Diet as a Factor Supporting Lung Cancer Treatment—A Systematic Review
Abstract
1. Introduction
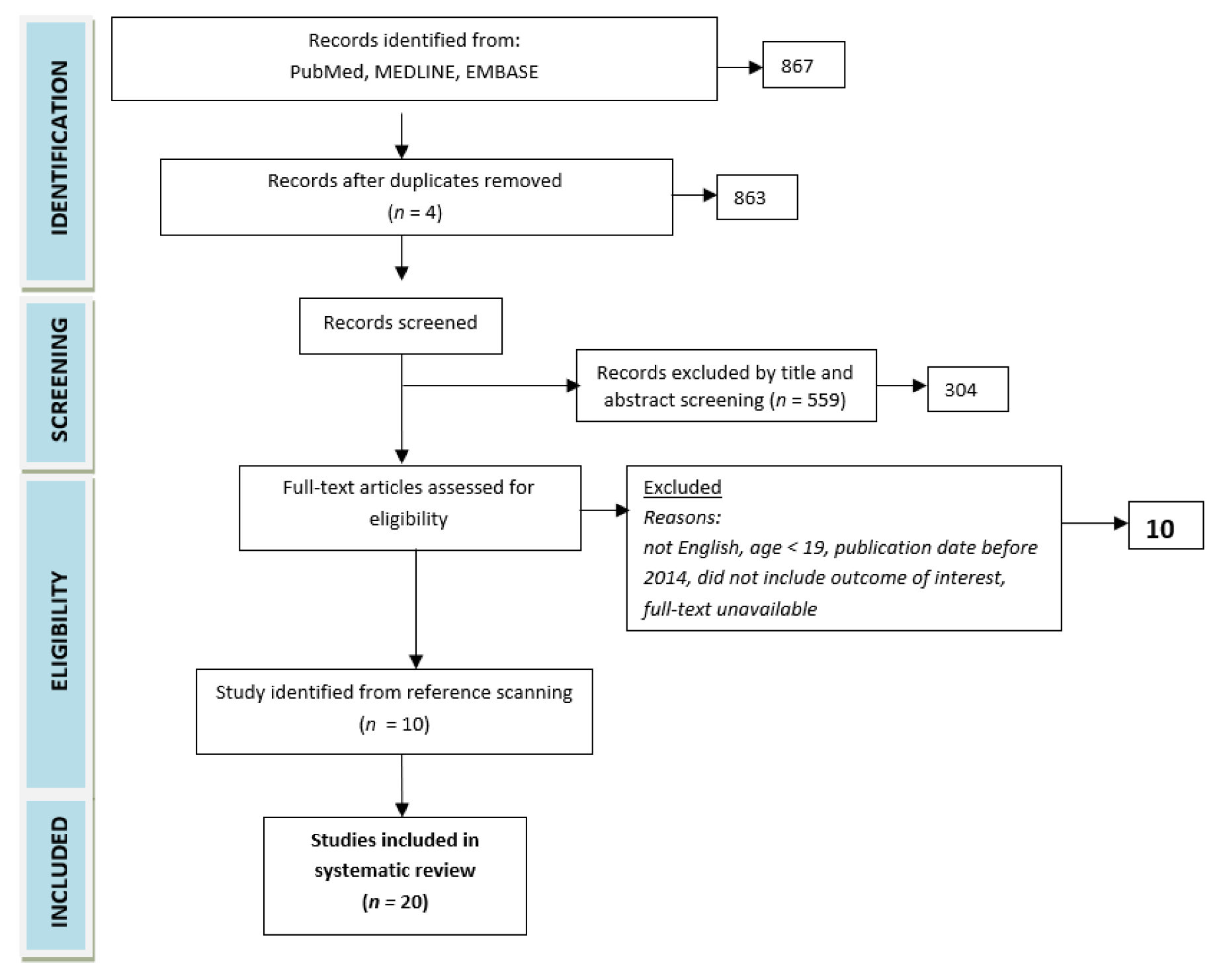
2. Methods
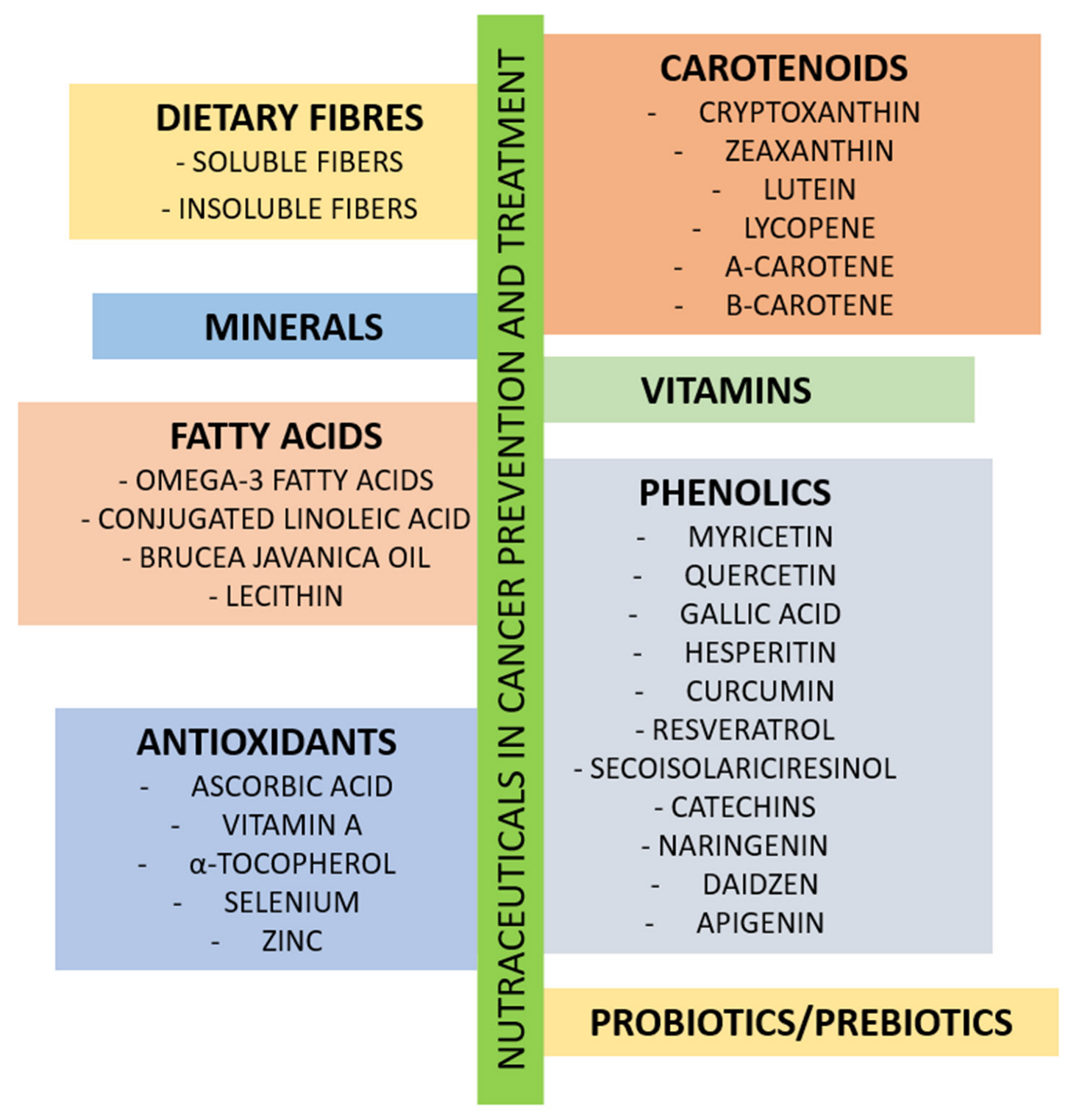
3. The Role of Nutrients in Cancer Treatment
4. Diet as a Factor Supporting Lung Cancer Treatment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Laversanne, M.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Tomorrow. Lyon, France: International Agency for Research on Cancer. 2020. Available online: https://gco.iarc.fr/tomorrow (accessed on 29 August 2022).
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Löfling, L.; Bahmanyar, S.; Kieler, H.; Lambe, M.; Wagenius, G. Temporal trends in lung cancer survival: A population-based study. Acta Oncol. 2021, 61, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Chabowski, M.; Polański, J.; Jankowska-Polańska, B.; Janczak, D.; Rosińczuk, J. Is nutritional status associated with the level of anxiety, depression and pain in patients with lung cancer? J. Thorac. Dis. 2018, 10, 2303–2310. [Google Scholar] [CrossRef]
