Abstract
We performed this study to clarify the dynamic changes in maternal manganese (Mn) concentration during pregnancy and its association with spontaneous preterm birth (SPB). A nested case–control study was conducted based on the Beijing Birth Cohort Study (BBCS) from 2018 to 2020. Singleton pregnancy women aged 18–44 (n = 488) were involved in the study, including 244 cases of SPB and 244 controls. All of the participants provided blood samples twice (in their first and third trimesters). Inductively coupled plasma mass spectrometry (ICP-MS) was used for the laboratory analysis, and unconditional logistic regression was used for the statistical analysis. We found that the maternal Mn levels were significantly higher in the third trimester than those in the first trimester (median: 1.23 vs. 0.81 ng/mL). The SPB risk was increased to 1.65 (95% CI: 1.04–2.62, p = 0.035) in the highest Mn level (third tertile) in the third trimester, especially in normal-weight women (OR: 2.07, 95% CI: 1.18–3.61, p = 0.011) or non-premature rupture of membrane (PROM) women (OR: 3.93, 95% CI: 2.00–7.74, p < 0.001). Moreover, a dose-dependent relationship exists between the SPB risk and maternal Mn concentration in non-PROM women (P trend < 0.001). In conclusion, dynamic monitoring of maternal Mn level during pregnancy would be helpful for SPB prevention, especially in normal-weight and non-PROM women.
1. Introduction
Preterm birth, defined as a live birth between 28 and 37 weeks of gestation, is the leading cause of perinatal death and the death of children under five years old worldwide, with an incidence of 6.4% [1,2]. Preterm birth can be classified into spontaneous and iatrogenic preterm birth; the former accounts for from 70% to 80% of total preterm births [1,2]. In contrast to iatrogenic preterm birth, which has a relatively specific cause, the causes and mechanisms that result in spontaneous preterm birth (SPB) remain unclear.
Manganese (Mn), as one of the essential trace elements, may play a vital role in fetal growth and development [3,4,5,6]. A national nutrition and health survey in the United States showed that the blood Mn concentration of pregnant women was higher than that of non-pregnant women, suggesting that Mn plays an essential role in pregnancy [7]. The adequate intake (AI) of Mn during pregnancy recommended by the Institute of Medicine (IOM) is 2 mg/day [8]. In comparison, the World Health Organization and the Food and Agriculture Organization (WHO/FAQ) designated 5 mg/day as the recommended dietary intake (RDI) [9]. We are exposed to Mn through diet (such as drinking water, leafy vegetables, nuts, grains, and tea) and environment (the respiratory inhalation of vehicle exhaust, skin absorption, etc.) [10]. As a result, Mn deficiency has been extremely rare in recent years, and there are no reports of Mn deficiency in non-experimental conditions. Conversely, environmental exposure is making an excess of Mn increasingly common. The harm that an excessive amount of Mn can cause to health is becoming gradually apparent. Labor (term and preterm) onset is characterized by increased myometrial contractility, cervical dilatation, and rupture of the chorioamnionitis membranes [11]. Inflammation and oxidative stress play essential roles in the process [11]. As molecular mechanisms of Mn toxicity include oxidative stress [12], maternally inappropriate Mn concentrations may be involved in the labor onset of SPB.
Several studies have discussed the relationship between Mn concentration and preterm birth. However, no consistent conclusions were made. Studies conducted in different countries suggested a positive correlation between Mn levels and preterm birth [13,14,15]. However, a study based on the Japan environment and children’s study (JECS) did not find relationships between high-level Mn exposure and an increased risk of premature birth [16]. Similarly, a study in Bangladesh also failed to conclude that Mn is related to the risk of premature birth [17]. These conflicting results may be due to ethnicity, region, exposure level, and sample time. Moreover, previous studies did not strictly distinguish between SPB and iatrogenic preterm birth, leading to a confusing conclusion. In addition, the sampling times used in existing studies were a single point in time, most of which occurred at delivery, and dynamic monitoring data during pregnancy were lacking.
Therefore, this study explores the relationship between the maternal plasma Mn level and SPB through repeated measurement data to discover SPB’s underlying drivers in micronutrient views. The measurement is carried out in the first and third trimesters of pregnancy. We aim for this study to be helpful for clinical prevention and intervention measures for preterm birth to reduce the occurrence of preterm birth and improve the quality of the birth population.
