Food Consumption of People with Sickle Cell Anemia in a Middle-Income Country
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Location
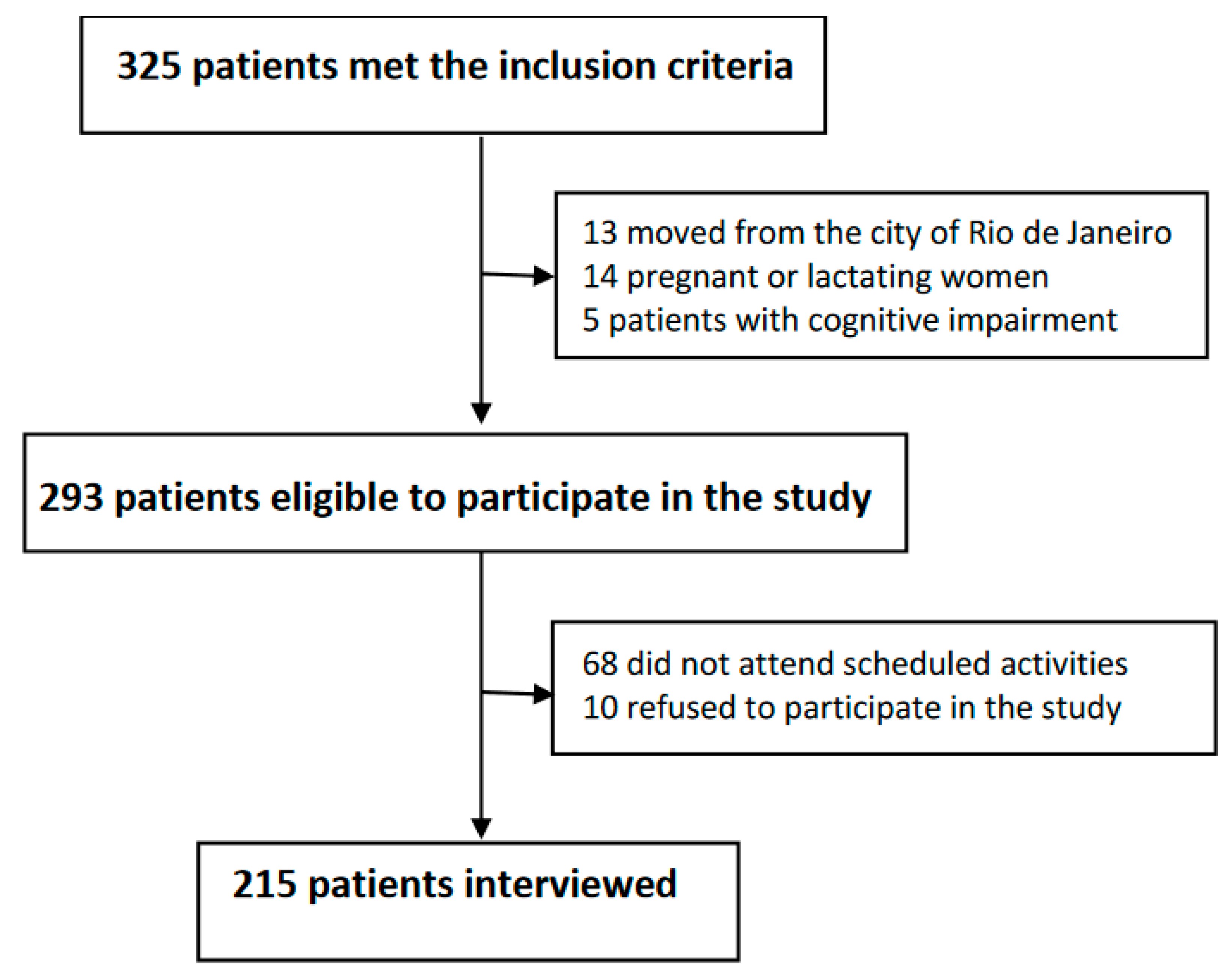
2.2. Data Collection, Study Variables, and Processing
2.3. Data Analysis
2.4. Ethical Aspects
3. Results
3.1. Sociodemographic and Clinical Characteristics of the Participants
3.2. Food Consumption According to the NOVA Classification
3.3. Food Consumption and Iron Intake According to Socioeconomic Classification
3.4. Iron Intake According to the Advice to Restrict Iron-Rich Foods
3.5. Prevalence of Inadequate Iron Intake
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ware, R.E.; Montalembert, M.; Tshilolo, L.; Abboud, M.R. Sickle cell disease. Lancet 2017, 390, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Brasil. Ministério da Saúde. Doença Falciforme: O Que se Deve Saber Sobre Herança Genética. 2014. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/doenca_falciforme_deve_saber_sobre_heranca.pdf (accessed on 21 September 2022).