- Bagan, P.; Berna, P.; De Dominicis, F.; Pereira, J.C.D.N.; Mordant, P.; De La Tour, B.; Le Pimpec-Barthes, F.; Riquet, M. Nutritional Status and Postoperative Outcome After Pneumonectomy for Lung Cancer. Ann. Thorac. Surg. 2013, 95, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Lis, C.G. Pretreatment serum albumin as a predictor of cancer survival: A systematic review of the epidemiological literature. Nutr. J. 2010, 9, 69. [Google Scholar] [CrossRef]
- Strasser, F.; Bruera, E.D. Update on anorexia and cachexia. Hematol./Oncol. Clin. North Am. 2002, 16, 589–617. [Google Scholar] [CrossRef] [PubMed]
- Puccio, M.; Nathason, L. The cancer cachexia syndrome. Semin. Oncol. 1997, 24, 277–287. [Google Scholar]
- Tisdale, M.J. Cancer cachexia: Metabolic alternations and clinical manifestations. Nutrition 1997, 13, 9058439. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Fedak, K.M.; Bernal, A.; Capshaw, Z.A.; Gross, S. Applying the Bradford Hill criteria in the 21st century: How data integration has changed causal inference in molecular epidemiology. Emerg. Themes Epidemiol. 2015, 12, 1–9. [Google Scholar] [CrossRef]
- Critical Appraisal Skills Programme (CASP). CASP Randomised Controlled Trial Checklist. 2013. Available online: http://www.casp-uk.net/checklists (accessed on 29 August 2022).