2. Materials and Methods
2.1. Study Design and Participants
A nested case–control study was conducted based on a prospective maternal and child health cohort, the Beijing Birth Cohort Study (ChiCTR2200058395), in Beijing, China. In total, 32,496 pregnant women were recruited in the cohort from 2018 to 2020 at Beijing Obstetrics and Gynecology Hospital, Capital Medical University. We recruited women with the following inclusive criteria: aged 18–44 years old, with gestational weeks ≤ 14 weeks during recruitment, with no mental illness, and who could provide signed informed consent. Participants were examined and followed up until delivery. We collected maternal and fetal information prospectively when participants came to the hospital for an antenatal examination, and the information was recorded in the electronic clinical system. The ethics committee of Beijing Obstetrics and Gynecology Hospital, Capital Medical University, approved the study (2018-ky-009-01).
Figure 1 depicts the screening process. Out of the 32,496 pregnant women in the cohort, excluding those who were lost to follow-up (n = 946), stillbirth (n = 46), fetal defects (n = 968), multiple pregnancies (n = 1030), and cervical insufficiency (n = 203), a total of 29,303 live singleton pregnant women with complete medical records remained, including premature births (n = 1599) and term births (n = 27,704). Among the preterm birth group, a final amount of 244 pregnant women were included in the SPB group after excluding iatrogenic preterm birth (n = 982) and a lack of plasma samples in the first or third trimester (n = 373). In the full-term birth group, women were excluded if they met the following criteria: gestational weeks were <39 weeks or >41 weeks (n = 8008), if they experienced pregnancy complications (n = 11,949), or if there was a lack of plasma samples in the first or third trimester (n = 2567). Then, the control group was randomly selected according to the blood sampling time (1:1).
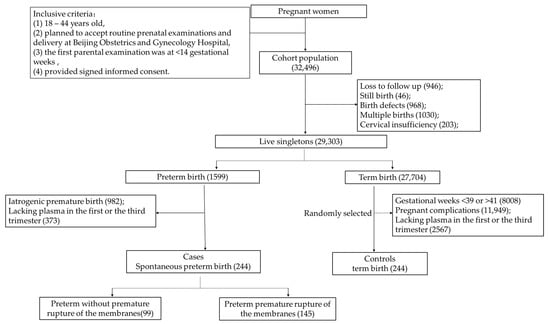
Figure 1.
Flow diagram of the selection of cases and controls from the cohort.
2.2. Definitions
In our study, preterm birth was defined as a live birth during the gestational ages of 28–36 + 6 weeks. SPB was defined as the spontaneous onset of labor at a gestational age of 28–36 + 6 weeks, including premature birth with a partial premature rupture of membranes and intact membranes. Iatrogenic premature birth refers to cases that require a medical intervention to give birth at 28–36 + 6 weeks. One reason for premature birth may be the mother’s or fetus’ poor health status that did not allow for pregnancy to continue (continuous deterioration of maternal pregnancy complications, infection, fetal distress, etc.). The gestational week was checked according to the last menstrual period (LMP), the course of pregnancy, and ultrasound measurement data in the first trimester. The first trimester referred to gestational weeks < 14 weeks, and the third trimester referred to gestational weeks ≥ 28 weeks. Body mass index (BMI) was classified into three grades based on expert consensus on medical nutrition treatment for overweight/obesity in China (2016) [18]. The criteria were as follows: slim: BMI < 18.5 kg/m2; normal: 18.5 ≤ BMI < 23.9 kg/m2; and overweight: BMI ≥ 24 kg/m2.
2.3. Laboratory Analysis
Plasma samples were chosen for Mn level determination to reflect nutrition intake to a certain degree. We collected plasma samples from the same pregnant women in their first and third trimesters. Maternal venous blood samples were collected by healthcare workers after 8–10 h of fasting. Then they were placed into vacuum blood collection vessels lined with sodium ethylene diamine tetraacetic acid (EDTA). All blood samples were centrifuged at 1680× g for 10 min within 1 h after collection, and plasma samples were extracted and stored at −80 °C until analysis. Inductively coupled plasma mass spectrometry (ICP-MS, 7700×, Agilent, Palo Alto, California, CA, USA) was used to detect plasma Mn concentration. A plasma sample of 0.1 mL was added to a 2 mL centrifuge tube. Then 0.1 mL of internal standard indium (In) (0.2 ng/mL, National Nonferrous Metals and Electronic Materials Analysis and Test Center, Beijing, China) and 1.8 mL of 1% nitric acid (UPS grade (68%), Suzhou Crystal Clear Chemical Co., Ltd., Suzhou, China) were added into the 2 mL tube. All of the samples were shaken sufficiently before determination. The median measured concentration of standard plasma samples (ClinChek®Plasma Control, Level II: 8884) was 15.3 ng/mL, which was within the range of the reference value: 15.2 (12.2–18.3) ng/mL. The detection limit of Mn was 0.008 ng/mL. We conducted the quantitative analysis in the Central Laboratory of biological elements of the Health Science Center of Peking University. The program has passed the China metrology certification system. Specific detection methods have been described in previously published studies [14].
