Effects of Probiotics on Autism Spectrum Disorder in Children: A Systematic Review and Meta-Analysis of Clinical Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategies
2.2. Inclusion Criteria and Exclusion Criteria
2.3. Study Selection and Data Extraction
2.4. Study Quality Assessment
2.5. Data Analysis
3. Results
3.1. Literature Search
3.2. Study Characteristics
3.3. Study Quality
3.4. Results of the Meta-Analysis
3.4.1. Effects of Probiotics on Autism-Related Behavioral Symptoms of Children with ASD
3.4.2. Subgroup Analyses
3.4.3. Publication Bias and Sensitivity Analysis
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, T.; Zhu, J.; Guo, M.; Lai, X.; Li, Y.Y.; Yang, T.; Chen, J.; Li, T.Y. Relationship of constipation and sleep disorders with emotional and behavioral problems in children with autism spectrum disorder. J. Chongqing Med. Univ. 2020, 45, 85–90. [Google Scholar] [CrossRef]
- Newschaffer, C.J.; Croen, L.A.; Daniels, J.; Giarelli, E.; Grether, J.K.; Levy, S.E.; Mandell, D.S.; Miller, L.A.; Pinto-Martin, J.; Reaven, J.; et al. The epidemiology of autism spectrum disorders. Annu. Rev. Public Health 2007, 28, 235–258. [Google Scholar] [CrossRef] [PubMed]
- Devlin, B.; Scherer, S.W. Genetic architecture in autism spectrum disorder. Curr. Opin. Genet. Dev. 2012, 22, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Ronemus, M.; Iossifov, I.; Levy, D.; Wigler, M. The role of de novo mutations in the genetics of autism spectrum disorders. Nat. Rev. Genet. 2014, 15, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Malkova, N.V.; Yu, C.Z.; Hsiao, E.Y.; Moore, M.J.; Patterson, P.H. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav. Immun. 2012, 26, 607–616. [Google Scholar] [CrossRef]
- Boggess, A.; Faber, S.; Kern, J.; Kingston, H.M.S. Mean serum-level of common organic pollutants is predictive of behavioral severity in children with autism spectrum disorders. Sci. Rep. 2016, 6, 26185. [Google Scholar] [CrossRef]
- Leigh, J.P.; Du, J. Brief Report: Forecasting the Economic Burden of Autism in 2015 and 2025 in the United States. J. Autism Dev. Disord. 2015, 45, 4135–4139. [Google Scholar] [CrossRef]
- Maenner, M.J.; Shaw, K.A.; Bakian, A.V.; Bilder, D.A.; Durkin, M.S.; Esler, A.; Furnier, S.M.; Hallas, L.; Hall-Lande, J.; Hudson, A.; et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2018. MMWR Surveill. Summ. 2021, 70, 1–16. [Google Scholar] [CrossRef]