- Sunshine, H.; Iruela-Arispe, M.L. Membrane lipids and cell signaling. Curr. Opin. Infect. Dis. 2017, 28, 408–413. [Google Scholar] [CrossRef]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Pappalardo, G.; Almeida, A.; Ravasco, P. Eicosapentaenoic acid in cancer improves body composition and modulates metabolism. Nutrition 2015, 31, 549–555. [Google Scholar] [CrossRef]
- Ravasco, P.; Lavriv, D.; Neves, P. Should omega-3 be used in cancer cachexia? Clin. Nutr. ESPEN 2018, 25, 18–25. [Google Scholar]
- Hering, J.; Garrean, S.; Dekoj, T.R.; Razzak, A.; Saied, A.; Trevino, J.; Babcock, T.A.; Espat, N.J. Inhibition of Proliferation by Omega-3 Fatty Acids in Chemoresistant Pancreatic Cancer Cells. Ann. Surg. Oncol. 2007, 14, 3620–3628. [Google Scholar] [CrossRef]
- Murphy, R.A.; Mourtzakis, M.; Mazurak, V. n-3 polyunsaturated fatty acids. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 246–251. [Google Scholar] [CrossRef]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef]
- Tao, X.; Zhou, Q.; Rao, Z. Efficacy of ω-3 Polyunsaturated Fatty Acids in Patients with Lung Cancer Undergoing Radiotherapy and Chemotherapy: A Meta-Analysis. Int. J. Clin. Pr. 2022, 2022, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Baracos, V.E.; Martin, L.; Korc, M.; Guttridge, D.C.; Fearon, K.C.H. Cancer-associated cachexia. Nat. Rev. Dis. Prim. 2018, 4, 29345251. [Google Scholar] [CrossRef] [PubMed]
- Troesch, B.; Eggersdorfer, M.; Laviano, A.; Rolland, Y.; Smith, A.; Warnke, I.; Weimann, A.; Calder, P. Expert Opinion on Benefits of Long-Chain Omega-3 Fatty Acids (DHA and EPA) in Aging and Clinical Nutrition. Nutrients 2020, 12, 2555. [Google Scholar] [CrossRef] [PubMed]
- Vega, O.M.; Abkenari, S.; Tong, Z.; Tedman, A.; Huerta-Yepez, S. Omega-3 Polyunsaturated Fatty Acids and Lung Cancer: Nutrition or Pharmacology? Nutr. Cancer 2020, 73, 541–561. [Google Scholar] [CrossRef]
- Kiss, N.; Isenring, E.; Gough, K.; Wheeler, G.; Wirth, A.; Campbell, B.A.; Krishnasamy, M. Early and Intensive Dietary Counseling in Lung Cancer Patients Receiving (Chemo)Radiotherapy—A Pilot Randomized Controlled Trial. Nutr. Cancer 2016, 68, 958–967. [Google Scholar] [CrossRef]
- Tozer, R.G.; Tai, P.; Falconer, W.; Ducruet, T.; Karabadjian, A.; Bounous, G.; Molson, J.H.; Dröge, W. Cysteine-Rich Protein Reverses Weight Loss in Lung Cancer Patients Receiving Chemotherapy or Radiotherapy. Antioxid. Redox Signal. 2008, 10, 395–402. [Google Scholar] [CrossRef]
- Klement, R.J.; Kämmerer, U. Is there a role for carbohydrate restriction in the treatment and prevention of cancer? Nutr. Metab. 2011, 8, 75. [Google Scholar] [CrossRef] [PubMed]
- Krejbich, P.; Birringer, M. The Self-Administered Use of Complementary and Alternative Medicine (CAM) Supplements and Antioxidants in Cancer Therapy and the Critical Role of Nrf-2—A Systematic Review. Antioxidants 2022, 11, 2149. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Espey, M.G.; Sun, A.Y.; Pooput, C.; Kirk, K.L.; Krishna, M.C.; Khosh, D.B.; Drisko, J.; Levine, M. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc. Natl. Acad. Sci. USA 2008, 105, 11105–11109. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Espey, M.G.; Krishna, M.C.; Mitchell, J.B.; Corpe, C.P.; Buettner, G.R.; Shacter, E.; Levine, M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro-drug to deliver hydrogen peroxide to tissues. Proc. Natl. Acad. Sci. USA 2005, 102, 13604–13609. [Google Scholar] [CrossRef]
- Carosio, R.; Zuccari, G.; Orienti, I.; Mangraviti, S.; Montaldo, P.G. Sodium Ascorbate induces apoptosis in neuroblastoma cell lines by interfering with iron uptake. Mol. Cancer 2007, 6, 55. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chapman, J.; Levine, M.; Polireddy, K.; Drisko, J.; Chen, Q. High-Dose Parenteral Ascorbate Enhanced Chemosensitivity of Ovarian Cancer and Reduced Toxicity of Chemotherapy. Sci. Transl. Med. 2014, 6, 222ra18. [Google Scholar] [CrossRef]
- Tokarski, S.; Rutkowski, M.; Godala, M.; Mejer, A.; Kowalski, J. Wpływ kwasu askorbinowego na stezenie witamin antyoksydacyjnych w osoczu chorych na niedrobnokomórkowego raka płuca poddanych chemioterapii pierwszorzutowej [The impact of ascorbic acid on the concentrations of antioxidative vitamins in the plasma of patients with non-small cell lung cancer undergoing first-line chemotherapy]. Pol. Merkur. Lekarski. 2013, 35, 136–140. [Google Scholar]
- Ou, J.; Zhu, X.; Chen, P.; Du, Y.; Lu, Y.; Peng, X.; Bao, S.; Wang, J.; Zhang, X.; Zhang, T.; et al. A randomized phase II trial of best supportive care with or without hyperthermia and vitamin C for heavily pretreated, advanced, refractory non-small-cell lung cancer. J. Adv. Res. 2020, 24, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, K.B.; Dutta, S.; Gupta, A.; Mondal, S.; Sharma, S. Comparative study among glutamine, acetyl-L-carnitine, vitamin-E and methylcobalamine for treatment of paclitaxel-induced peripheral neuropathy. Clin. Cancer Investig. J. 2014, 3, 213. [Google Scholar] [CrossRef]