2.4. Sample Size Estimation
According to a previous search of the literature, the exposure rate of high-level Mn in the term birth group was about 50%. The SPB risk was significantly increased to 2.46 (95% CI: 1.08–5.62) at the highest level of Mn in the first trimester [14]. Assuming that the risk-odds ratio is 2, the significance level of the bilateral test is α = 0.05, β = 0.1. The SPB group sample size was at least 179, calculated using the PASS2021 software (PASS 2021 Power Analysis and Sample Size Software (2021). NCSS, LLC. Kaysville, Utah, UT, USA). As a 1:1 case–control study design was used here, 244 women in the SPB group and 244 women in the term birth group were eventually selected for final analysis.
2.5. Statistical Analysis
Student’s t-tests or Pearson’s chi-square test were used for the comparison of the maternal baseline information. As a result of the skewed distribution of Mn concentration, the median with an interquartile range (IQR) was used to describe Mn exposure level. The Mann–Whitney U test was used to compare the two groups’ differences. Wilcoxon’s signed-rank test was used to compare the difference in Mn concentrations between the first and third trimesters. In the dose–response analysis, we divided all women into three equal groups based on the Mn concentration: low, medium, and high. Unconditional binary or multiple logistic regression was used to analyze the relationship between Mn level and SPB risk in different populations with or without adjusting for confounders. The odds ratio (OR) and a 95% confidence interval (CI) were used to quantify the SPB risk. Adjusted confounders included age, BMI, education, economy, nationality, gravida, parity, sampling time, and fetal gender. All the confounders involved have been reported to have effects on preterm birth [19,20,21]. As inflammation may affect SPB, we screened out factors in the 1st and 3rd trimester blood routine test that may affect SPB as inflammatory confounders. Univariate logistic regression was used in this process. Stratified analyses were carried out according to pre-pregnancy BMI and parity. We also conducted a sensitivity analysis to verify the stability of the statistical results. Women with vaginal group B streptococcus (GBS) infection (n = 9 in the SPB group and n = 16 in the term labor group) were excluded from the sensitivity analysis. Spss26.0 (Released 2019. IBM SPSS Statistics for Windows, Version 26.0, IBM Corp, Armonk, New York, NY, USA) was used for data analysis, and a two-tailed p-value < 0.05 was considered statistically significant. Regarding multiple testing by analyzing various subgroups, Bonferroni correction was used here to adjust the significant p-value [22].
3. Results
3.1. Baseline Information
In this study, a total of 488 women were included in the final analysis, including 244 cases (SPB) and 244 controls (term birth). The primary characteristics of the mothers and fetuses are summarized in Table 1. All of the women were non-smokers. The average age of all the participants was 31.51 ± 3.91 years old. The SPB group had a slightly higher average BMI and a higher proportion of overweight women (25% vs. 13.1%, p = 0.003). The SPB group had a lower proportion of nulliparous than the controls (64.3% vs. 76.2%, p = 0.004). All of the women in the SPB group were late preterm (34–36 + 6 weeks), and the cesarean section rate was higher than that in the term labor group (39.8% vs. 27.2%, p = 0.003). There was a similarity between the two groups in terms of education, economy, nationality, sampling time (8 weeks in the first trimester and 34 weeks in the third trimester), and fetal gender. All women were not antibiotic-treated during pregnancy.

Table 1.
Characteristics of participants.
