05) | 0.01 (0.05) | 0.294 | 0.01 (0.03) | 0.01 (0.05) | 0.500 |
| 2 digits miss | 0.06 (0.05) | 0.08 (0.05) | 0.194 | 0.05 (0.04) | 0.07 (0.05) | 0.06 (0.06) | 0.08 (0.07) | −0.01 (0.05) | −0.01 (0.06) | 0.833 | 0.00 (0.05) | 0.00 (0.08) | 0.933 |
| 3 digits miss | 0.10 (0.07) | 0.11 (0.07) | 0.473 | 0.08 (0.07) | 0.09 (0.06) | 0.08 (0.07) | 0.10 (0.08) | −0.02 (0.07) | −0.02 (0.07) | 0.949 | −0.01 (0.07) | −0.01 (0.08) | 0.676 |
| Rate of change (2digits) | 0.87 (0.15) | 0.93 (0.18) | 0.14 | 0.78 (0.08) | 0.85 (0.12) | 0.82 (0.10) | 0.81 (0.08) | −0.09 (0.12) | −0.08 (0.16) | 0.952 | −0.05 (0.14) | −0.12 (0.16) | 0.093 |
| Rate of change (3digits) | 0.66 (0.12) | 0.71 (0.16) | 0.211 | 0.62 (0.09) | 0.64 (0.12) | 0.64 (0.09) | 0.63 (0.10) | −0.04 (0.07) | −0.08 (0.15) | 0.275 | −0.03 (0.12) | −0.08 (0.15) | 0.111 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogawa, T.; Sawane, K.; Ookoshi, K.; Kawashima, R. Supplementation with Flaxseed Oil Rich in Alpha-Linolenic Acid Improves Verbal Fluency in Healthy Older Adults. Nutrients 2023, 15, 1499. https://doi.org/10.3390/nu15061499
Ogawa T, Sawane K, Ookoshi K, Kawashima R. Supplementation with Flaxseed Oil Rich in Alpha-Linolenic Acid Improves Verbal Fluency in Healthy Older Adults. Nutrients. 2023; 15(6):1499. https://doi.org/10.3390/nu15061499
Chicago/Turabian StyleOgawa, Toshimi, Kento Sawane, Kouta Ookoshi, and Ryuta Kawashima. 2023. "Supplementation with Flaxseed Oil Rich in Alpha-Linolenic Acid Improves Verbal Fluency in Healthy Older Adults" Nutrients 15, no. 6: 1499. https://doi.org/10.3390/nu15061499
APA StyleOgawa, T., Sawane, K., Ookoshi, K., & Kawashima, R. (2023). Supplementation with Flaxseed Oil Rich in Alpha-Linolenic Acid Improves Verbal Fluency in Healthy Older Adults. Nutrients, 15(6), 1499. https://doi.org/10.3390/nu15061499