- Centers for Disease Control and Prevention. Data & Statistics on Sickle Cell Disease. 2020. Available online: https://www.cdc.gov/ncbddd/sicklecell/data.html (accessed on 21 September 2022).
- Kato, G.J.; Piel, F.B.; Reid, C.D.; Gaston, M.H.; Ohene-Frempong, K.; Krishnamurti, L.; Smith, W.R.; Panepinto, J.A.; Weatherall, D.J.; Costa, F.F. Sickle cell disease. Nat. Rev. Dis. Prim. 2018, 4, 18010. [Google Scholar] [CrossRef]
- Piel, F.B.; Hay, S.L.; Gupta, S.; Weatherall, D.J.; Williams, T.N. Global burden of sickle cell anaemia in children under five, 2010–2015: Modelling based on demographics, excess mortality, and interventions. PLoS Med. 2013, 10, e1001484. [Google Scholar] [CrossRef]
- Brasil. Ministério da Saúde. Protocolo Clínico e Diretrizes Terapêuticas da Doença Falciforme. 2018. Available online: https://www.gov.br/saude/pt-br/assuntos/protocolos-clinicos-e-diretrizes-terapeuticas-pcdt/arquivos/2018/doenca-falciforme-pcdt.pdf (accessed on 21 September 2022).
- United States Census Bureau. Current Population Survey Annual Social and Economic Supplement. Poverty Rates for Blacks and Hispanics Reached Historic Lows in Washington. 2019. Available online: https://www.census.gov/library/stories/2020/09/poverty-rates-for-blacks-and-hispanics-reached-historic-lows-in-2019.html (accessed on 13 December 2022).
- Instituto Brasileiro de Geografia e Estatística. Síntese de Indicadores Sociais: Uma ANÁLISE DAS Condições de Vida da População Brasileira. 2020. Available online: https://biblioteca.ibge.gov.br/visualizacao/livros/liv101760.pdf (accessed on 13 December 2022).
- Santos, I.N.; Damião, J.J.; Fonseca, M.J.M.; Cople-Rodrigues, C.S.; Aguiar, O.B. Food insecurity and social support in families of children with sickle-cell disease. J. Pediatr. 2019, 95, 306–313. [Google Scholar] [CrossRef]
- Araújo, M.L.; Mendonça, R.D.; Lopes Filho, J.D.; Lopes, A.C.S. Association between food insecurity and food intake. Nutrition 2018, 54, 54–59. [Google Scholar] [CrossRef]
- Cox, S.E.; Makani, J.; Fulford, A.J.; Komba, A.N.; Soka, D.; Williams, T.N.; Newton, C.; Marsh, K.; Prentice, A.M. Nutritional status, hospitalization and mortality among patients with sickle cell anemia in Tanzania. Haematologica 2011, 96, 948–953. [Google Scholar] [CrossRef]
- Omena, J.; Curioni, C.; Cople-Rodrigues, C.D.S.; Citelli, M. The effect of food and nutrients on iron overload: What do we know so far? Eur. J. Clin. Nutr. 2021, 75, 1771–1780. [Google Scholar] [CrossRef] [PubMed]
- Omena, J.; Cople-Rodrigues, C.S.; Cardoso, J.D.A.; Soares, A.R.; Fleury, M.F.; Brito, F.S.B.; Koury, J.C.; Citelli, M. Serum hepcidin concentration in individuals with sickle cell anemia: Basis for the dietary recommendation of iron. Nutrients 2018, 10, 498. [Google Scholar] [CrossRef] [PubMed]
- Ohemeng, A.; Boadu, I. The role of nutrition in the pathophysiology and management of sickle cell disease among children: A review of literature. Crit. Rev. Food Sci. Nutr. 2017, 58, 2299–2305. [Google Scholar] [CrossRef]
- Brissot, P.; Ropert, M.; Lan, C.L.; Loréal, O. Non-transferrin bound iron: A key role in iron overload and iron toxicity. Biochim. Biophys. Acta. 2012, 1820, 403–410. [Google Scholar] [CrossRef]
- World Bank. Prevalence of Anemia among Children (% of Children under 5). 2016. Available online: https://data.worldbank.org/indicator/SH.ANM.CHLD.ZS (accessed on 13 December 2022).