3.2. Mn Concentration in the First and Third Trimesters
The median plasma Mn concentration for all women was 0.81 ng/mL (IQR: 0.63 ng/mL) in the first trimester, and it was 1.23 ng/mL (IQR: 0.67 ng/mL) in the third trimester. Despite the fact that there was a slightly higher Mn median concentration in the SPB group than that of the controls, it was not statistically significant (0.82 vs. 0.79 ng/mL, p = 0.441 in the first trimester; 1.27 vs. 1.20 ng/mL, p = 0.119 in the third trimester). When comparing Mn levels between the first and third trimesters, a cumulative effect during pregnancy was found, as the Mn concentration in the third trimester was significantly higher than that in the first trimester (p < 0.001) (see Table 2).

Table 2.
Median Mn concentrations of women who had SPB and term birth.
3.3. Relationship between Mn Level in the Third Trimester and SPB Risk
Table 3 displays the association between Mn levels and SPB risk. We divided all the women into three equal groups based on Mn concentration for the dose–response analysis. Overall, we found no significant stable relationship between the plasma Mn level in the first trimester and SPB risk. SPB risk was increased to 1.54 (95% CI: 1.00–2.39, p = 0.052) in the highest Mn concentration in the third trimester. After adjustment for clinical confounders, including age, BMI, education, economy, nationality, gravida, parity, sampling time, and fetal gender, the association became significant (OR: 1.60, 95% CI: 1.02–2.53, p = 0.043).

Table 3.
Association of maternal plasma Mn levels with SPB.
As inflammation may affect SPB, we conducted univariate logistic regression to analyze the relationship between inflammatory factors and SPB. The results are summarized in Table S1. We finally selected the white blood cell count (WBC), platelet count (PLT), granulocyte ratio (GR), and platelet crit (PCT) as inflammatory confounders in the first trimester; and WBC, PLT, PCT, and neutrophil (NE) as inflammatory confounders in the third trimester. After adjustment for the inflammatory confounders, alone or combined with the clinical confounders, the association between the plasma Mn level in the third trimester and SPB remained (OR: 1.63, 95% CI: 1.04–2.55, p = 0.032; OR: 1.65, 95% CI: 1.04–2.62, p = 0.035).
3.4. Relationship between Mn Level in the Third Trimester and SPB Risk Stratified by BMI Grade
Stratified analyses were carried out based on BMI grade and parity. The results from the stratified analysis by BMI showed no significant difference between the SPB group and the controls (Table 4). Though a higher Mn concentration in the SPB group was found in the normal weight layer (1.30 vs. 1.15 ng/mL, p = 0.043; see Table 4), it was nonsignificant after Bonferroni correction (significance level α = 0.017 (0.05/3)). SPB risk was increased in the highest tertile Mn level in normal weight women (OR: 1.89, 95%CI: 1.12–3.20, p = 0.014). After adjustment for the clinical confounders, the SPB risk increased to 1.96 (95% CI: 1.13–3.38, p = 0.014). Moreover, a combined adjustment for clinical and inflammatory confounders made the risk association much more apparent (OR: 2.07, 95% CI: 1.18–3.61, p = 0.011) (see Table 5). However, there was no statistically significant relationship between the plasma Mn level and SPB risk when stratified by parity (Tables S2 and S3).

Table 4.
Mn concentrations of women who had SPB or term birth stratified by BMI grade.

Table 5.
Association of maternal plasma Mn levels with SPB stratified by BMI grade.
3.5. Relationship between Mn Level in the Third Trimester and SPB Risk in Non-PROM Women
Table 6 depicts the multiple logistic regression results. When it was limited to non-PROM participants, the SPB risk increased to 2.13 (95% CI: 1.11–4.10, p = 0.023) and 3.48 (95% CI: 1.84–6.57, p < 0.001) for the second and third tertile, respectively. Additionally, there was a clear dose-dependent relationship (p trend < 0.001) (see Figure 2). After a combined adjustment for clinical and inflammatory confounders, the SPB risk increased to 2.11 (95% CI: 1.05–4.22, p = 0.031) and 3.93 (95% CI: 2.00–7.74, p < 0.001) for the second and third tertile, respectively.

Table 6.
Association of maternal plasma Mn levels with SPB stratified by PROM.
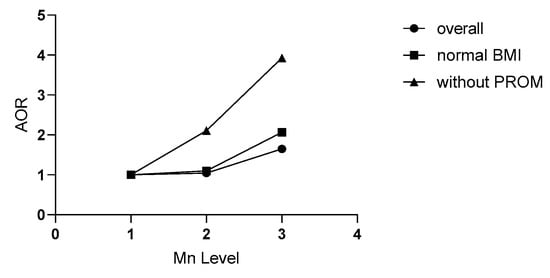
Figure 2.