- Da, Z.Q.; Wang, Y.F.; Wang, L.X.; Yue, X.; Li, W.; Wang, W.K.; Yang, L. Prevalence and trend analysis of autism spectrum disorders in China based on the big data of the Global Burden of Disease. J. Lanzhou Univ. (Med. Sci.) 2022, 48, 38–44. [Google Scholar] [CrossRef]
- Masi, A.; DeMayo, M.M.; Glozier, N.; Guastella, A.J. An Overview of Autism Spectrum Disorder, Heterogeneity and Treatment Options. Neurosci. Bull. 2017, 33, 183–193. [Google Scholar] [CrossRef]
- Hume, K.; Steinbrenner, J.R.; Odom, S.L.; Morin, K.L.; Nowell, S.W.; Tomaszewski, B.; Szendrey, S.; McIntyre, N.S.; Yücesoy-Özkan, S.; Savage, M.N. Evidence-Based Practices for Children, Youth, and Young Adults with Autism: Third Generation Review. J. Autism Dev. Disord. 2021, 51, 4013–4032. [Google Scholar] [CrossRef] [PubMed]
- Lemmon, M.E.; Gregas, M.; Jeste, S.S. Risperidone use in autism spectrum disorders: A retrospective review of a clinic-referred patient population. J. Child Neurol. 2011, 26, 428–432. [Google Scholar] [CrossRef]
- Dean, O.M.; Gray, K.M.; Villagonzalo, K.A.; Dodd, S.; Mohebbi, M.; Vick, T.; Tonge, B.J.; Berk, M. A randomised, double blind, placebo-controlled trial of a fixed dose of N-acetyl cysteine in children with autistic disorder. Aust. N. Z. J. Psychiatry 2017, 51, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Eslamzadeh, M.; Hebrani, P.; Behdani, F.; Dadgar Moghadam, M.; Panaghi, L.; Mirzadeh, M.; Arabgol, F. Assessment the Efficacy of Atomoxetine in Autism Spectrum Disorders: A Randomized, Double-Blind, Placebo-Controlled Trial. Iran. J. Psychiatry Behav. Sci. 2018, 12, e10596. [Google Scholar] [CrossRef]
- Liang, S.; Wu, X.; Hu, X.; Wang, T.; Jin, F. Recognizing Depression from the Microbiota–Gut–Brain Axis. Int. J. Mol. Sci. 2018, 19, 1592. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Si, C.; Sun, Z.; Chen, Y.; Zhang, X. The Intervention of Prebiotics on Depression via the Gut-Brain Axis. Molecules 2022, 27, 3671. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, F.; Fan, B.; Wang, R.; Ren, J.; Jia, S.; Wang, L.; Chen, Z.; Liu, X.A. The Molecular Gut-Brain Axis in Early Brain Development. Int. J. Mol. Sci. 2022, 23, 15389. [Google Scholar] [CrossRef]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A novel class of psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef]
- Sgritta, M.; Dooling, S.W.; Buffington, S.A.; Momin, E.N.; Francis, M.B.; Britton, R.A.; Costa-Mattioli, M. Mechanisms Underlying Microbial-Mediated Changes in Social Behavior in Mouse Models of Autism Spectrum Disorder. Neuron 2019, 101, 246–259.e6. [Google Scholar] [CrossRef]