- Monteiro, C.A.; Cannon, G.; Moubarac, J.C.; Levy, R.B.; Louzada, M.L.C.; Jaime, P.C. The un decade of nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. 2017, 21, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Martínez Leo, E.E.; Peñafiel, A.M.; Hernández Escalante, V.M.; Cabrera Araujo, Z.M. Ultra-processed diet, systemic oxidative stress, and breach of immunologic tolerance. Nutrition 2021, 91–92, 111419. [Google Scholar] [CrossRef]
- Delesderrier, E.; Curioni, C.; Omena, J.; Macedo, C.R.; Cople-Rodrigues, C.; Citelli, M. Antioxidant nutrients and hemolysis in sickle cell disease. Clin. Chim. Acta 2020, 510, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.A.; Andrade, G.C.; Oliveira, M.F.B.; Rauber, F.; de Castro, I.R.R.; Couto, M.T.; Levy, R.B. “Healthy”, “Usual” and “Convenience” cooking practices patterns: How do they influence children’s food consumption? Appetite 2020, 158, 105018. [Google Scholar] [CrossRef] [PubMed]
- Moshfegh, A.J.; Rhodes, D.G.; Baer, D.J.; Murayi, T.; Clemens, J.C.; Rumpler, W.V.; Paul, D.R.; Sebastian, R.S.; Kuczynski, K.J.; Ingwersen, L.A.; et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am. J. Clin. Nutr. 2008, 88, 324–332. [Google Scholar] [CrossRef]
- Crispim, S.P.; Fisberg, R.M.; Almeida, C.C.B.; Geneviève, N.; Knaze, V.; Pereira, R.A.; Slimani, N. Manual Fotográfico de Quantificação Alimentar—Curitiba; Universidade Federal do Paraná: Curitiba, Brazil, 2017. [Google Scholar]
- Tabela Brasileira de Composição de Alimentos. 2011. Available online: https://www.cfn.org.br/wp-content/uploads/2017/03/taco_4_edicao_ampliada_e_revisada.pdf (accessed on 21 September 2022).
- Associação Brasileira de Empresas de Pesquisa. Critérios Classificação Econômica. 2019. Available online: http://www.abep.org.br/site/ (accessed on 11 December 2022).
- Institute of Medicine. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. 2001. Available online: https://www.ncbi.nlm.nih.gov/books/NBK222310/ (accessed on 11 December 2022).
- Vieira, D.A.; Steluti, J.; Verly-Jr, E.; Marchioni, D.M.; Fisberg, R.M. Brazilans’ experiences with iron fortification: Evidence of effectiveness for reducing inadequate iron intakes with fortified flour policy. Public Heath Nutr. 2016, 20, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Wastnedge, E.; Waters, D.; Patel, S.; Morrison, K.; Goh, M.Y.; Adeloye, D.; Rudan, I. The global burden of sickle cell disease in children under five years of age: A systematic review and meta-analysis. J. Glob. Health 2018, 8, 021103. [Google Scholar] [CrossRef] [PubMed]
- Tshilolo, L.; Kafando, E.; Sawadogo, M.; Cotton, F.; Vertongen, F.; Ferster, A.; Gulbis, B. Neonatal screening and clinical care programmes for sickle cell disorders in sub-Saharan Africa: Lessons from pilot studies. Public Health 2008, 122, 933–941. [Google Scholar] [CrossRef]
- McGann, P.T. Time to invest in sickle cell anemia as a global health priority. Pediatrics 2016, 137, e20160348. [Google Scholar] [CrossRef]
- Instituto Brasileiro de Geografia e Estatística. Pesquisa de Orçamentos Familiares 2017–2018, Análise do Consumo Alimentar Pessoal no Brasil. 2020. Available online: https://biblioteca.ibge.gov.br/visualizacao/livros/liv101742.pdf (accessed on 11 December 2022).
