Increased Dietary Intakes of Total Protein, Animal Protein and White Meat Protein Were Associated with Reduced Bone Loss—A Prospective Analysis Based on Guangzhou Health and Nutrition Cohort, South China
Abstract
1. Introduction
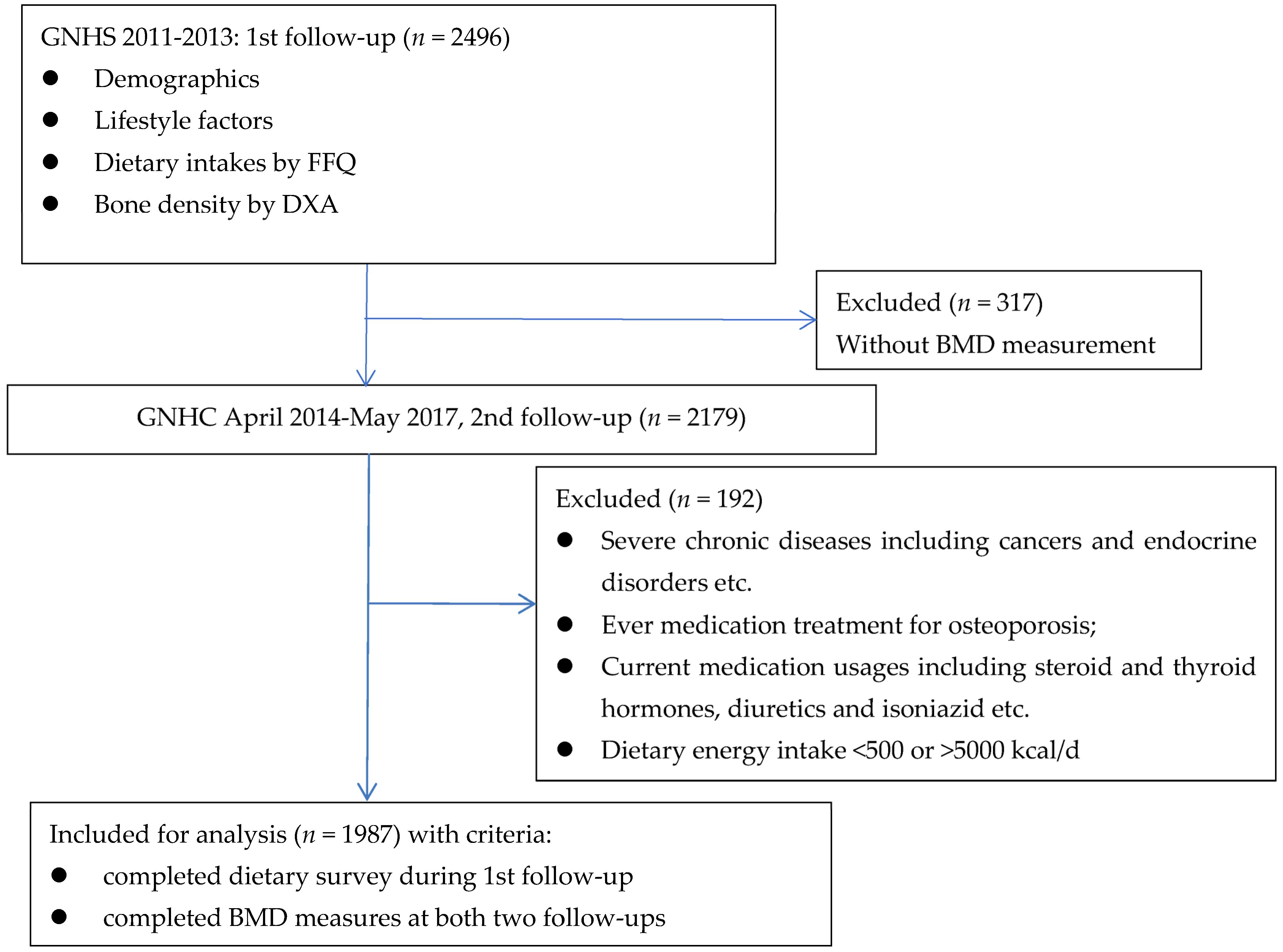
2. Methodology
2.1. Questionnaire Survey and Anthropometric Measures
2.2. Dietary Assessment
2.3. Bone Mineral Density (BMD) Measurements
2.4. Statistical Analysis
3. Results
Participants’ Characteristics in the First and Second Trimesters
4. Discussion
4.1. Summary of Current Findings
4.2. Protein Intake among the Elderly Chinese Population
4.3. Mechanisms of White Meat Protein and the Slowing Down of Bone Loss
4.4. Study Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, F.; Xia, W. The epidemiology of osteoporosis, associated fragility fractures, and management gap in China. Arch. Osteoporos. 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Kung, A.W.; Fan, T.; Xu, L.; Xia, W.B.; Park, I.H.; Kim, H.S.; Chan, S.P.; Lee, J.K.; Koh, L.; Soong, Y.K.; et al. Factors influencing diagnosis and treatment of osteoporosis after a fragility fracture among postmenopausal women in Asian countries: A retrospective study. BMC Womens Health 2013, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R.; Biver, E.; Bonjour, J.P.; Coxam, V.; Goltzman, D.; Kanis, J.A.; Lappe, J.; Rejnmark, L.; Sahni, S.; Weaver, C.; et al. Benefits and safety of dietary protein for bone health-an expert consensus paper endorsed by the European Society for Clinical and Economical Aspects of Osteoporosis, Osteoarthritis, and Musculoskeletal Diseases and by the International Osteoporosis Foundation. Osteoporos. Int. 2018, 29, 1933–1948. [Google Scholar] [CrossRef] [PubMed]
- Bonjour, J.P. Protein intake and bone health. Int. J. Vitam. Nutr. Res. 2011, 81, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.C.; Frankenfeld, C.L. Dietary protein intake above the current rda and bone health: A systematic review and meta-analysis. J. Am. Coll. Nutr. 2017, 36, 481–496. [Google Scholar] [CrossRef]
- Thorpe, M.P.; Evans, E.M. Dietary protein and bone health: Harmonizing conflicting theories. Nutr. Rev. 2011, 69, 215–230. [Google Scholar] [CrossRef]
- Groenendijk, I.; den Boeft, L.; van Loon, L.J.C.; de Groot, L. High versus low dietary protein intake and bone health in older adults: A systematic review and meta-analysis. Comput. Struct. Biotechnol. J. 2019, 17, 1101–1112. [Google Scholar] [CrossRef]
- Darling, A.L.; Manders, R.J.F.; Sahni, S.; Zhu, K.; Hewitt, C.E.; Prince, R.L.; Millward, D.J.; Lanham-New, S.A. Dietary protein and bone health across the life-course: An updated systematic review and meta-analysis over 40 years. Osteoporos. Int. 2019, 30, 741–761. [Google Scholar] [CrossRef] [PubMed]
- Shams-White, M.M.; Chung, M.; Fu, Z.; Insogna, K.L.; Karlsen, M.C.; LeBoff, M.S.; Shapses, S.A.; Sackey, J.; Shi, J.; Wallace, T.C.; et al. Animal versus plant protein and adult bone health: A systematic review and meta-analysis from the National Osteoporosis Foundation. PLoS ONE 2018, 13, e0192459. [Google Scholar] [CrossRef]
- Remer, T.; Krupp, D.; Shi, L. Dietary protein’s and dietary acid load’s influence on bone health. Crit. Rev. Food Sci. Nutr. 2014, 54, 1140–1150. [Google Scholar] [CrossRef]