- Fritz, H.; Kennedy, D.; Fergusson, D.; Fernandes, R.; Doucette, S.; Cooley, K.; Seely, A.; Sagar, S.; Wong, R.; Seely, D. Vitamin A and Retinoid Derivatives for Lung Cancer: A Systematic Review and Meta Analysis. PLoS ONE 2011, 6, e21107. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, U.; Infante, M.; Maioli, M.; Chiesa, G.; Buyse, M.; Firket, P.; Rosmentz, N.; Clerici, M.; Soresi, E.; Valente, M. Adjuvant treatment of stage I lung cancer with high-dose vitamin A. J. Clin. Oncol. 1993, 11, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, A.; Kaya, Y.; Tanriverdi, O. Effect of the Interaction Between Selenium and Zinc on DNA Repair in Association with Cancer Prevention. J. Cancer Prev. 2019, 24, 146–154. [Google Scholar] [CrossRef]
- Piccinini, L.; Borella, P.; Bargellini, A.; Medici, C.I.; Zoboli, A. A case-control study on selenium, zinc, and copper in plasma and hair of subjects affected by breast and lung cancer. Biol. Trace Element Res. 1996, 51, 23–30. [Google Scholar] [CrossRef]
- Tian, J.; Wei, X.; Zhang, W.; Xu, A. Effects of Selenium Nanoparticles Combined with Radiotherapy on Lung Cancer Cells. Front. Bioeng. Biotechnol. 2020, 8, 598997. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Takata, Y.; Smith-Warner, S.A.; Blot, W.; Sawada, N.; White, E.; Freedman, N.; Robien, K.; Giovannucci, E.; Zhang, X.; et al. Prediagnostic Calcium Intake and Lung Cancer Survival: A Pooled Analysis of 12 Cohort Studies. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1060–1070. [Google Scholar] [CrossRef] [PubMed]
- Berghmans, T.; Paesmans, M.; Body, J.-J. A prospective study on hyponatraemia in medical cancer patients: Epidemiology, aetiology and differential diagnosis. Support. Care Cancer 2000, 8, 192–197. [Google Scholar] [CrossRef]
- Sengupta, A.; Banerjee, S.N.; Biswas, N.M.; Jash, D.; Saha, K.; Maji, A.; Bandyopadhyaya, A.; Agarwal, S. The incidence of hyponatraemia and its effect on the ECOG performance status among lung cancer patients. J. Clin. Diagn. Res. 2013, 7, 1678–1682. [Google Scholar]
- Rawson, N.S.; Peto, J. An overview of prognostic factors in small cell lung cancer. A report from the subcommittee for the management of lung cancer of the United Kingdom Coordinating Committee on Cancer Research. Br. J. Cancer 1990, 61, 597–604. [Google Scholar] [CrossRef]
- Jeppesen, A.N.; Jensen, H.K.; Donskov, F.; Marcussen, N.; Von Der Maase, H. Hyponatremia as a prognostic and predictive factor in metastatic renal cell carcinoma. Br. J. Cancer 2010, 102, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Berardi, R.; Santoni, M.; Newsom-Davis, T.; Caramanti, M.; Rinaldi, S.; Tiberi, M.; Morgese, F.; Torniai, M.; Pistelli, M.; Onofri, A.; et al. Hyponatremia normalization as an independent prognostic factor in patients with advanced non-small cell lung cancer treated with first-line therapy. Oncotarget 2016, 8, 23871–23879. [Google Scholar] [CrossRef]
- Berardi, R.; Caramanti, M.; Castagnani, M.; Guglielmi, S.; Marcucci, F.; Savini, A.; Morgese, F.; Rinaldi, S.; Ferrini, C.; Tiberi, M.; et al. Hyponatremia is a predictor of hospital length and cost of stay and outcome in cancer patients. Support. Care Cancer 2015, 23, 3095–3101. [Google Scholar] [CrossRef] [PubMed]
- Girault, A.; Privé, A.; Trinh, N.T.N.; Bardou, O.; Ferraro, P.; Joubert, P.; Bertrand, R.; Brochiero, E. Identification of KvLQT1 K+ channels as new regulators of non-small cell lung cancer cell proliferation and migration. Int. J. Oncol. 2013, 44, 838–848. [Google Scholar] [CrossRef]
- Lajer, H.; Daugaard, G. Cisplatin and hypomagnesemia. Cancer Treat. Rev. 1999, 25, 47–58. [Google Scholar] [CrossRef]
- Muraki, K.; Koyama, R.; Honma, Y.; Yagishita, S.; Shukuya, T.; Ohashi, R.; Takahashi, F.; Kido, K.; Iwakami, S.; Sasaki, S.; et al. Hydration with magnesium and mannitol without furosemide prevents the nephrotoxicity induced by cisplatin and pemetrexed in patients with advanced non-small cell lung cancer. J. Thorac. Dis. 2012, 4, 562–568. [Google Scholar] [CrossRef]
- Yoshida, T.; Niho, S.; Toda, M.; Goto, K.; Yoh, K.; Umemura, S.; Matsumoto, S.; Ohmatsu, H.; Ohe, Y. Protective Effect of Magnesium Preloading on Cisplatin-induced Nephrotoxicity: A Retrospective Study. Jpn. J. Clin. Oncol. 2014, 44, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Oka, T.; Kimura, T.; Suzumura, T.; Yoshimoto, N.; Nakai, T.; Yamamoto, N.; Matsuura, K.; Mitsuoka, S.; Yoshimura, N.; Kudoh, S.; et al. Magnesium supplementation and high volume hydration reduce the renal toxicity caused by cisplatin-based chemotherapy in patients with lung cancer: A toxicity study. BMC Pharmacol. Toxicol. 2014, 15, 70. [Google Scholar] [CrossRef]
- Zahra, A.; Fath, M.A.; Opat, E.; Mapuskar, K.A.; Bhatia, S.K.; Ma, D.C.; Iii, S.N.R.; Snyders, T.P.; Chenard, C.A.; Eichenberger-Gilmore, J.M.; et al. Consuming a Ketogenic Diet while Receiving Radiation and Chemotherapy for Locally Advanced Lung Cancer and Pancreatic Cancer: The University of Iowa Experience of Two Phase 1 Clinical Trials. Radiat. Res. 2017, 187, 743–754. [Google Scholar] [CrossRef]