- Levy, R.B.; Andrade, G.C.; Cruz, G.L.D.; Rauber, F.; Louzada, M.L.D.C.; Claro, R.M.; Monteiro, C.A. Three decades of household food availability according to NOVA—Brazil, 1987–2018. Rev. Saude Publica 2022, 56, 75. [Google Scholar] [CrossRef]
- Cediel, G.; Reyes, M.; Louzada, M.L.C.; Steele, E.M.; Monteiro, C.A.; Corvalán, C.; Uauy, R. Ultra-processed foods and added sugars in the Chilean diet. Public Health Nutr. 2010, 21, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Marrón-Ponce, J.; Sánchez-Pimienta, T.; Louzada, M.; Batis, C. Energy contribution of NOVA food groups and sociodemographic determinants of ultra-processed food consumption in the Mexican population. Public Health Nutr. 2018, 21, 87–93. [Google Scholar] [CrossRef]
- Zupo, R.; Castellana, F.; Guerra, V.; Donghia, R.; Bortone, I.; Griseta, C.; Lampignano, L.; Dibello, V.; Lozupone, M.; Coelho-Júnior, H.J.; et al. Associations between nutritional frailty and 8-year all-cause mortality in older adults: The Salus in Apulia Study. J. Intern. Med. 2021, 290, 1071–1082. [Google Scholar] [CrossRef] [PubMed]
- Parrow, N.L.; Violet, P.C.; George, N.A.; Ali, F.; Bhanvadia, S.; Wong, R.; Tisdale, J.F.; Fitzhugh, C.; Levine, M.; Thein, S.L.; et al. Dietary iron restriction improves markers of disease severity in murine sickle cell anemia. Blood 2021, 137, 1553–1555. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Kazmi, J.S.; Lee, S.; Zhang, D.; Gao, X.; Maryanovich, M.; Torres, L.; Verma, D.; Kelly, L.; Ginzburg, Y.Z.; et al. Dietary iron restriction protects against vaso-occlusion and organ damage in murine sickle cell disease. Blood 2023, 141, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Bailey, Z.D.; Krieger, N.; Agenor, M.; Graves, J.; Linos, N.; Bassett, M. Structural racism and health inequities in the USA: Evidence and interventions. Lancet 2017, 389, 1453–1463. [Google Scholar] [CrossRef]
- Creary, M.S. Biocultural citizenship and embodying exceptionalism: Biopolitics for sickle cell disease in Brazil. Soc. Sci. Med. 2018, 199, 123–131. [Google Scholar] [CrossRef]
- Layrisse, M.; Chaves, J.F.; Mendez-Castellano, B.V.; Tropper, E.; Bastardo, B.; Gonzalez, E. Early response to the effect of iron fortification in the Venezuelan population. Am. J. Clin. Nutr. 1996, 64, 903–907. [Google Scholar] [CrossRef]
- Martorell, R.; Ascencio, M.; Tacsan, L.; Alfaro, T.; Young, M.F.; Addo, O.Y.; Flores-Ayala, R. Effectiveness evaluation of the food fortification program of Costa Rica: Impact on anemia prevalence and hemoglobin concentrations in women and children. Am. J. Clin. Nutr. 2014, 101, 210–217. [Google Scholar] [CrossRef]
- Beaton, G.H.; Milner, J.; Corey, P.; Mcguire, V.; Cousins, M.; Stewart, E.; De Ramos, M.; Hewitt, D.; Grambsch, P.V. Sources of variance in 24-hour dietary recall data: Implications for nutrition study design and interpretation. Am. J. Clin. Nutr. 1979, 32, 2556–2559. [Google Scholar] [CrossRef]
- National Institutes of Health, National Cancer Institute. 24-Hour Dietary Recall (24HR) at a Glance. 2014. Available online: https://dietassessmentprimer.cancer.gov/ (accessed on 11 December 2022).