Relationships of maternal plasma Mn in the third trimester with SPB in different populations. Plasma Mn concentration was classified into three levels by the tertile of all subjects (L1, L2, and L3). Without PROM refers to the population without premature rupture of membranes (PROM); normal BMI refers to the people with a pre-pregnancy body mass index (BMI) in the 18.5–23.9 kg/m2 range. AOR refers to the odds ratio adjusted for age, BMI, education, economy, nationality, parity, gravida, fetal gender, blood week, and inflammatory factors related to SPB in routine blood tests. SPB: spontaneous preterm birth.
To test the robustness of our results, we conducted a sensitivity analysis by excluding women with vaginal GBS infection (n = 9 in the SPB group; n = 16 in the term labor group). The results did not change significantly (see Table 7).

Table 7.
Sensitivity analysis.
4. Discussion
In this prospective nested case–control study, we identified for the first time that a high maternal plasma Mn level in the third trimester was positively associated with an increased risk of SPB, especially in normal-weight women and non-PROM women. Moreover, there is a dose-dependent relationship in the non-PROM population. The higher the level of plasma Mn in the third trimester, the higher the risk of SPB.
Solubilized Mn is absorbed in the intestine and transported across the microvilli to the blood via divalent metal transporter 1 (DMT1). They are absorbed in the form of Mn2+ or Mn3+, and Mn2+ is the most common form in the body [23,24]. In the blood, most Mn2+ is combined with albumin for transport and distribution [23]. Mn is distributed from plasma to the liver (accounting for 30% of total Mn), kidney (5%), pancreas (5%), colon (1%), urinary system (0.2%), bone (0.5%), brain (0.1%), red blood cells (0.02%), and the rest in soft tissue via DMT1 or transferrin receptor [23]. Most excessive Mn is combined with bile, transmitted into the intestine, and excreted. Trace Mn can also be detected in urine, sweat, and breast milk [23]. A study used rats as an animal model to explore Mn distribution during pregnancy. The results showed that the maternal liver accounts for most of the Mn, followed by the lung, brain, femur, and blood [25]. The distribution is similar to that of the non-pregnant subject. In addition, some of the Mn is distributed in the placenta and can be transported from maternal to fetal [25].
Blood Mn concentration, including whole blood and plasma/serum samples, is an effective and representative biomarker of Mn exposure [26,27]. Plasma/serum Mn accounts for 4% of the whole blood Mn concentration [27]. Most studies used whole blood samples for detection. The study results from different countries ranged from 10 to 22.5 ng/mL [4,7,16,28,29,30,31,32,33,34]. Several studies used plasma or serum for detection, ranging from 1.18 to 5.44 ng/mL [14,35,36,37,38]. In this study, the plasma Mn concentrations in both the first and third trimesters were significantly lower than those reported by the China Nutrition and Health Survey 2010–2012 of pregnant women (2.4 ng/mL, 95% CI: first trimester 1.1–9.2 ng/mL; third trimester 1.4–7.7 ng/mL) [39]. However, the plasma Mn concentrations in the third trimester observed in this study were similar to the Mn levels in the first trimester reported in a Shanxi and Anhui cohort study in China [14,15,37]. Due to the development of the coal mining industry, the environmental exposure risk of Mn is remarkably higher in Anhui and Shanxi provinces than in Beijing. Otherwise, our observed plasma Mn concentration in the third trimester was higher than that reported in an Indonesian cohort study (premature birth group: 1.09 ng/mL; term birth group: 1.0 ng/mL) [37]. Our results in the third trimester were similar to those reported in the Pakistan cohort study (1.38 ng/mL) [36]. The results may be affected by the participants’ age, BMI, gestational week, gravida, parity, sampling time, region, eating habits, laboratory testing methods, and environmental exposure. In addition, we found that there was a cumulative effect of the blood Mn levels during pregnancy, which was consistent with the research results in Taiwan, China [40], Wuhan [30], Anhui [37], and Costa Rica [4]. This may be related to the average growth and development of the fetus and the increased demand for the balance of the redox system during pregnancy. In addition, as it is necessary for bone formation [41], Mn concentration may increase in the third trimester for fetal growth. Since there is no clear evidence of a link between maternal Mn level and fetal development, further studies must be conducted.