- Sakoda, L.C.; Loomis, M.M.; Doherty, J.A.; Neuhouser, M.L.; Barnett, M.J.; Thornquist, M.D.; Weiss, N.S.; Goodman, G.E.; Chen, C. Chromosome 15q24-25.1 variants, diet, and lung cancer susceptibility in cigarette smokers. Cancer Causes Control. 2011, 22, 449–461. [Google Scholar] [CrossRef]
- Lee, B.W.; Wain, J.C.; Kelsey, K.T.; Wiencke, J.K.; Christiani, D.C. Association between Diet and Lung Cancer Location. Am. J. Respir. Crit. Care Med. 1998, 158, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.C.; Bueno-De-Mesquita, H.B.; Fidanza, F.; Nissinen, A.M.; Menotti, A.; Kok, F.J.; Kromhout, D. Cohort analysis of fruit and vegetable consumption and lung cancer mortality in European men. Int. J. Cancer 2001, 92, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Mulder, I.; Jansen, M.C.; Smit, H.A.; Jacobs, D.R., Jr.; Menotti, A.; Nissinen, A.; Fidanza, F.; Kromhout, D. Role of smoking and diet in the cross-cultural variation in lung-cancer mortality: The seven countries study. Int. J. Cancer 2000, 88, 665–671. [Google Scholar] [CrossRef]
- Shike, M.; Russell, D.M.; Detsky, A.S.; Harrison, J.E.; McNeill, K.G.; Shepherd, F.A.; Feld, R.; Evans, W.K.; Jeejeebhoy, K.N. Changes in Body Composition in Patients with Small-Cell Lung Cancer. Ann. Intern. Med. 1984, 101, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Leedo, E.; Gade, J.; Granov, S.; Mellemgaard, A.; Klausen, T.W.; Rask, K.; Astrup, A. The Effect of a Home Delivery Meal Service of Energy- and Protein-Rich Meals on Quality of Life in Malnourished Outpatients Suffering from Lung Cancer: A Randomized Controlled Trial. Nutr. Cancer 2017, 69, 444–453. [Google Scholar] [CrossRef]
- Cheng, T.Y.; Neuhouser, M.L. Serum 25-hydroxyvitamin D, interaction with vitamin A and lung cancer mortality in the U.S. population. Cancer Causes Control. 2012, 23, 1557–1565. [Google Scholar] [CrossRef]
- Menezes, R.J.; Cheney, R.T.; Husain, A.; Tretiakova, M.; Loewen, G.; Johnson, C.S.; Jayaprakash, V.; Moysich, K.B.; Salgia, R.; Reid, M.E. Vitamin D Receptor Expression in Normal, Premalignant, and Malignant Human Lung Tissue. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 1104–1110. [Google Scholar] [CrossRef]
- Sánchez-Lara, K.; Turcott, J.G.; Juárez-Hernández, E.; Nuñez-Valencia, C.; Villanueva, G.; Guevara, P.; De la Torre-Vallejo, M.; Mohar, A.; Arrieta, O. Effects of an oral nutritional supplement containing eicosapentaenoic acid on nutritional and clinical outcomes in patients with advanced non-small cell lung cancer: Randomised trial. Clin. Nutr. 2014, 33, 1017–1023. [Google Scholar] [CrossRef]
- Arrieta, O.; Ortega, R.M.M.; Villanueva-Rodríguez, G.; Serna-Thomé, M.G.; Flores-Estrada, D.; Diaz-Romero, C.; Rodríguez, C.M.; Martínez, L.; Sánchez-Lara, K. Association of nutritional status and serum albumin levels with development of toxicity in patients with advanced non-small cell lung cancer treated with paclitaxel-cisplatin chemotherapy: A prospective study. BMC Cancer 2010, 10, 50. [Google Scholar] [CrossRef]
- Sánchez-Lara, K.; Turcott, J.G.; Juárez, E.; Guevara, P.; Núñez-Valencia, C.; Oñate-Ocaña, L.F.; Flores, D.; Arrieta, O. Association of Nutrition Parameters Including Bioelectrical Impedance and Systemic Inflammatory Response with Quality of Life and Prognosis in Patients with Advanced Non-Small-Cell Lung Cancer: A Prospective Study. Nutr. Cancer 2012, 64, 526–534. [Google Scholar] [CrossRef]
- Van der Meij, B.S.; Langius, J.A.; Spreeuwenberg, M.D.; Slootmaker, S.M.; Paul, M.A.; Smit, E.F.; van Leeuwen, P.A. Oral nutritional supplements containing n-3 polyunsaturated fatty acids affect quality of life and functional status in lung cancer patients during multi-modality treatment: An RCT. Eur. J. Clin. Nutr. 2012, 66, 399e404. [Google Scholar] [CrossRef]
- Finocchiaro, C.; Segre, O.; Fadda, M.; Monge, T.; Scigliano, M.; Schena, M.; Canuto, R.A. Effect of n-3 fatty acids on patients with advanced lung cancer: A double-blind, placebocontrolled study. Br. J. Nutr. 2012, 108, 327.e33. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.A.; Mourtzakis, M.; Chu, Q.; Baracos, V.E.; Reiman, T.; Mazurak, V.C. Nutritional intervention with fish oil provides benefit over standard of care for weight and skeletal muscle mass in patients wirh nonsmall cell lung cancer receiving chemotherapy. Cancer 2011, 1117, 1775.e82. [Google Scholar]
- Murphy, R.A.; Mourtzakis, M.; Chu, Q.S.C.; Baracos, V.E.; Reiman, T.; Mazurak, V.C. Supplementation with fish oil increases first-line chemotherapy efficacy in patients with advanced nonsmall cell lung cancer. Cancer 2011, 117, 3774–3780. [Google Scholar] [CrossRef]
- Sun, A.S.; Ostadal, O.; Ryznar, V.; Dulik, I.; Dušek, J.; Vaclavik, A.; Yeh, H.-C.; Hsu, C.; Bruckner, H.W.; Fasy, T.M. Phase I/II Study of Stage III and IV Non-Small Cell Lung Cancer Patients Taking a Specific Dietary Supplement. Nutr. Cancer 1999, 34, 62–69. [Google Scholar] [CrossRef]