- Gibson, R. Principles of Nutritional Assessment; Oxford University Press: New York, NY, USA, 2005. [Google Scholar]

| Variables | Frequencies | |
|---|---|---|
| Sex | N | % |
| Female | 126 | 58.6 |
| Male | 89 | 41.4 |
| Age (Years) | ||
| 19–30 | 89 | 41.4 |
| 31–50 | 98 | 45.6 |
| 51–59 | 28 | 13.0 |
| Color/race | ||
| Black | 133 | 61.9 |
| Brown | 65 | 30.2 |
| White | 9 | 4.2 |
| Yellow | 6 | 2.8 |
| Indigenous | 2 | 0.9 |
| Education | ||
| Elementary | 65 | 30.2 |
| Secondary school | 124 | 57.7 |
| Tertiary/University | 26 | 12.1 |
| Benefit from federal financial aid programs | ||
| Yes | 58 | 27.0 |
| Advice to restrict iron source foods * | ||
| Yes | 123 | 57.2 |
| Occurrence of pica | ||
| Yes | 40 | 18.6 |
| Performing multiple blood transfusions ** | ||
| Yes | 139 | 64.7 |
| Use of iron chelators | ||
| Yes | 16 | 7.4 |
| Regular alcohol consumption | ||
| Yes | 46 | 21.4 |
| Food Groups and Subgroups | Energy | Iron | Vitamin C | |||
|---|---|---|---|---|---|---|
| kcal/Day (M ± SD) * | % of Total Intake | mg/Day (M ± SD) * | % of Total Intake | mg/Day (M ± SD) * | % of Total Intake | |
| In natura or minimally processed foods ** | 1330.8 ± 1364.0 | 58.4 | 9.6 ± 9.5 | 72.7 | 57.9 ± 109.3 | 68.8 |
| Beef or pork | 239.3 | 10.5 | 2.5 | 18.9 | ||
| Rice | 225.8 | 9.9 | 0.2 | 1.5 | ||
| Poultry | 185.4 | 8.1 | 1.0 | 7.6 | ||
| Beans | 142.9 | 6.3 | 3.1 | 23.5 | ||
| Other cereals a# | 88.6 | 3.9 | 0.6 | 4.5 | 1.5 | 1.8 |
| Fruits b | 82.9 | 3.6 | 0.3 | 2.3 | 39.2 | 46.6 |
| Roots and tubers | 82.3 | 3.6 | 0.3 | 2.3 | 4.6 | 5.5 |
| Milk | 43.0 | 1.9 | - | - | ||
| Eggs | 41.6 | 1.8 | 0.2 | 1.5 | ||
| Vegetables | 39.5 | 1.7 | 0.4 | 3.0 | 11.2 | 13.3 |
| Viscera | 38.6 | 1.7 | 0.6 | 4.5 | ||
| Fish | 29.3 | 1.3 | 0.1 | 0.8 | ||
| Fermented foods | 22.9 | 1.0 | - | - | ||
| Homemade cakes # | 20.0 | 0.9 | - | - | ||
| Other in natura or minimally processed foods c | 48.8 | 2.1 | 0.3 | 2.3 | 1.5 | 1.8 |
| Processed foods | 146.1 ± 181.5 | 6.4 | 0.5 ± 0.6 | 3.8 | 0.5 ± 3.1 | 0.6 |
| Bread (fresh unpackaged) # | 106.6 | 4.7 | 0.4 | 3.1 | ||
| Cheeses | 20.6 | 0.9 | - | - | ||
| Salted, dried, cured, or smoked meats | 10.7 | 0.5 | - | - | 0.1 | 0.1 |
| Fruit in syrup (with or without added antioxidants) | 6.8 | 0.3 | - | - | 0.4 | 0.5 |
| Other processed foods d | 1.3 | 0.1 | 0.1 | 0.7 | ||
| Ultra-processed foods | 800.8 ± 849.8 | 35.2 | 3.1 ± 4.7 | 23.5 | 25.8 ± 52.4 | 30.6 |
| Carbonated soft drinks and industrialized juices | 156.1 | 6.9 | 0.2 | 1.5 | 18.2 | 21.5 |
| Ready or semi-ready dishes e# | 105.9 | 4.6 | 0.8 | 6.1 | 0.9 | 1.1 |
| Cakes, pies, and sweet cookies | 95.6 | 4.2 | 0.5 | 3.8 | - | - |