Stratified by BMI grade, it showed a relationship between the two groups only in the normal weight layer. The reason may be that being overweight or thin is a risk factor for premature birth and would modify the effect of Mn on SPB. Researchers should fully evaluate these confounders when conducting further research. All the above findings suggested that the plasma Mn level in the third trimester may be associated with SPB, and the effect is significant in specific populations.
Several previous studies have reported that an excessive Mn concentration was associated with a higher preterm birth risk. Most studies did not strictly distinguish SPB from iatrogenic preterm birth, and most sampling times were at the time of delivery. Only one study conducted in a Shanxi cohort study estimated the relationship between the serum Mn level and SPB in the first and second trimesters. However, their serum samples in the first and second trimesters were from different populations. They reported a positive risk association between serum Mn levels and SPB in a positive dose-dependent manner in the first trimester, not the second trimester [14]. However, we found that a high concentration of plasma Mn (third tertile) in the third trimester of pregnancy, but not in the first trimester, was a risk factor for SPB. The serum Mn concentration in the first trimester of pregnant women in Shanxi was close to that in the third trimester in our study. Therefore, this may further indicate that a high concentration of Mn is associated with a higher risk of SPB, with no correlation to the pregnancy stage. In contrast, other previous studies did not find an association between the Mn concentration and preterm birth risk. Those inconsistent results may be related to population, study design, sample type, sampling time, and preterm birth type. Our study proved that the Mn concentration increases with gestational age when dynamically monitoring levels in pregnancy. However, most previous studies collected serum samples at delivery, whether there was a preterm birth (<37 weeks) or a term birth (≥37 weeks), resulting in different sampling times in the two groups. In our study, sampling times were similar in the SPB and term birth groups. Therefore, our results better clarify the relationship between a high maternal Mn concentration and SPB risk.
Considering that a vaginal GBS infection may impact SPB, we performed a sensitivity analysis after excluding women with the infection. The results showed that the relationship between the Mn concentration and SPB risk was still significant, further confirming the aforementioned results that a high Mn concentration is a risk factor for SPB.
The current understanding of the SPB mechanism is related to inflammation and oxidative stress [11], such as inflammatory factors, genetic variants, and predisposition [42]; alterations in inflammation and energy metabolism [43]; transcriptomics-determined chemokine-cytokine pathways [44]; telomeres and oxidative stress [45]; and so on. Mn toxicity is mediated, at least in part, by reactive oxygen species (ROS); depletion of cellular antioxidant defense mechanisms; and alterations in mitochondrial function and ATP production [12]. As part of metalloenzymes or their activators, Mn participates in maintaining the balance of the redox system in some critical physiological processes. An abnormal concentration of Mn may lead to premature birth by inducing an imbalance in the redox system in the body [46]. We hypothesized that higher levels of Mn during pregnancy might lead to oxidative stress caused by the imbalance between ROS and antioxidants. Higher levels of ROS may attack telomeres or another possible pathway, resulting in a higher risk of SPB eventually. All in all, the possible mechanism of the Mn effect on SPB needs further study.
There are some advantages to our study. First, we are the first to use a repeated measurement design to clarify the dynamic changes in plasma Mn during pregnancy in the related studies. Second, iatrogenic preterm birth would confuse the study results with a specific reason for preterm birth. In this study, only SPB cases were selected for analysis and strictly distinguished from iatrogenic preterm birth. Third, the sampling time was similar in the two groups, which could reduce the effect of the gestational week on element concentration. Fourth, this nested case–control study relied on a prospective cohort study conducted in Beijing; the same exposure backgrounds helped to reduce confounding in the region. Additionally, we were able to obtain the accurate information we required in the survey, which minimized recall bias.
Still, several limitations should be considered when explaining our results. First, we included no information on diet and nutrient supplementation, and we could not evaluate the pregnant women’s dietary Mn exposure level and its relationship with the plasma Mn concentration. Second, when the stratified analysis was carried out according to specific factors, some subgroups’ relatively small sample size may have affected the power to determine an association between the Mn level and SPB. Third, in this study, all cases were late-preterm (labor week ≥34 weeks), meaning they were close to full-term. The pathological condition related to SPB may be too small to observe a meaningful difference between the two groups. In future studies, it will be helpful to include early preterm women to clarify the relationship between plasma Mn levels and SPB. Fourth, parameters of oxidative stress were not assessed. As Mn may play an important role in SPB through oxidative stress, it would be better if the oxidative stress parameters were evaluated in our study. In future studies, we could further optimize the experimental design and try to assess related parameters of oxidative stress.