- Guo, M.; Li, R.; Wang, Y.; Ma, S.; Zhang, Y.; Li, S.; Zhang, H.; Liu, Z.; You, C.; Zheng, H. Lactobacillus plantarum ST-III modulates abnormal behavior and gut microbiota in a mouse model of autism spectrum disorder. Physiol. Behav. 2022, 257, 113965. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Zhang, M.; Teng, L.; Wang, Y.; Zhu, L. Prebiotics and probiotics for autism spectrum disorder: A systematic review and meta-analysis of controlled clinical trials. J. Med. Microbiol. 2022, 71, 001510. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Liong, M.T.; Chung, Y.E.; Huang, H.Y.; Peng, W.S.; Cheng, Y.F.; Lin, Y.S.; Wu, Y.Y.; Tsai, Y.C. Effects of Lactobacillus plantarum PS128 on Children with Autism Spectrum Disorder in Taiwan: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2019, 11, 820. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Li, Q.; Zhang, J.; Wen, F.; Dang, W.; Duan, G.; Li, H.; Ruan, W.; Yang, P.; Guan, C.; et al. Characterization of Intestinal Microbiota and Probiotics Treatment in Children With Autism Spectrum Disorders in China. Front. Neurol. 2019, 10, 1084. [Google Scholar] [CrossRef]
- Li, Y.Q.; Sun, Y.H.; Liang, Y.P.; Zhou, F.; Yang, J.; Jin, S.L. Effect of probiotics combined with applied behavior analysis in the treatment of children with autism spectrum disorder: A prospective randomized controlled trial. Zhongguo Dang Dai Er Ke Za Zhi 2021, 23, 1103–1110. (In Chinese) [Google Scholar] [CrossRef]
- Wang, Y.; Li, N.; Yang, J.J.; Zhao, D.M.; Chen, B.; Zhang, G.Q.; Chen, S.; Cao, R.F.; Yu, H.; Zhao, C.Y.; et al. Probiotics and fructo-oligosaccharide intervention modulate the microbiota-gut brain axis to improve autism spectrum reducing also the hyper-serotonergic state and the dopamine metabolism disorder. Pharmacol. Res. 2020, 157, 104784. [Google Scholar] [CrossRef] [PubMed]
- Santocchi, E.; Guiducci, L.; Prosperi, M.; Calderoni, S.; Gaggini, M.; Apicella, F.; Tancredi, R.; Billeci, L.; Mastromarino, P.; Grossi, E.; et al. Effects of Probiotic Supplementation on Gastrointestinal, Sensory and Core Symptoms in Autism Spectrum Disorders: A Randomized Controlled Trial. Front. Psychiatry 2020, 11, 550593. [Google Scholar] [CrossRef]
- Arnold, L.E.; Luna, R.A.; Williams, K.; Chan, J.; Parker, R.A.; Wu, Q.; Hollway, J.A.; Jeffs, A.; Lu, F.; Coury, D.L.; et al. Probiotics for Gastrointestinal Symptoms and Quality of Life in Autism: A Placebo-Controlled Pilot Trial. J. Child Adolesc. Psychopharmacol. 2019, 29, 659–669. [Google Scholar] [CrossRef]