- Shintani, Y.; Ikeda, N.; Matsumoto, T.; Kadota, Y.; Okumura, M.; Ohno, Y.; Ohta, M. Nutritional status of patients undergoing chemoradiotherapy for lung cancer. Asian Cardiovasc. Thorac. Ann. 2012, 20, 172–176. [Google Scholar] [CrossRef]
- Jagoe, R.; Goodship, T.H.; Gibson, G. The influence of nutritional status on complications after operations for lung cancer. Ann. Thorac. Surg. 2001, 71, 936–943. [Google Scholar] [CrossRef]
- Kaya, S.O.; Akcam, T.I.; Ceylan, K.C.; Samancılar, O.; Ozturk, O.; Usluer, O. Is preoperative protein-rich nutrition effective on postoperative outcome in non-small cell lung cancer surgery? A prospective randomized study. J. Cardiothorac. Surg. 2016, 11, 14. [Google Scholar] [CrossRef] [PubMed]
| No. | Author, Year | Study Design | Study Group | Intervention | Findings and Conclusions |
|---|---|---|---|---|---|
| 1 | Zahra et al., 2017 [52] | Clinical trial, phase 1 (ketolung) | 7 patients diagnosed with NSCLC (inoperable stage III or oligometastatic stage IV) | combination of standard radiation therapy and chemotherapy with ketogenic diet (duration: 6 wks.) | Mean actual duration of ketogenic diet use was 16.9 days (0–42 d) of the planned 42 d. Median PFS for patients who discontinued the diet early was 7.5 mos. and median OS was 22 mos. (3.7–33.3 mos.). For one known participant who completed the entire dietary intervention, median PFS was 4.6 mos, and median OS was 17.7 mos. (9.4–26 mos.). |
| 2 | Sakoda et al., 2011 [53] | Nested case–control | IG: 746 patients with lung cancer CG: 1477 participants | n/a | No relationship between diet and lung cancer risk among smokers with the 15q24-25 chromosome. |
| 3 | Lee et al., 1998 [54] | Case-control | 328 patients with lung cancer Lower-lobe tumors: 93 patients, mean age 67.1 ± 9.7 years Upper-lobe tumors: 235 patients, mean age 66.1 ± 9.7 years | n/a | Upper-lobe tumors were significantly more common in patients who consumed less vitamin E (p = 0.05) and yellow-orange vegetables (p = 0.04). |
| 4 | Jansen et al., 2001 [55] | Prospective follow-up cohort study | 3108 men from three European countries: non-smokers (never smoked or quit) + smokers eating fruit and vegetables, depending on culture and location Finland: non-smokers n = 651 smokers n = 637 Netherlands: non-smokers n = 288 smokers n = 325 Italy: non-smokers n = 591 smokers n = 616 | n/a | Over 25 years, lung cancer mortality was the highest in the Netherlands, moderate in Finland, and the lowest in Italy. Fruit intake was inversely proportional to mortality due to lung cancer in smokers (especially in the Dutch population). Vegetable consumption has no impact on lung cancer risk and mortality in smokers. |
| 5 | Mulder et al., 2000 [56] | Comparative study | 12,763 men from 7 countries and 16 cohorts, aged 40–59 years (Finland, Italy, Greece, former Yugoslavia, Japan, Netherlands, USA) | n/a | Consumption of saturated fat significantly increased lung cancer mortality in smokers (HR 1.10, 95% CI: 1.04–1.17 for an increase of 4.6 g). |
| 6 | Leedo et al., 2017 [58] | Randomized controlled clinical trial | 40 patients with lung cancer, with an NRS-2002 score of ≥3 IG (n = 21): protein- and energy-rich diet CG (n = 19): usual diet | Primary endpoint—QoL after 6 and 12 weeks, follow-up after 3 and 6 mos. Secondary endpoints—performance status, functional score, depression, symptoms, lower body strength, grip strength, body weight after 6 and 12 weeks, follow-up after 3 and 6 mos. | Increased supply of energy and protein in patients with lung cancer shows a tendency towards QoL improvement but does not produce a statistically significant effect; it may improve lower body strength and performance status. |
| 7 | Cheng et al., 2012 [59] | Non-human subjects research | Data of 16 693 male and female patients from the NHANES III study, 1988–1994. 258 subjects, 104—former smokers, 23—nonsmokers (data from the National Death Index database) | n/a | In non-smokers, 25(OH)D levels were inversely proportional to mortality due to lung cancer. Benefits were smaller in patients with abnormally high vitamin A intake or blood levels of vitamin A/beta-carotene. |
| 8 | Menezes et al., 2008 [60] | Non-human subjects research | Immunohistochemical expression of VDR in 180 precancerous or cancerous bronchial biopsies from bronchoscopy of 78 high-risk subjects, 63 tumor biopsies from 35 patients with lung cancer | n/a | No cytoplasmic VDR found in 38/61 (62%) of tumor biopsies, with nuclear expression observed in 49/62 (79%). All-sample analysis showed a positive linear trend, comparing samples with higher nuclear VDR expression and higher histologic grade (p < 0.01). The findings suggest a potential chemoprotective effect of calcitriol on the course of lung cancer. |