5. Conclusions
The increased plasma Mn concentration in the third trimester of pregnancy is associated with higher risks of SPB. The effect is much more significant in normal-weight women and non-PROM women. Our results provide a unique insight into SPB prevention in terms of monitoring and managing essential micronutrient elements during pregnancy. In addition, as the functioning of micronutrients especially tends to be in groups, further studies should be conducted to identify whether it is Mn alone or another compound that accompanies it that is associated with an increased risk of SPB.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15061413/s1. Table S1: Association of inflammatory factors in routine blood tests with SPB; Table S2: Median Mn concentrations of women who had SPB and term birth stratified by parity; Table S3: Association of maternal plasma Mn level with SPB stratified by parity.
Author Contributions
Conceptualization, Z.L. and G.L.; Data curation, A.W., J.W., J.H., Y.M., K.Z. and R.Y.; Formal analysis, W.H., W.Z. and J.C.; Funding acquisition, W.Z., Z.L. and G.L.; Investigation, J.C., H.A. and L.Y.; Methodology, W.Z. and L.Y.; Supervision, L.Y., Z.L. and G.L.; Writing—original draft, W.H.; Writing—review and editing, W.H., W.Z., L.Y., Z.L. and G.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (82171671, National Natural Science Foundation of China), the Scientific Research Common Program of Beijing Municipal Commission of Education (KM202110025007, Beijing Municipal Education Commission), the Beijing Hospitals Authority’ Ascent Plan (DFL20191402, Beijing Hospital Authority), the National Key Research and Development Program of China (2016YFC1000304, Ministry of Finance of the People’s Republic of China), and the Beijing Natural Science Foundation (7222248, Beijing Municipal Natural Science Foundation Committee).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of Beijing Obstetrics and Gynecology Hospital, Capital Medical University (2018-ky-009-01).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We send great thanks to professional English editing services for helping us improve the language and presentation quality.
Conflicts of Interest
The authors declare no conflict of interests. The sponsors had no role in the design, execution, interpretation, or writing of the study.
References
- Deng, K.; Liang, J.; Mu, Y.; Liu, Z.; Wang, Y.; Li, M.; Li, X.; Dai, L.; Li, Q.; Chen, P.; et al. Preterm births in China between 2012 and 2018: An observational study of more than 9 million women. Lancet Glob. Health 2021, 9, e1226–e1241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, K.; Zhang, Y. The rising preterm birth rate in China: A cause for concern. Lancet Glob. Health 2021, 9, e1179–e1180. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez, L.; García-Vicent, C.; López, J.; Torró, M.I.; Lurbe, E. Assessment of ten trace elements in umbilical cord blood and maternal blood: Association with birth weight. J. Transl. Med. 2015, 13, 291. [Google Scholar] [CrossRef]
- Mora, A.M.; Joode, B.V.W.D.; Mergler, D.; Córdoba, L.; Cano, C.; Quesada, R.; Smith, D.R.; Menezes-Filho, J.A.; Eskenazi, B. Maternal blood and hair manganese concentrations, fetal growth, and length of gestation in the ISA cohort in Costa Rica. Environ. Res. 2015, 136, 47–56. [Google Scholar] [CrossRef]
- Eum, J.-H.; Cheong, H.-K.; Ha, E.-H.; Ha, M.; Kim, Y.; Hong, Y.-C.; Park, H.; Chang, N. Maternal blood manganese level and birth weight: A MOCEH birth cohort study. Environ. Health 2014, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhang, J.; Qi, X.; Wang, Z.; Zheng, M.; Liu, P.; Jiang, S.; Guo, J.; Wu, C.; Zhou, Z. Cord Blood Manganese Concentrations in Relation to Birth Outcomes and Childhood Physical Growth: A Prospective Birth Cohort Study. Nutrients 2021, 13, 4304. [Google Scholar] [CrossRef]
- Oulhote, Y.; Mergler, D.; Bouchard, M.F. Sex- and age-differences in blood manganese levels in the U.S. general population: National health and nutrition examination survey 2011–2012. Environ. Health 2014, 13, 87. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press (US): Washington, DC, USA, 2001.