- Sanctuary, M.R.; Kain, J.N.; Chen, S.Y.; Kalanetra, K.; Lemay, D.G.; Rose, D.R.; Yang, H.T.; Tancredi, D.J.; German, J.B.; Slupsky, C.M.; et al. Pilot study of probiotic/colostrum supplementation on gut function in children with autism and gastrointestinal symptoms. PLoS ONE 2019, 14, e0210064. [Google Scholar] [CrossRef]
- Available online: https://clinicaltrials.gov/ct2/show/results/NCT03369431 (accessed on 17 January 2023).
- Parracho, H.; Gibson, G.R.; Knott, F.J.; Bosscher, D.; Kleerebezem, M.; McCartney, A.L. A double-blind, placebo-controlled, crossover-designed probiotic feeding study in children diagnosed with autistic spectrum disorders. Int. J. Probiotics Prebiotics 2010, 5, 69–74. [Google Scholar]
- EI-Alfy, M.S.E.; Youssef, A.; Sabrey, R. A Study on Effect of Probiotic Supplementation on Gastrointestinal Symptoms, Cognition and Behavior in Egyptian Children with Autism Spectrum Disorder. Egypt. J. Paediatr. 2019, 36, 327–337. [Google Scholar] [CrossRef]
- Mahapatra, S.; Khokhlovich, E.; Martinez, S.; Kannel, B.; Edelson, S.M.; Vyshedskiy, A. Longitudinal Epidemiological Study of Autism Subgroups Using Autism Treatment Evaluation Checklist (ATEC) Score. J. Autism Dev. Disord. 2020, 50, 1497–1508. [Google Scholar] [CrossRef]
- Aman, M.G.; Singh, N.N.; Stewart, A.W.; Field, C.J. The aberrant behavior checklist: A behavior rating scale for the assessment of treatment effects. Am. J. Ment. Defic. 1985, 89, 485–491. [Google Scholar] [PubMed]
- Kaat, A.J.; Zelko, F.; Wilkening, G.; Berg, A.T. Evaluation of the Aberrant Behavior Checklist for Developmental and Epileptic Encephalopathies. Epilepsy Behav. 2021, 119, 107958. [Google Scholar] [CrossRef] [PubMed]
- Janvier, D.; Choi, Y.B.; Klein, C.; Lord, C.; Kim, S.H. Brief Report: Examining Test-Retest Reliability of the Autism Diagnostic Observation Schedule (ADOS-2) Calibrated Severity Scores (CSS). J. Autism Dev. Disord. 2022, 52, 1388–1394. [Google Scholar] [CrossRef]
- Ng, Q.X.; Loke, W.; Venkatanarayanan, N.; Lim, D.Y.; Soh, A.Y.S.; Yeo, W.S. A Systematic Review of the Role of Prebiotics and Probiotics in Autism Spectrum Disorders. Medicina 2019, 55, 129. [Google Scholar] [CrossRef]