| 9 | Sánchez-Lara et al., 2014 [61] | Randomized clinical trial | 92 patients with advanced NSCLC: IG: 46 (ONS-EPA) CG: 46 (normocaloric diet) | Comparison of an isocaloric diet and diet enriched with ONS-EPA in patients undergoing paclitaxel + cisplatin/carboplatin chemotherapy Response to chemotherapy and overall survival was measured. | IG diet provided much more protein and calories than CG diet. IG gained 1.65 kg of lean body weight; CG lost 2.06. Symptoms, i.e., fatigue, appetite loss, and neuropathy decreased in IG (p ≤ 0.05). The groups did not differ in terms of overall survival. |
| 10 | Arrieta et al., 2010 [62] | Prospective study | 100 patients with CS-IV NSCLC receiving paclitaxel (175 mg/m2) and cisplatin (80 mg/m2) chemotherapy (mean age 58 ± 10 years). SGA was used to determine nutritional status before treatment. | n/a | Malnutrition in 51% of patients, albumin concentration of ≤3.0 mg/mL in 50%. Neutrophil-lymphocyte ratio ≥ 5 associated with hypoalbuminemia (mean ranks, 55.7 vs. 39 p = 0.006), ECOG = 2 (47.2 vs. 55.4 p = 0.026), and platelet-lymphocyte ratio ≥ 150 significantly correlated with BMI ≤20 (56.6 vs. 43.5; p = 0.02) and hypoalbuminemia (58.9 vs. 41.3; p = 0.02). Malnourished and hypoalbuminemic patients had a higher likelihood of developing symptoms of chemotherapy toxicity than normally nourished patients (31 vs. 22; p = 0.02) and those with normal albumin levels (mean ranks, 62 vs. 43; p = 0.002). Chemotherapy induces more adverse effects in malnourished and hypoalbuminemic NSCLC patients. |
| 11 | Sánchez-Lara et al., 2012 [63] | Prospective study | 119 patients; 55 female, 64 male (median age 60.5 ± 12.5 y/o, mean BMI 24.8 ± 4.5 kg/m2) (A) 40.3% normally nourished per the SGA (B) 32.8% at risk of malnutrition (C) 26.9% severely malnourished Albumin levels were 1.7–4.5 mg/dL, mean 3.3 ± 0.5 mg/dL. Median NLR and PLR 4.7 ± 4.6 and 231.2 ± 162.3, respectively, median CRP level 3.9 mg/dL. Serum proinflammatory factor levels 18.4 ± 31.7 and 21.16 ± 6.4 pg/mL, respectively, for TNF and IL-6. | n/a | Measurement of pre-treatment nutritional parameters contributes to prognosis in patients with lung cancer; this may be a first step towards the unification of studies on the introduction of interventions to improve HRQoL and overall survival. In summary, malnutrition results in poorer HRQoL, the latter being an independent prognostic variable in advanced NSCLC. |
| 12 | Van der Meij et al., 2012 [64] | Randomized clinical trial | IG/CG (20/20): 40 patients with CS-III NCSLC | IG: 2 cans/d of calorie- and protein-rich oral nutritional supplements containing n-3 PUFAs CG: isocaloric nutritional supplements. | IG had significantly higher QoL, physical and cognitive function parameters (p < 0.01), overall health (p = 0.04), and social functioning (p = 0.04) than CG after 5 weeks. Higher KPS (p = 0.04) observed in IG than CG after 3 weeks. No significant differences in grip strength between the groups. IG patients were more physically active than CG patients after 3 and 5 weeks (p = 0.05). In summary, n-3 PUFAs may positively influence QoL, functioning, and physical activity in NSCLC patients receiving multimodal cancer treatment. |
| 13 | Finocchiaro et al., 2012 [65] | Randomized double-blind clinical trial | 33 patients aged 46–70 IG: 13 patients out of 19 who completed the trial, aged 46–66 (mean 55.56, SD 7.35 yrs.), took a supplement rich in n-3 fatty acids CG: 14 patients aged 50–70 (mean 60.57, SD 7.43 years) | Clinical condition, inflammation, and oxidative stress levels compared over 66 days of observation, until the end of chemotherapy. | Body weight increased significantly in IG patients, who also had reduced systemic inflammatory response and oxidative stress. |
| 14 | Murphy et al., 2011 [66] | Clinical trial | 40 patients with advanced NSCLC, from diagnosis to completion of 1st line chemotherapy. | IG (n = 16) supplemented with fish oil at 2.2 g EPA/d CG (n = 24) standard of care Muscle and fat tissue content was compared using computed tomography. Data on blood counts and body weight were collected throughout the treatment period. | In the CG, mean body weight loss was 2.3 ± 0.9 kg, compared to 0.5 ± 1.0 kg in the IG (p = 0.05). Patients with a greater increase in blood EPA levels in the IG achieved greater benefits in terms of muscle mass (p = 0.01). Muscle mass gain or maintenance recorded in about 69% of patients from IG. In total, 1 kg total muscle loss observed in the SOC group, and only 29% of patients in that group maintained their muscle mass. No statistically significant change in fat tissue was found in either group. In summary, the nutritional intervention with 2.2 g of EPA daily seems superior to standard care, resulting in the maintenance of body weight and muscle mass during chemotherapy. |