- Mangano, K.M.; Walsh, S.J.; Kenny, A.M.; Insogna, K.L.; Kerstetter, J.E. Dietary acid load is associated with lower bone mineral density in men with low intake of dietary calcium. J. Bone Miner. Res. 2014, 29, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.D.; Zhu, Y.Y.; Cao, Y.; Liu, J.; Shi, W.Q.; Liu, Z.M.; Chen, Y.M. Association of dietary consumption and serum levels of vitamin A and beta-carotene with bone mineral density in Chinese adults. Bone 2015, 79, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.X.; Ho, S.C. Validity and reproducibility of a food frequency Questionnaire among Chinese women in Guangdong province. Asia Pac. J. Clin. Nutr. 2009, 18, 240–250. [Google Scholar] [PubMed]
- Yang, Y.X.; Wang, Y.G.; Pan, X.C. Chinese Food Composition Table, 2nd ed.; The Chinese Center for Diseases Prevention and Control, Ed.; Institute of Nutrition and Food Safety, Beijing Medical University: Beijing, China, 2009; p. 384. [Google Scholar]
- Crandall, C.J.; Larson, J.; Wright, N.C.; Laddu, D.; Stefanick, M.L.; Kaunitz, A.M.; Watts, N.B.; Wactawski-Wende, J.; Womack, C.R.; Johnson, K.C.; et al. Serial Bone Density Measurement and Incident Fracture Risk Discrimination in Postmenopausal Women. JAMA Intern. Med. 2020, 180, 1232–1240. [Google Scholar] [CrossRef]
- Phillips, S.M. Current Concepts and Unresolved Questions in Dietary Protein Requirements and Supplements in Adults. Front. Nutr. 2017, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Deutz, N.E.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznariç, Z.; Nair, K.S.; et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef]
- Rizzoli, R.; Biver, E.; Brennan-Speranza, T.C. Nutritional intake and bone health. Lancet Diabetes Endocrinol. 2021, 9, 606–621. [Google Scholar] [CrossRef]
- Protein and Amino Acid Requirements in Human Nutrition; World Health Organization Technical Report Series; World Health Organization: Geneva, Switzerland, 2007; pp. 1–265.
- Beasley, J.M.; LaCroix, A.Z.; Larson, J.C.; Huang, Y.; Neuhouser, M.L.; Tinker, L.F.; Jackson, R.; Snetselaar, L.; Johnson, K.C.; Eaton, C.B.; et al. Biomarker-calibrated protein intake and bone health in the Women’s Health Initiative clinical trials and observational study. Am. J. Clin. Nutr. 2014, 99, 934–940. [Google Scholar] [CrossRef]
- Steell, L.; Sillars, A.; Welsh, P.; Iliodromiti, S.; Wong, S.C.; Pell, J.P.; Sattar, N.; Gill, J.M.R.; Celis-Morales, C.A.; Gray, S.R. Associations of dietary protein intake with bone mineral density: An observational study in 70,215 UK Biobank participants. Bone 2019, 120, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Shams-White, M.M.; Chung, M.; Du, M.; Fu, Z.; Insogna, K.L.; Karlsen, M.C.; LeBoff, M.S.; Shapses, S.A.; Sackey, J.; Wallace, T.C.; et al. Dietary protein and bone health: A systematic review and meta-analysis from the National Osteoporosis Foundation. Am. J. Clin. Nutr. 2017, 105, 1528–1543. [Google Scholar] [CrossRef] [PubMed]
- Dawson-Hughes, B. Calcium and protein in bone health. Proc. Nutr. Soc. 2003, 62, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Liu, S.; Xia, J.; Xu, L.; Cheng, Y.; Li, W.; Zhang, Y.; Wang, G.; Wei, W.; Shi, H.; et al. The Mechanism of Dietary Protein Modulation of Bone Metabolism via Alterations in Members of the GH/IGF Axis. Curr. Protein Pept. Sci. 2019, 20, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Langsetmo, L.; Shikany, J.M.; Cawthon, P.M.; Cauley, J.A.; Taylor, B.C.; Vo, T.N.; Bauer, D.C.; Orwoll, E.S.; Schousboe, J.T.; Ensrud, K.E.; et al. The Association Between Protein Intake by Source and Osteoporotic Fracture in Older Men: A Prospective Cohort Study. J. Bone Miner. Res. 2017, 32, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.M.; Huang, Q.; Li, S.Y.; Liu, Y.P.; Wu, Y.; Zhang, S.J.; Li, B.L.; Chen, Y.M. A 1:1 matched case-control study on dietary protein intakes and hip fracture risk in Chinese elderly men and women. Osteoporos. Int. 2021, 32, 2205–2216. [Google Scholar] [CrossRef]