- Mintál, K.; Tóth, A.; Hormay, E.; Kovács, A.; László, K.; Bufa, A.; Marosvölgyi, T.; Kocsis, B.; Varga, A.; Vizvári, Z.; et al. Novel probiotic treatment of autism spectrum disorder associated social behavioral symptoms in two rodent models. Sci. Rep. 2022, 12, 5399. [Google Scholar] [CrossRef]
- Nettleton, J.E.; Klancic, T.; Schick, A.; Choo, A.C.; Cheng, N.; Shearer, J.; Borgland, S.L.; Rho, J.M.; Reimer, R.A. Prebiotic, Probiotic, and Synbiotic Consumption Alter Behavioral Variables and Intestinal Permeability and Microbiota in BTBR Mice. Microorganisms 2021, 9, 1833. [Google Scholar] [CrossRef]
- Tan, Q.; Orsso, C.E.; Deehan, E.C.; Kung, J.Y.; Tun, H.M.; Wine, E.; Madsen, K.L.; Zwaigenbaum, L.; Haqq, A.M. Probiotics, prebiotics, synbiotics, and fecal microbiota transplantation in the treatment of behavioral symptoms of autism spectrum disorder: A systematic review. Autism Res. 2021, 14, 1820–1836. [Google Scholar] [CrossRef]
- Sharon, G.; Cruz, N.J.; Kang, D.W.; Gandal, M.J.; Wang, B.; Kim, Y.M.; Zink, E.M.; Casey, C.P.; Taylor, B.C.; Lane, C.J.; et al. Human Gut Microbiota from Autism Spectrum Disorder Promote Behavioral Symptoms in Mice. Cell 2019, 177, 1600–1618.e17. [Google Scholar] [CrossRef]
- Wang, H.; Lee, I.S.; Braun, C.; Enck, P. Effect of Probiotics on Central Nervous System Functions in Animals and Humans: A Systematic Review. J. Neurogastroenterol. Motil. 2016, 22, 589–605. [Google Scholar] [CrossRef]
- Mazurak, N.; Broelz, E.; Storr, M.; Enck, P. Probiotic Therapy of the Irritable Bowel Syndrome: Why Is the Evidence Still Poor and What Can Be Done About It? J. Neurogastroenterol. Motil. 2015, 21, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Buffington, S.A.; Di Prisco, G.V.; Auchtung, T.A.; Ajami, N.J.; Petrosino, J.F.; Costa-Mattioli, M. Microbial Reconstitution Reverses Maternal Diet-Induced Social and Synaptic Deficits in Offspring. Cell 2016, 165, 1762–1775. [Google Scholar] [CrossRef]
- Alharthi, A.; Alhazmi, S.; Alburae, N.; Bahieldin, A. The Human Gut Microbiome as a Potential Factor in Autism Spectrum Disorder. Int. J. Mol. Sci. 2022, 23, 1363. [Google Scholar] [CrossRef]
- Oono, I.P.; Honey, E.J.; McConachie, H. Parent-mediated early intervention for young children with autism spectrum disorders (ASD). Cochrane Database Syst. Rev. 2013, 4, CD009774. [Google Scholar] [CrossRef]
- Reichow, B.; Hume, K.; Barton, E.E.; Boyd, B.A. Early intensive behavioral intervention (EIBI) for young children with autism spectrum disorders (ASD). Cochrane Database Syst. Rev. 2018, 5, CD009260. [Google Scholar] [CrossRef] [PubMed]