| Sausages and other reconstituted meat products | 77.7 | 3.4 | 0.4 | 3.0 | - | - |
| Milk drinks | 75.2 | 3.3 | - | - | 4.7 | 5.6 |
| Salty cookies and snack chips # | 72.4 | 3.2 | 0.3 | 2.3 | 1.0 | 1.2 |
| Margarine | 64.7 | 2.8 | - | - | - | - |
| Breads (packaged) # | 51.2 | 2.2 | 0.5 | 3.8 | - | - |
| Candies (confectionery) f | 47.8 | 2.1 | 0.1 | 0.8 | - | - |
| Fast food g# | 43.3 | 1.9 | 0.4 | 3.0 | - | - |
| Other ultra-processed foods h# | 11.2 | 0.5 | 0.1 | 0.8 | 1.0 | 1.2 |
| Total | 2277.8 ± 1634.9 | 100.0 | 13.2 ± 10.5 | 100.0 | 84.2 ± 120.0 | 100.0 |
| Independent Variable | Dependent Variable Food Groups | F | p | Effect Size η2 |
|---|---|---|---|---|
| Socioeconomic classes * | In natura or minimally processed foods | 0.477 | 0.699 | 0.007 |
| Processed foods | 1.691 | 0.170 | 0.023 | |
| Ultra-processed foods | 2.340 | 0.074 | 0.032 | |
| Monthly household income ** | In natura or minimally processed foods | 5.123 | <0.0001 | 0.870 |
| Processed foods | 0.765 | 0.917 | 0.499 | |
| Ultra-processed foods | 1.151 | 0.239 | 0.600 |
| Classes A + B1 + B2 (n = 25) | Class C1 (n = 70) | Class C2 (n = 82) | Classes D + E (n = 25) | p Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | median | IQR | median | IQR | median | IQR | median | IQR | |
| Ultra-processed foods | 4.4a | 2.1–8.6 | 2.1b | 1.0–4.5 | 1.8b | 0.9–4.0 | 2.2b | 0.6–4.1 | 0.004 |
| Meats * | 3.1a | 0.8–5.1 | 1.9ac | 0.7–6.0 | 1.3bc | 0.0–3.8 | 1.0b | 0.0–2.7 | 0.041 |
| Bean | 2.0 | 0.0–6.5 | 2.7 | 0.0–4.7 | 2.3 | 0.0–3.1 | 2.3 | 0.0–3.7 | 0.913 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira, T.V.; Da Silva, A.C.F.; Rodrigues, C.d.S.C.; Brito, F.d.S.B.; Canella, D.S.; Citelli, M. Food Consumption of People with Sickle Cell Anemia in a Middle-Income Country. Nutrients 2023, 15, 1478. https://doi.org/10.3390/nu15061478
Teixeira TV, Da Silva ACF, Rodrigues CdSC, Brito FdSB, Canella DS, Citelli M. Food Consumption of People with Sickle Cell Anemia in a Middle-Income Country. Nutrients. 2023; 15(6):1478. https://doi.org/10.3390/nu15061478
Chicago/Turabian StyleTeixeira, Tamara Vilhena, Ana Carolina Feldenheimer Da Silva, Cláudia dos Santos Cople Rodrigues, Flávia dos Santos Barbosa Brito, Daniela Silva Canella, and Marta Citelli. 2023. "Food Consumption of People with Sickle Cell Anemia in a Middle-Income Country" Nutrients 15, no. 6: 1478. https://doi.org/10.3390/nu15061478
APA StyleTeixeira, T. V., Da Silva, A. C. F., Rodrigues, C. d. S. C., Brito, F. d. S. B., Canella, D. S., & Citelli, M. (2023). Food Consumption of People with Sickle Cell Anemia in a Middle-Income Country. Nutrients, 15(6), 1478. https://doi.org/10.3390/nu15061478