- Groenendijk, I.; Grootswagers, P.; Santoro, A.; Franceschi, C.; Bazzocchi, A.; Meunier, N.; Caille, A.; Malpuech-Brugere, C.; Bialecka-Debek, A.; Pietruszka, B.; et al. Protein intake and bone mineral density: Cross-sectional relationship and longitudinal effects in older adults. J. Cachexia Sarcopenia Muscle 2023, 14, 116–125. [Google Scholar] [CrossRef]
- Li, C.Y.; Fang, A.P.; Ma, W.J.; Wu, S.L.; Li, C.L.; Chen, Y.M.; Zhu, H.L. Amount Rather than Animal vs Plant Protein Intake Is Associated with Skeletal Muscle Mass in Community-Dwelling Middle-Aged and Older Chinese Adults: Results from the Guangzhou Nutrition and Health Study. J. Acad. Nutr. Diet 2019, 119, 1501–1510. [Google Scholar] [CrossRef]
- Deane, C.S.; Bass, J.J.; Crossland, H.; Phillips, B.E.; Atherton, P.J. Animal, Plant, Collagen and Blended Dietary Proteins: Effects on Musculoskeletal Outcomes. Nutrients 2020, 12, 2670. [Google Scholar] [CrossRef] [PubMed]
- Cuenca-Sanchez, M.; Navas-Carrillo, D.; Orenes-Pinero, E. Controversies surrounding high-protein diet intake: Satiating effect and kidney and bone health. Adv. Nutr. 2015, 6, 260–266. [Google Scholar] [CrossRef]
- Fenton, T.R.; Lyon, A.W. Milk and acid-base balance: Proposed hypothesis versus scientific evidence. J. Am. Coll. Nutr. 2011, 30, 471s–475s. [Google Scholar] [CrossRef]
- Chen, H.; Kubo, K.Y. Bone three-dimensional microstructural features of the common osteoporotic fracture sites. World J. Orthop. 2014, 5, 486–495. [Google Scholar] [CrossRef]
- Wang, T.; He, C. TNF-α and IL-6: The Link between Immune and Bone System. Curr. Drug Targets 2020, 21, 213–227. [Google Scholar] [CrossRef]
- Bonjour, J.P. The dietary protein, IGF-I, skeletal health axis. Horm. Mol. Biol. Clin. Investig. 2016, 28, 39–53. [Google Scholar] [CrossRef]
- Su, Y.; Elshorbagy, A.; Turner, C.; Refsum, H.; Chan, R.; Kwok, T. Circulating amino acids are associated with bone mineral density decline and ten-year major osteoporotic fracture risk in older community-dwelling adults. Bone 2019, 129, 115082. [Google Scholar] [CrossRef] [PubMed]
- Jennings, A.; MacGregor, A.; Spector, T.; Cassidy, A. Amino Acid Intakes Are Associated With Bone Mineral Density and Prevalence of Low Bone Mass in Women: Evidence From Discordant Monozygotic Twins. J. Bone Miner. Res. 2016, 31, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, V.; Khandelwal, M.; Manglani, K.; Gupta, S.; Surolia, A. Methionine down-regulates TLR4/MyD88/NF-κB signalling in osteoclast precursors to reduce bone loss during osteoporosis. Br. J. Pharmacol. 2014, 171, 107–121. [Google Scholar] [CrossRef]
- Sharan, K.; Lewis, K.; Furukawa, T.; Yadav, V.K. Regulation of bone mass through pineal-derived melatonin-MT2 receptor pathway. J. Pineal. Res. 2017, 63, e12423. [Google Scholar] [CrossRef]
- Zhao, Q.; Shen, H.; Su, K.J.; Zhang, J.G.; Tian, Q.; Zhao, L.J.; Qiu, C.; Zhang, Q.; Garrett, T.J.; Liu, J.; et al. Metabolomic profiles associated with bone mineral density in US Caucasian women. Nutr. Metab. 2018, 15, 57. [Google Scholar] [CrossRef]