- Iovene, M.R.; Bombace, F.; Maresca, R.; Sapone, A.; Iardino, P.; Picardi, A.; Marotta, R.; Schiraldi, C.; Siniscalco, D.; Serra, N.; et al. Intestinal Dysbiosis and Yeast Isolation in Stool of Subjects with Autism Spectrum Disorders. Mycopathologia 2017, 182, 349–363. [Google Scholar] [CrossRef]
- Ristori, M.V.; Quagliariello, A.; Reddel, S.; Ianiro, G.; Vicari, S.; Gasbarrini, A.; Putignani, L. Autism, Gastrointestinal Symptoms and Modulation of Gut Microbiota by Nutritional Interventions. Nutrients 2019, 11, 2812. [Google Scholar] [CrossRef]
- Mensi, M.M.; Rogantini, C.; Marchesi, M.; Borgatti, R.; Chiappedi, M. Lactobacillus plantarum PS128 and Other Probiotics in Children and Adolescents with Autism Spectrum Disorder: A Real-World Experience. Nutrients 2021, 13, 2036. [Google Scholar] [CrossRef] [PubMed]




| Author (Year) | Country/Region | Type of Trial | Age (Year) | Sample Size | Intervention Group | Compare Group | Intervention Duration | Scale on Autism | GI Symptoms Are Measured |
|---|---|---|---|---|---|---|---|---|---|
| Niu M (2019) [24] | China | RCT | 3–8 | 65 | n = 37 probiotics +ABA 3.6 ×1010 CFU/day | n = 28 ABA | 4 weeks | ATEC | Yes |
| Li YQ (2021) [25] | China | RCT | 3–6 | 41 | n = 21 Bifidobacterium triple live dispersion +ABA 9 × 107 CFU/day | n = 20 ABA | 3 months | ATEC | Yes |
| Liu YW (2019) [23] | Taiwan (China) | RCT | 7–15 | 71 | n = 36 Lactobacillus plantarum PS128 3 × 107 CFU/day | n = 35 placebo | 4 weeks | CGI-I ABC-T SRS SNAP-IV | No |
| Santocchi E (2020) [27] | Italy | RCT | 1.5–6 | 63 | n = 32 DSF 9 × 1011 CFU/day | n = 31 placebo | 6 months | ADOS-CSS, GI Severity Index Score | Yes |
| Wang Y (2020) [26] | China | RCT | 2–8 | 11 | n = 7 probiotics * + FOS 1010 CFU/day | n = 4 placebo | 108 days | ATEC 6-GSI | Yes |
| Arnold LE (2018) [28] | America | crossover controlled trials | 3–12 | 10 | n = 10 DSF 4.5–9 × 1012 CFU/day | n = 10 placebo | 8 weeks | ABC, SRS | Yes |
| Parracho H (2010) [31] | Britain | crossover controlled trials | 3–16 | 17 | n = 17 Lactobacillus plantarum WCFS1 4.5 × 1010 CFU/day | n = 17 placebo | 6 weeks | DBC | Yes |
| Sanctuary MR (2019) [29] | America | crossover controlled trials | 2–11 | 8 | n = 8 Bifidobacterium infantis +BCP 2 × 1010 CFU/day | n = 8 BCP | 5 months | ABC, GIH | Yes |
| Simmons S (2022) [30] | America | crossover controlled trials | 5–11 | 64 | n = 64 DSF 4.5 × 1011 CFU/y | n = 64 placebo | 12 weeks | ATEC GHI ABC | Yes |