| 15 | Murphy et al., 2011b [67] | Clinical trial | 46 patients with advanced NSCLC | IG (n = 15) supplemented with fish oil at 2.5 g EPA and DHA/d during chemotherapy (carboplatin + vinorelbine or gemcitabine) CG (n = 31) standard of care | Greater clinical benefit in IG than in CG (60.0% vs. 25.8%, p = 0.008; 80.0% vs, 41.9%, p = 0.02) No difference in DLT incidence between the two groups (p = 0.46). One-year survival seemed higher in IG (60.0% vs. 38.7%, p = 0.15). In summary, supplementation with EPA/DHA-rich fish oil is associated with increased effectiveness of chemotherapy, with no impact on toxicity profile, and may contribute to overall survival. |
| 16 | Sun et al., 1999 [68] | Clinical trial | CG: 5 patients stage IV, 4 stage IIIB, 4 stage IIIA (54.3 ± 8.8). SVG: 6 patients: 2 stage IV, 3 stage IIIB, 1 stage IIIA (49.2 ± 4.7). | Vegetables with anti-tumor components (SV) were added to the daily diet of TG and SVG, and not added to the diet of CG. | KPS decreased in CG patients but increased in SVG patients within 1–3 mos. of inclusion in the study. Changes in body weight observed for CG, SVG, and TG were as follows: –12 ± 5%, –2 ± 2%, and +4 ± 4%. The median survival time and mean survival were 4 and 4.8 mos. in the CG but 15.5 and 15 mos. in the SVG (p < 0.01). The authors found that the addition of SV to the daily diet of NSCLC patients is not toxic and is related to enhanced body weight maintenance, KPS, and survival in patients with stage III and IV NSCLC. |
| 17 | Shintani et al., 2012 [69] | Comparative study | IG: 15 patients (aged 62 ± 8 years) with resectable clinical N2 or N3 NSCLC CG: 15 patients (aged 62 ± 6 years) with resectable clinical N2 or N3 NSCLC | IG: received Impact orally (750–1000 mL daily) for 5 days prior to surgery; amount was specified by dietitians based on each patient’s diet during this period. CG: conventional diet before surgery. | Patients undergoing chemoradiotherapy had lower lymphocyte counts, lower BMI, and a higher risk of serious postoperative complications compared to those not undergoing chemoradiotherapy. After chemoradiotherapy, the postoperative decrease in transferrin levels and lymphocyte counts was reduced in the IG. Immunonutrition administered before surgery may enhance perioperative nutritional status after first-line chemoradiotherapy in patients undergoing surgery for lung cancer. Such intervention can also contribute to less severe postoperative complications. |
| 18 | Jagoe et al., 2001 [70] | Prospective study | 52 patients undergoing surgical lung cancer resection in the years 1995–1997. | n/a | Impaired respiration was associated with nutritional status, poorer lung function, and lower maximum expiratory pressure. |
| 19 | Kaya et al., 2016 [71] | Randomized clinical trial | 58 patients treated with lung resection | IG: n = 31; immunomodulating formulas (with n-3 fatty acids, arginine and nucleotides) for 10 days; n = 20—anatomic resection by thoracotomy; n = 11—videothoracoscopy CG: n = 27; normal diet; n = 16—thoracotomy; n = 11—videothoracoscopy | Three days after the surgery, a decrease in albumin levels down to 25.71% of the baseline was observed in the CG. In contrast, the decrease was limited to 14.69% in the IG (p < 0.001). In total, 12 patients from the CG experienced complications (44.4%) against 6 patients in the IG (p = 0.049). The mean chest tube drainage duration was 6 (1–42) days and 4 (2–15) days for the CG and IG, respectively (p = 0.019). |
| 20 | Shike et al., 1984 [57] | Clinical trial | 31 patients undergoing chemotherapy for SCLC: IG: 15 patients aged 57.5 ± 2.2 years CG: 16 patients aged 60.4 ± 2.05 years | Total parenteral nutrition for 4 weeks (IG) or continued self-regulated oral diet (CG). | Significant increase in body weight, total adipose tissue, and total body potassium in the IG over those 4 weeks (p < 0.001). No such increase was observed for total body nitrogen. Once parenteral nutrition was discontinued, both body weight and potassium levels in the IG decreased significantly, just as in the CG. Overall, both groups saw a decrease in nitrogen concentrations during the 32 weeks of study. CG experienced a significantly larger adipose tissue reduction compared to the IG once the parenteral nutrition period was over (p < 0.05). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polański, J.; Świątoniowska-Lonc, N.; Kołaczyńska, S.; Chabowski, M. Diet as a Factor Supporting Lung Cancer Treatment—A Systematic Review. Nutrients 2023, 15, 1477. https://doi.org/10.3390/nu15061477
Polański J, Świątoniowska-Lonc N, Kołaczyńska S, Chabowski M. Diet as a Factor Supporting Lung Cancer Treatment—A Systematic Review. Nutrients. 2023; 15(6):1477. https://doi.org/10.3390/nu15061477
Chicago/Turabian StylePolański, Jacek, Natalia Świątoniowska-Lonc, Sylwia Kołaczyńska, and Mariusz Chabowski. 2023. "Diet as a Factor Supporting Lung Cancer Treatment—A Systematic Review" Nutrients 15, no. 6: 1477. https://doi.org/10.3390/nu15061477
APA StylePolański, J., Świątoniowska-Lonc, N., Kołaczyńska, S., & Chabowski, M. (2023). Diet as a Factor Supporting Lung Cancer Treatment—A Systematic Review. Nutrients, 15(6), 1477. https://doi.org/10.3390/nu15061477