- Miyamoto, T.; Hirayama, A.; Sato, Y.; Koboyashi, T.; Katsuyama, E.; Kanagawa, H.; Miyamoto, H.; Mori, T.; Yoshida, S.; Fujie, A.; et al. A serum metabolomics-based profile in low bone mineral density postmenopausal women. Bone 2017, 95, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Hyeon, J.; Lee, S.A.; Kwon, S.O.; Lee, H.; Keum, N.; Lee, J.K.; Park, S.M. Role of total, red, processed, and white meat consumption in stroke incidence and mortality: A systematic review and meta-analysis of prospective cohort studies. J. Am. Heart Assoc. 2017, 6, e005983. [Google Scholar] [CrossRef] [PubMed]
- Rosendahl-Riise, H.; Sulo, G.; Karlsson, T.; Drevon, C.; Dierkes, J.; Tell, G. The limited benefit of fish consumption on risk of hip fracture among men in the community-based Hordaland health study. Nutrients 2018, 10, 873. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Ho, S.C.; Lam, S.S. Higher sea fish intake is associated with greater bone mass and lower osteoporosis risk in postmenopausal Chinese women. Osteoporos. Int. 2010, 21, 939–946. [Google Scholar] [CrossRef]
- Palomeras-Vilches, A.; Viñals-Mayolas, E.; Bou-Mias, C.; Jordà-Castro, M.; Agüero-Martínez, M.; Busquets-Barceló, M.; Pujol-Busquets, G.; Carrion, C.; Bosque-Prous, M.; Serra-Majem, L.; et al. Adherence to the Mediterranean diet and bone fracture risk in middle-aged women: A case control study. Nutrients 2019, 11, 2508. [Google Scholar] [CrossRef]
- Pérez-Rey, J.; Roncero-Martín, R.; Rico-Martín, S.; Rey-Sánchez, P.; Pedrera-Zamorano, J.D.; Pedrera-Canal, M.; López-Espuela, F.; Lavado García, J.M. Adherence to a Mediterranean diet and bone mineral density in spanish premenopausal women. Nutrients 2019, 11, 555. [Google Scholar] [CrossRef]
- Virtanen, J.; Mozaffarian, D.; Cauley, J.; Mukamal, K.; Robbins, J.; Siscovick, D. Fish consumption, bone mineral density, and risk of hip fracture among older adults: The cardiovascular health study. J. Bone Miner. Res. 2010, 25, 1972–1979. [Google Scholar] [CrossRef] [PubMed]
- Rodopaios, N.E.; Mougios, V.; Koulouri, A.A.; Vasara, E.; Papadopoulou, S.K.; Skepastianos, P.; Hassapidou, M.; Kafatos, A.G. Dietary protein intake from different animal and plant sources plays a minor role in the bone health of adults with or without intermittent fasting for decades. Int. J. Food Sci. Nutr. 2021, 72, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Park, Y. The Association between the Consumption of Fish/Shellfish and the Risk of Osteoporosis in Men and Postmenopausal Women Aged 50 Years or Older. Nutrients 2016, 8, 113. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, O.; Djafarian, K.; Ghorabi, S.; Khodadost, M.; Nasiri, M.; Shab-Bidar, S. Dietary intake of fish, n-3 polyunsaturated fatty acids and risk of hip fracture: A systematic review and meta-analysis on observational studies. Crit. Rev. Food Sci. Nutr. 2019, 59, 1320–1333. [Google Scholar] [CrossRef]
- Chen, J.; Jayachandran, M.; Bai, W.; Xu, B. A critical review on the health benefits of fish consumption and its bioactive constituents. Food Chem. 2022, 369, 130874. [Google Scholar] [CrossRef]
- The Food and Agriculture Organization of the United Nations (FAO). Poultry Development Review. 2013. Available online: https://www.fao.org/3/i3531e/i3531e.pdf (accessed on 20 December 2022).