| Alfy MSE (2019) [32] | Egypt | RCT | 2–10 | 100 | n = 50 Lacteol Fort 2 × 108 CFU/day | n = 50 standard treatment | 12 weeks | ATEC 6-GSI | Yes |
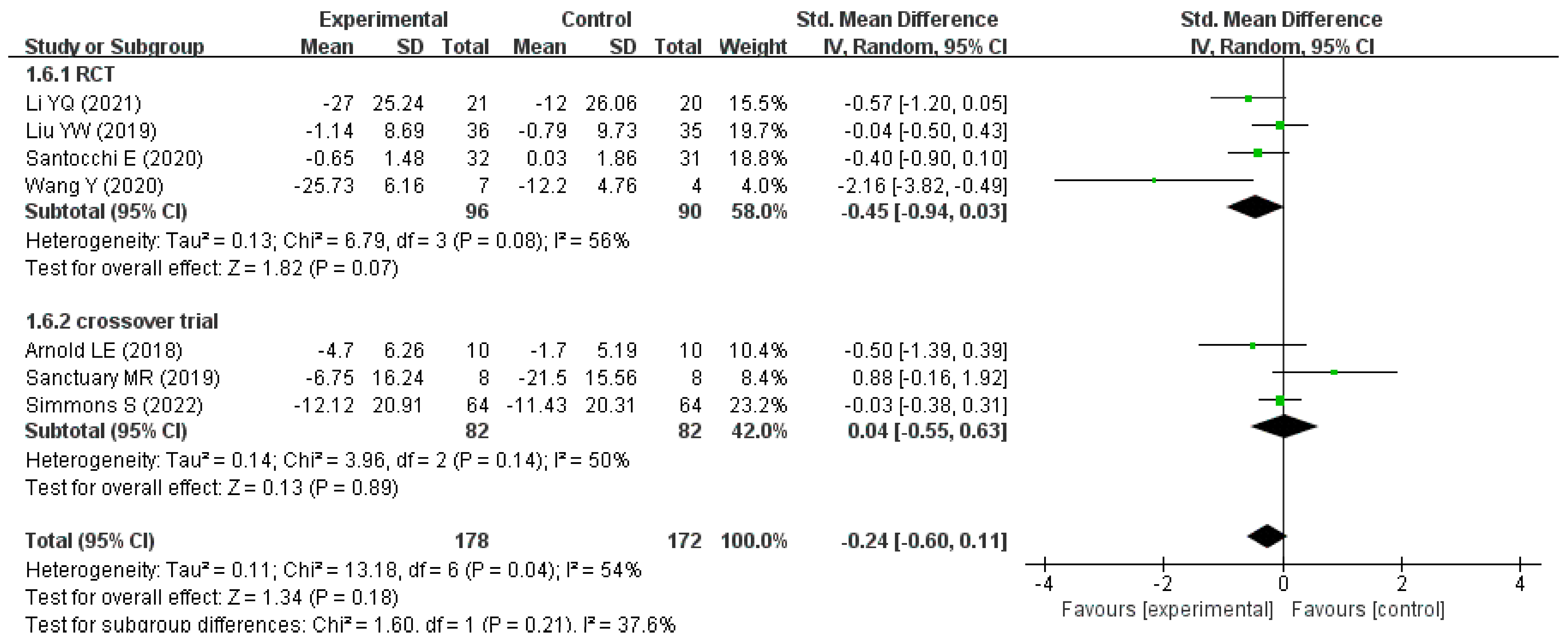
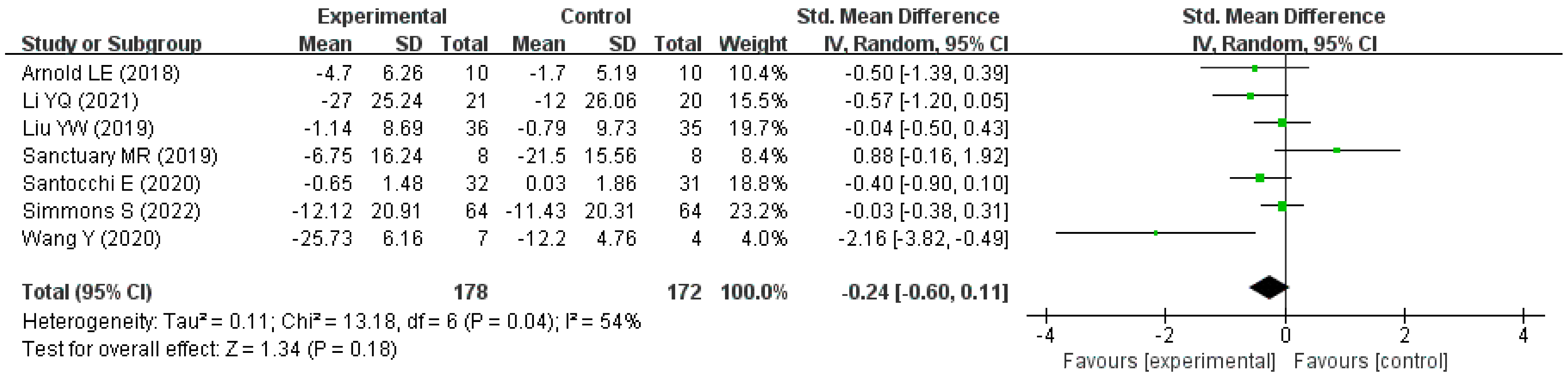
| Author (Year) | Country | Type of Trial | Sample Size (Intervention /Compare) | Age (Year) | Intervention Measure/Compare Measure | Intervention Duration | Change in Score (Intervention /Compare) | Scale |
|---|---|---|---|---|---|---|---|---|
| Li YQ (2021) [25] | China | RCT | 21/20 | 3–6 | Bifidobacterium triple live dispersion +ABA/ABA | 3 months | −27.00 (25.24)/ −12.00 (26.06) * | ATEC |
| Liu YW (2019) [23] | Taiwan (China) | RCT | 36/35 | 7–15 | Lactobacillus plantarum PS128/Placebo | 4 weeks | −1.14 (8.69)/ −0.79 (9.73) * | ABC-T |
| Santocchi E (2020) [27] | Italy | RCT | 32/31 | 1.5–6 | DSF/ Placebo | 6 months | −0.65 (1.48)/ 0.03 (1.86) * | ADOS-CSS |
| Wang Y (2020) [26] | China | RCT | 7/4 | 2–8 | Probiotics + FOS/Placebo | 108 days | −25.73 (6.16)/ −12.20 (4.76) * | ATEC |
| Arnold LE (2018) [28] | America | Crossover controlled trial | 10/10 | 3–12 | DSF/ Placebo | 8 weeks | −4.70 (6.26)/ −1.70 (5.19) | SRS |
| Sanctuary MR (2019) [29] | America | Crossover controlled trial | 8/8 | 2–11 | Bifidobacterium infantis + BCP/BCP | 5 weeks | −6.75 (16.24)/ −21.5 (15.56) | ABC |
| Simmons S (2022) [30] | America | Crossover controlled trial | 64/64 | 5–11 | DSF/ Placebo | 12 weeks | −12.12 (20.91)/ −11.43 (20.31) | ATEC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Liu, W.; Tang, F.; Chen, X.; Song, G. Effects of Probiotics on Autism Spectrum Disorder in Children: A Systematic Review and Meta-Analysis of Clinical Trials. Nutrients 2023, 15, 1415. https://doi.org/10.3390/nu15061415
He X, Liu W, Tang F, Chen X, Song G. Effects of Probiotics on Autism Spectrum Disorder in Children: A Systematic Review and Meta-Analysis of Clinical Trials. Nutrients. 2023; 15(6):1415. https://doi.org/10.3390/nu15061415
Chicago/Turabian StyleHe, Xiao, Wenxi Liu, Fengrao Tang, Xin Chen, and Guirong Song. 2023. "Effects of Probiotics on Autism Spectrum Disorder in Children: A Systematic Review and Meta-Analysis of Clinical Trials" Nutrients 15, no. 6: 1415. https://doi.org/10.3390/nu15061415
APA StyleHe, X., Liu, W., Tang, F., Chen, X., & Song, G. (2023). Effects of Probiotics on Autism Spectrum Disorder in Children: A Systematic Review and Meta-Analysis of Clinical Trials. Nutrients, 15(6), 1415. https://doi.org/10.3390/nu15061415