- Cross, A.J.; Pollock, J.R.; Bingham, S.A. Haem, not protein or inorganic iron, is responsible for endogenous intestinal N-nitrosation arising from red meat. Cancer Res. 2003, 63, 2358–2360. [Google Scholar]
- Misra, R.; Balagopal, P.; Raj, S.; Patel, T.G. Red Meat Consumption (Heme Iron Intake) and Risk for Diabetes and Comorbidities? Curr. Diab. Rep. 2018, 18, 100. [Google Scholar] [CrossRef]
- Goethals, S.; Rombouts, C.; Hemeryck, L.Y.; Van Meulebroek, L.; Van Hecke, T.; Vossen, E.; Van Camp, J.; De Smet, S.; Vanhaecke, L. Untargeted Metabolomics to Reveal Red versus White Meat-Associated Gut Metabolites in a Prudent and Western Dietary Context. Mol. Nutr. Food Res. 2020, 64, e2000070. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liang, S.; Chen, X.; Yang, J.; Zhou, Y.; Du, L.; Li, K. Red/processed meat consumption and non-cancer-related outcomes in humans: Umbrella review. Br. J. Nutr. 2022, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Palermo, A.; Tuccinardi, D.; Defeudis, G.; Watanabe, M.; D’Onofrio, L.; Lauria Pantano, A.; Napoli, N.; Pozzilli, P.; Manfrini, S. BMI and BMD: The Potential Interplay between Obesity and Bone Fragility. Int. J. Environ. Res. Public Health 2016, 13, 544. [Google Scholar] [CrossRef] [PubMed]
- Gkastaris, K.; Goulis, D.G.; Potoupnis, M.; Anastasilakis, A.D.; Kapetanos, G. Obesity, osteoporosis and bone metabolism. J. Musculoskelet. Neuronal. Interact. 2020, 20, 372–381. [Google Scholar] [PubMed]
| Total Protein Intakes (g·kg−1·d−1) | ||||||
|---|---|---|---|---|---|---|
| Q1 (<0.96) | Q2 (0.96~) | Q3 (1.10~) | Q4 (≥1.26) | Total | p | |
| n | 497 | 497 | 497 | 496 | 1987 | |
| Age, year | 60.3 ± 5.0 | 60.4 ± 4.7 | 60.3 ± 4.8 | 60.1 ± 5.1 | 60.3 ± 4.9 | 0.757 |
| Women, n (%) | 327 (65.8) | 347 (69.8) | 359 (72.2) | 391 (78.8) | 1424 (71.7) | <0.001 |
| BMI, kg/m2 | 26.1 ± 3.1 | 24.1 ± 2.4 | 22.8 ± 2.3 | 21.1 ± 2.4 | 23.5 ± 3.2 | <0.001 |
| Income ≤ 3000 Yuan·month−1·person−1, n (%) | 288 (58.3) | 268 (54.1) | 277 (56.1) | 309 (62.3) | 1142 (57.5) | 0.126 |
| Married, n (%) | 447 (89.9) | 452 (91.1) | 433 (87.1) | 427 (86.1) | 1759 (88.5) | 0.043 |
| Smoking, n (%) | 58 (11.7) | 37 (7.4) | 40 (8.0) | 30 (6.0) | 165 (8.3) | 0.011 |
| Habitual alcohol drinking, n (%) | 41 (8.2) | 35 (7.0) | 36 (7.2) | 33 (6.7) | 145 (7.3) | 0.796 |
| Habitual tea drinking, n (%) | 299 (60.2) | 274 (55.1) | 262 (52.7) | 248 (50.0) | 1083 (54.5) | 0.011 |
| Calcium supplements, n (%) | 150 (30.2) | 138 (27.8) | 153 (30.8) | 152 (30.6) | 593 (29.8) | 0.716 |
| Medical history | ||||||
| Hypertension, n (%) | 146 (29.4) | 122 (24.6) | 126 (25.5) | 112 (22.6) | 506 (25.5) | 0.094 |
| Stroke, n (%) | 10 (2.0) | 8 (1.6) | 9 (1.8) | 9 (1.8) | 36 (1.8) | 0.973 |
| Hyperlipidemia, n (%) | 192 (38.7) | 207 (41.7) | 188 (38.0) | 191 (38.6) | 778 (39.3) | 0.621 |
| HRT, n (%) women only | 14 (4.3) | 27 (7.8) | 20 (5.6) | 33 (8.4) | 94 (6.6) | 0.095 |
| Multivitamin usage, n (%) | 85 (17.1) | 87 (17.5) | 111 (22.3) | 109 (22.0) | 392 (19.7) | 0.059 |
| Total physical activity, Mets/week | 24.1 ± 6.1 | 25.3 ± 6.4 | 25.2 ± 6.4 | 25.7 ± 6.8 | 25.1 ± 6.5 | 0.001 |
| Dietary intakes | ||||||
| Total energy, kcal/d | 1663.8 ± 496.9 | 1595.1 ± 456.2 | 1629.5 ± 460.2 | 1591.8 ± 531.8 | 1620.1 ± 487.7 | 0.067 |
| Fat, % total energy | 31.2 ± 14.4 | 32.1 ± 16.3 | 31.6 ± 14.8 | 32.5 ± 15.6 | 31.9 ± 15.3 | 0.552 |
| Carbohydrate, % total energy | 57.6 ± 22.1 | 59.1 ± 25.4 | 58.3 ± 24.9 | 61.2 ± 25.8 | 59.1 ± 24.6 | 0.110 |
| Total protein, % total energy | 14.9 ± 4.7 | 16.9 ± 4.9 | 17.8 ± 5.5 | 20.3 ± 6.6 | 17.5 ± 5.8 | <0.001 |
| Animal protein, % total protein | 43.59 ± 11.26 | 47.93 ± 10.85 | 50.79 ± 10.92 | 54.22 ± 11.12 | 49.13 ± 11.70 | <0.001 |
| Calcium, mg/d | 447.1 ± 129.1 | 510.1 ± 147.1 | 529.1 ± 153.1 | 564.3 ± 183.0 | 512.3 ± 159.7 | <0.001 |
| Vitamin D, μg/d | 3.44 ± 2.20 | 3.75 ± 2.29 | 4.16 ± 3.20 | 4.08 ± 2.88 | 3.86 ± 2.69 | <0.001 |
| Total protein, g·kg−1·d−1 | 0.85 ± 0.09 | 1.04 ± 0.04 | 1.19 ± 0.05 | 1.46 ± 0.17 | 1.13 ± 0.24 | <0.001 |
| Animal protein, g·kg−1·d−1 | 0.37 ± 0.11 | 0.50 ± 0.12 | 0.60 ± 0.13 | 0.80 ± 0.21 | 0.57 ± 0.21 | <0.001 |
| Red-meat protein, g·kg−1·d−1 | 0.15 ± 0.08 | 0.19 ± 0.09 | 0.23 ± 0.11 | 0.30 ± 0.17 | 0.22 ± 0.13 | <0.001 |
| White-meat protein, g·kg−1·d−1 | 0.13 ± 0.07 | 0.18 ± 0.08 | 0.22 ± 0.10 | 0.32 ± 0.17 | 0.21 ± 0.13 | <0.001 |
| Milk and dairy protein, g·kg−1·d−1 | 0.05 ± 0.04 | 0.07 ± 0.06 | 0.08 ± 0.07 | 0.09 ± 0.09 | 0.07 ± 0.07 | <0.001 |
| Egg protein, g·kg−1·d−1 | 0.05 ± 0.03 | 0.06 ± 0.04 | 0.07 ± 0.05 | 0.09 ± 0.06 | 0.07 ± 0.05 | <0.001 |
| Plant protein, g·kg−1·d−1 | 0.48 ± 0.10 | 0.54 ± 0.11 | 0.58 ± 0.13 | 0.66 ± 0.16 | 0.57 ± 0.15 | <0.001 |
| Soy protein, g·kg−1·d−1 | 0.05 ± 0.04 | 0.06 ± 0.05 | 0.08 ± 0.07 | 0.11 ± 0.11 | 0.07 ± 0.08 | <0.001 |
| Changes of BMD, 3 mg/cm2 | ||||||
| Whole body | −262.4 ± 145.0 | −286.7 ± 148.0 | −287.9 ± 207.7 | −323.4 ± 151.1 | −290.1 ± 166.3 | <0.001 |
| Spine L1–L4 | 262.4 ± 152.1 | 284.6 ± 148.6 | 281.8 ± 164.0 | 307.0 ± 152.0 | 283.9 ± 155.0 | <0.001 |
| Total hip | −40.4 ± 252.1 | −7.0 ± 251.7 | −8.7 ± 233.2 | 16.4 ± 225.3 | −10.0 ± 241.5 | 0.003 |
| Femur neck | −456.8 ± 155.4 | −434.8 ± 160.7 | −431.4 ± 177.6 | −403.0 ± 136.5 | −431.5 ± 159.3 | <0.001 |
| Trochanter | −15.9 ± 36.6 | −14.7 ± 35.1 | −15.9 ± 48.8 | −12.1 ± 30.9 | −14. 7 ± 38. 5 | 0.356 |
| Changes in Bone Mineral Density (mg/cm2) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Protein Intakes, g·kg−1·d−1 | Whole Body | Spine L1–L4 | Total Hip | Femur Neck | Trochanter | |||||
| B ± SE | β | B ± SE | β | B ± SE | β | B ± SE | β | B ± SE | β | |
| Total protein | −4.71 ± 20.86 | −0.007 | 8.54 ± 19.21 | 0.014 | 31.28 ± 30.51 | 0.032 | 67.79 ± 20.07 *** | 0.104 | 18.73 ± 4.90 *** | 0.118 |
| Animal protein | 11.11 ± 20.12 | 0.014 | −7.99 ± 18.52 | −0.011 | 34.66 ± 29.44 | 0.031 | 53.61 ± 19.37 ** | 0.073 | 12.05 ± 4.73 * | 0.067 |
| Red-meat protein | −5.09 ± 30.46 | −0.004 | 12.90 ± 28.04 | 0.011 | 29.06 ± 44.61 | 0.016 | 18.16 ± 29.38 | 0.015 | 5.85 ± 7.18 | 0.020 |
| White−meat protein | 23.89 ± 30.16 | 0.019 | −30.61 ± 27.77 | −0.026 | 65.55 ± 44.26 | 0.036 | 89.44 ± 29.03 ** | 0.074 | 19.77 ± 7.10 ** | 0.067 |
| Milk and dairy protein | 61.19 ± 80.67 | 0.025 | −25.16 ± 74.28 | −0.011 | −85.04 ± 117.87 | −0.024 | 76.60 ± 77.82 | 0.033 | 12.29 ± 19.01 | 0.022 |
| Egg protein | −20.46 ± 86.76 | −0.006 | 28.92 ± 79.88 | 0.009 | −31.51 ± 126.90 | −0.006 | 21.43 ± 83.71 | 0.006 | −1.01 ± 20.45 | −0.001 |
| Plant protein | −40.51 ± 32.53 | −0.035 | 41.69 ± 29.95 | 0.039 | −14.45 ± 47.64 | −0.009 | 24.67 ± 31.39 | 0.022 | 14.04 ± 7.66 | 0.052 |
| Soy protein | −47.73 ± 52.49 | −0.022 | 19.27 ± 48.34 | 0.009 | 93.98 ± 76.79 | 0.029 | −28.07 ± 50.65 | −0.013 | 9.03 ± 12.37 | 0.018 |
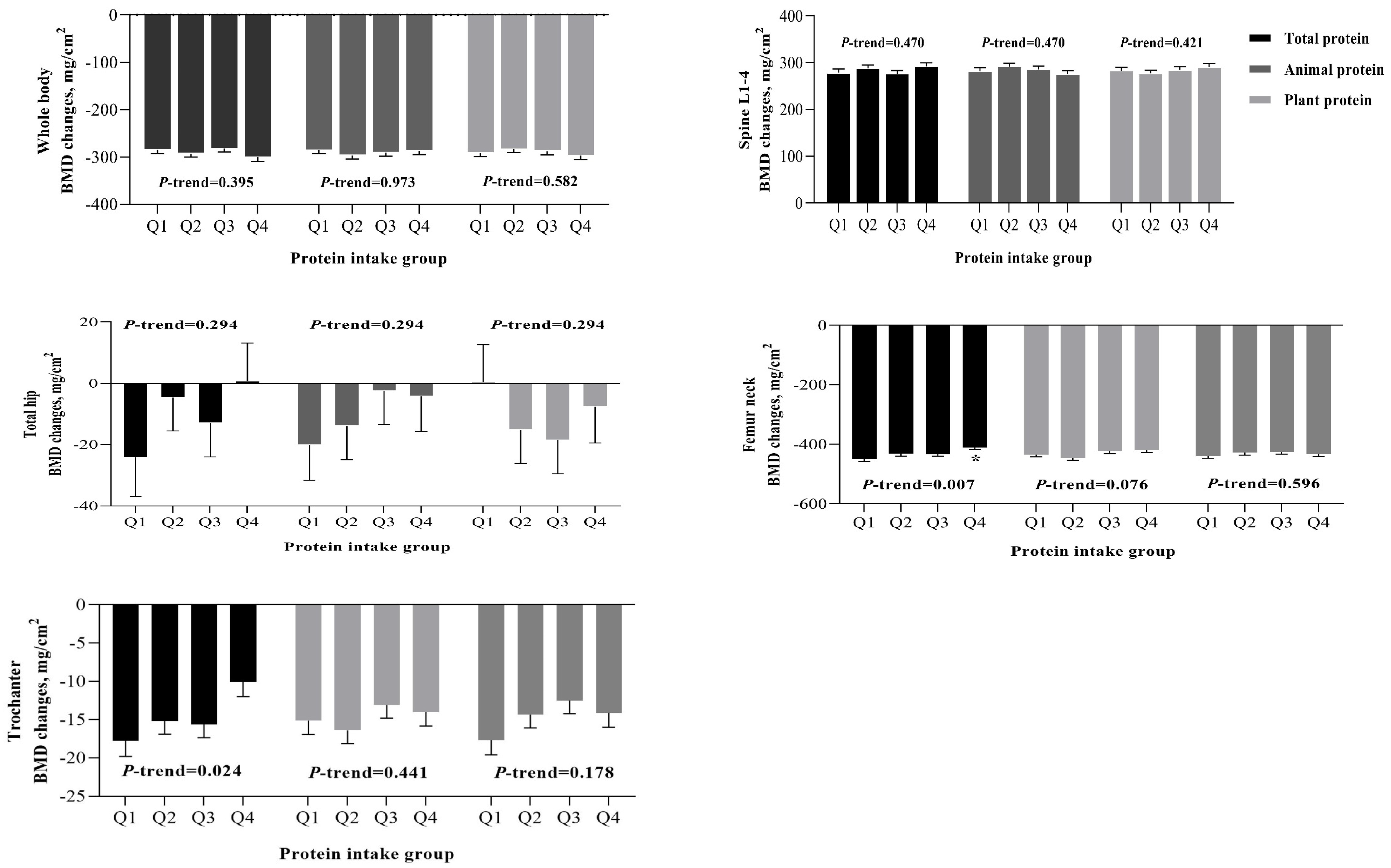
| Total Protein Intakes (g·kg−1·d−1) | |||||||
|---|---|---|---|---|---|---|---|
| Q1 (<0.96) | Q2 (0.96~) | Q3 (1.10~) | Q4 (≥1.26) | % Difference | p-ANCOVA | p Trend | |
| n = 497 | n = 497 | n = 497 | n = 496 | ||||
| BMD changes, mg/cm2 | |||||||
| Whole body | |||||||
| Model 1 | −287.79 ± 8.35 | −291.13 ± 7.47 | −280.25 ± 7.55 | −295.67 ± 8.30 | −2.74 | 0.531 | 0.756 |
| Model 2 | −283.36 ± 8.81 | −291.61 ± 7.57 | −281.66 ± 7.64 | −298.60 ± 8.59 | −5.38 | 0.435 | 0.416 |
| Spine L1-4 | |||||||
| Model 1 | 282.11 ± 7.72 | 287.18 ± 6.91 | 275.37 ± 6.98 | 289.24 ± 7.68 | 2.53 | 0.503 | 0.801 |
| Model 2 | 279.19 ± 8.12 | 287.18 ± 6.97 | 275.85 ± 7.03 | 290.74 ± 7.91 | 4.14 | 0.443 | 0.565 |
| Total hip | |||||||
| Model 1 | −23.77 ± 12.13 | −4.88 ± 10.99 | −12.36 ± 11.13 | 0.24 ± 12.21 | 101.01 | 0.561 | 0.285 |
| Model 2 | −27.29 ± 12.89 | −7.14 ± 11.07 | −11.52 ± 11.24 | 2.52 ± 12.33 | 109.23 | 0.485 | 0.187 |
| Femur neck | |||||||
| Model 1 | −445.31 ± 8.07 | −428.32 ± 7.22 | −432.53 ± 7.30 | −410.37 ± 8.03 * | 7.85 | 0.037 | 0.011 |
| Model 2 | −449.67 ± 8.49 | −427.74 ± 7.28 | −431.23 ± 7.35 | −407.05 ± 8.27 ** | 9.48 | 0.012 | 0.003 |
| Trochanter | |||||||
| Model 1 | −17.39 ± 1.97 | −14.73 ± 1.76 | −15.66 ± 1.78 | −10.13 ± 1.96 | 41.74 | 0.083 | 0.033 |
| Model 2 | −18.02 ± 2.08 | −14.75 ± 1.78 | −15.55 ± 1.80 | −9.61 ± 2.02 | 46.67 | 0.049 | 0.018 |
| Dietary Animal Protein Intakes (g·kg−1·d−1) | |||||||
|---|---|---|---|---|---|---|---|
| Q1 (<0.42) | Q2 (0.42~) | Q3 (0.54~) | Q4 (≥0.68) | % Difference | p-ANCOVA | p Trend | |
| n = 497 | n = 496 | n = 497 | n = 497 | ||||
| BMD changes, mg/cm2 | |||||||
| Whole body | |||||||
| Model 1 | −286.02 ± 7.77 | −294.29 ± 7.49 | −289.75 ± 7.45 | −284.70 ± 7.80 | 0.42 | 0.805 | 0.815 |
| Model 2 | −284.21 ± 8.01 | −294.30 ± 7.57 | −290.71 ± 7.55 | −285.95 ± 8.05 | −0.61 | 0.775 | 0.966 |
| Spine L1-4 | |||||||
| Model 1 | 280.64 ± 7.18 | 292.41 ± 6.92 | 285.39 ± 6.88 | 275.36 ± 7.20 | −1.88 | 0.359 | 0.496 |
| Model 2 | 279.47 ± 7.37 | 292.67 ± 6.97 | 285.71 ± 6.94 | 275.03 ± 7.41 | −1.59 | 0.312 | 0.565 |
| Total hip | |||||||
| Model 1 | −22.10 ± 11.38 | −13.38 ± 11.01 | −3.36 ± 10.95 | −2.09 ± 11.48 | 90.54 | 0.594 | 0.189 |
| Model 2 | −24.40 ± 11.70 | −14.75 ± 11.11 | −2.91 ± 11.05 | −1.43 ± 11.83 | 94.14 | 0.514 | 0.151 |
| Femur neck | |||||||
| Model 1 | −429.57 ± 7.51 | −443.97 ± 7.24 | −424.13 ± 7.20 | −419.15 ± 7.54 | 2.43 | 0.099 | 0.145 |
| Model 2 | −431.04 ± 7.72 | −445.04 ± 7.29 | −422.44 ± 7.27 | −417.33 ± 7.75 | 3.18 | 0.057 | 0.084 |
| Trochanter | |||||||
| Model 1 | −15.58 ± 1.84 | −15.96 ± 1.77 | −13.43 ± 1.76 | −13.07 ± 1.84 | 16.11 | 0.604 | 0.241 |
| Model 2 | −15.24 ± 1.89 | −16.12 ± 1.78 | −13.11 ± 1.78 | −13.50 ± 1.90 | 11.42 | 0.633 | 0.363 |
| Dietary White Meat Protein Intakes (g·kg−1·d−1) | |||||||
|---|---|---|---|---|---|---|---|
| Q1 (<0.12) | Q2(0.12~) | Q3(0.17~) | Q4(≥0.26) | % Difference | p-ANCOVA | p Trend | |
| n = 496 | n = 498 | n = 496 | n = 497 | ||||
| BMD changes, mg/cm2 | |||||||
| Whole body | |||||||
| Model 1 | −287.55 ± 7.59 | −291.38 ± 7.49 | −288.93 ± 7.45 | −286.94 ± 7.56 | 0.21 | 0.976 | 0.901 |
| Model 2 | −287.76 ± 7.68 | −290.91 ± 7.58 | −289.19 ± 7.53 | −287.34 ± 7.69 | 0.15 | 0.987 | 0.933 |
| Spine L1-4 | |||||||
| Model 1 | 280.54 ± 7.01 | 283.91 ± 6.92 | 291.38 ± 6.89 | 278.02 ± 6.98 | −0.90 | 0.547 | 0.998 |
| Model 2 | 281.34 ± 7.07 | 282.96 ± 6.97 | 291.50 ± 6.93 | 277.17 ± 7.08 | −1.48 | 0.520 | 0.902 |
| Total hip | |||||||
| Model 1 | −14.70 ± 11.14 | −15.88 ± 10.97 | −14.49 ± 10.96 | 3.99 ± 11.12 | 127.14 | 0.537 | 0.255 |
| Model 2 | −16.24 ± 11.23 | −16.74 ± 11.23 | −15.26 ± 11.06 | 4.49 ± 11.28 | 127.65 | 0.486 | 0.218 |
| Femur neck | |||||||
| Model 1 | −443.62 ± 7.33 | −429.37 ± 7.24 | −428.91 ± 7.20 | −415.19 ± 7.30 * | 6.41 | 0.062 | 0.010 |
| Model 2 | −445.60 ± 7.39 | −429.92 ± 7.29 | −426.95 ± 7.25 | −413.68 ± 7.40 * | 7.16 | 0.030 | 0.004 |
| Trochanter | |||||||
| Model 1 | −16.99 ± 1.79 | −16.38 ± 1.77 | −13.07 ± 1.76 | −11.66 ± 1.78 | 31.37 | 0.107 | 0.017 |
| Model 2 | −17.08 ± 1.81 | −16.28 ± 1.78 | −12.92 ± 1.77 | −11.75 ± 1.81 | 31.21 | 0.122 | 0.020 |
| Dietary Amino Acids Intakes (mg·kg−1d−1) | Whole Body | Spine L1-4 | Total Hip | Femur Neck | Trochanter |
|---|---|---|---|---|---|
| Lysine | 0.03 ± 0.25 | 0.04 ± 0.23 | 0.36 ± 0.36 | 0.44 ± 0.24 | 0.09 ± 0.06 |
| Tryptophan | −0.68 ± 1.44 | 1.37 ± 1.33 | 2.58 ± 2.10 | 1.95 ± 1.39 | 0.68 ± 0.34 * |
| Phenylalanine | −0.33 ± 0.44 | 0.46 ± 0.40 | 0.82 ± 0.64 | 0.85 ± 0.42 * | 0.25 ± 0.10 * |
| Threonine | −0.09 ± 0.46 | 0.22 ± 0.42 | 0.65 ± 0.67 | 0.90 ± 0.44 * | 0.19 ± 0.11 |
| Isoleucine | 0.01 ± 0.44 | 0.19 ± 0.41 | 0.73 ± 0.65 | 0.94 ± 0.43 * | 0.21 ± 0.10 * |
| Leucine | −0.06 ± 0.24 | 0.15 ± 0.22 | 0.37 ± 0.36 | 0.49 ± 0.23 * | 0.12 ± 0.06 * |
| Valine | −0.18 ± 0.40 | 0.27 ± 0.36 | 0.63 ± 0.58 | 0.83 ± 0.38 * | 0.21 ± 0.09 * |
| Histidine | −0.20 ± 0.67 | 0.36 ± 0.62 | 0.77 ± 0.98 | 1.22 ± 0.65 | 0.25 ± 0.16 |
| Methionine | −0.23 ± 0.82 | 0.38 ± 0.75 | 1.05 ± 1.20 | 1.59 ± 0.79 * | 0.31 ± 0.19 |
| Cystine | 0.35 ± 1.35 | −0.06 ± 1.24 | 3.04 ± 1.98 | 3.82 ± 1.30 ** | 0.51 ± 0.32 |
| Sulfur amino acids | −0.12 ± 0.55 | 0.25 ± 0.50 | 0.90 ± 0.80 | 1.17 ± 0.53 * | 0.22 ± 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.-M.; Huang, Q.; Long, H.-H.; Li, S.-Y.; Wu, Y.; Zhang, S.-J.; Tang, X.-Y.; Chen, Y.-M. Increased Dietary Intakes of Total Protein, Animal Protein and White Meat Protein Were Associated with Reduced Bone Loss—A Prospective Analysis Based on Guangzhou Health and Nutrition Cohort, South China. Nutrients 2023, 15, 1432. https://doi.org/10.3390/nu15061432
Liu Z-M, Huang Q, Long H-H, Li S-Y, Wu Y, Zhang S-J, Tang X-Y, Chen Y-M. Increased Dietary Intakes of Total Protein, Animal Protein and White Meat Protein Were Associated with Reduced Bone Loss—A Prospective Analysis Based on Guangzhou Health and Nutrition Cohort, South China. Nutrients. 2023; 15(6):1432. https://doi.org/10.3390/nu15061432
Chicago/Turabian StyleLiu, Zhao-Min, Qi Huang, Huan-Huan Long, Shu-Yi Li, Yi Wu, Su-Juan Zhang, Xin-Yi Tang, and Yu-Ming Chen. 2023. "Increased Dietary Intakes of Total Protein, Animal Protein and White Meat Protein Were Associated with Reduced Bone Loss—A Prospective Analysis Based on Guangzhou Health and Nutrition Cohort, South China" Nutrients 15, no. 6: 1432. https://doi.org/10.3390/nu15061432
APA StyleLiu, Z.-M., Huang, Q., Long, H.-H., Li, S.-Y., Wu, Y., Zhang, S.-J., Tang, X.-Y., & Chen, Y.-M. (2023). Increased Dietary Intakes of Total Protein, Animal Protein and White Meat Protein Were Associated with Reduced Bone Loss—A Prospective Analysis Based on Guangzhou Health and Nutrition Cohort, South China. Nutrients, 15(6), 1432. https://doi.org/10.3390/nu15061432