- WHO; FAO. Vitamin and Mineral Requirements in Human Nutrition, 2nd ed.; World Health Organization: Geneva, Switzerland; FAO: Rome, Italy, 2004. [Google Scholar]
- Williams, M.; Todd, G.D.; Roney, N.; Crawford, J.; Coles, C.; McClure, P.R.; Garey, J.D.; Zaccaria, K.; Citra, M. Toxicological Profile for Manganese. Atlanta (GA): Agency for Toxic Substances and Disease Registry (US); Agency for Toxic Substances and Disease Registry (ATSDR): Atlanta, GA, USA, 2012; (PMID: 24049862; Bookshelf ID: NBK158872). Available online: https://www.atsdr.cdc.gov/ToxProfiles/tp151.pdf (accessed on 12 March 2023).
- Di Renzo, G.C.; Tosto, V.; Giardina, I. The biological basis and prevention of preterm birth. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 52, 13–22. [Google Scholar] [CrossRef]
- Martinez-Finley, E.J.; Gavin, C.E.; Aschner, M.; Gunter, T.E. Manganese neurotoxicity and the role of reactive oxygen species. Free. Radic. Biol. Med. 2013, 62, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Ashrap, P.; Watkins, D.J.; Mukherjee, B.; Boss, J.; Richards, M.J.; Rosario, Z.; Velez-Vega, C.M.; Alshawabkeh, A.; Cordero, J.F.; Meeker, J.D. Maternal blood metal and metalloid concentrations in association with birth outcomes in Northern Puerto Rico. Environ. Int. 2020, 138, 105606. [Google Scholar] [CrossRef]
- Hao, Y.; Yan, L.; Pang, Y.; Yan, H.; Zhang, L.; Liu, J.; Li, N.; Wang, B.; Zhang, Y.; Li, Z.; et al. Maternal serum level of manganese, single nucleotide polymorphisms, and risk of spontaneous preterm birth: A nested case-control study in China. Environ. Pollut. 2020, 262, 114187. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Meng, X.; Pang, Y.; An, H.; Wang, B.; Zhang, L.; Ye, R.; Ren, A.; Li, Z.; Gong, J. Associations of maternal exposure to 41 metals/metalloids during early pregnancy with the risk of spontaneous preterm birth: Does oxidative stress or DNA methylation play a crucial role? Environ. Int. 2022, 158, 106966. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, M.; Shibata, E.; Morokuma, S.; Tanaka, R.; Senju, A.; Araki, S.; Sanefuji, M.; Koriyama, C.; Yamamoto, M.; Ishihara, Y.; et al. The association between whole blood concentrations of heavy metals in pregnant women and premature births: The Japan Environment and Children’s Study (JECS). Environ. Res. 2018, 166, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wei, L.; Chen, X.; Zhang, R.; Su, L.; Rahman, M.; Mostofa, G.; Qamruzzaman, Q.; Zhao, Y.; Yu, H.; et al. Cord serum elementomics profiling of 56 elements depicts risk of preterm birth: Evidence from a prospective birth cohort in rural Bangladesh. Environ. Int. 2021, 156, 106731. [Google Scholar] [CrossRef]
- China Expert Panel of Medical Nutrition Therapy for Overweight/Obesity. Expert consensus on medical nutrition therapy for overweight/obesity in China. Chin. J. Diabetes Mellit. 2016, 8, 525–540. (In Chinese) [Google Scholar]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Blencowe, H.; Cousens, S.; Chou, D.; Born Too Soon Preterm Birth Action Group. Born too soon: The global epidemiology of 15 million preterm births. Reprod. Health 2013, 10 (Suppl. 1), S2. [Google Scholar] [CrossRef]
- Peelen, M.J.C.S.; Kazemier, B.M.; Ravelli, A.C.J.; de Groot, C.J.M.; van der Post, J.A.M.; Mol, B.W.J.; Kok, M.; Hajenius, P.J. Ethnic differences in the impact of male fetal gender on the risk of spontaneous preterm birth. J. Perinatol. 2021, 41, 2165–2172. [Google Scholar] [CrossRef]
- Armstrong, R.A. When to use the Bonferroni correction. Ophthalmic Physiol. Opt. 2014, 34, 502–508. [Google Scholar] [CrossRef]
- Horning, K.J.; Caito, S.W.; Tipps, K.G.; Bowman, A.B.; Aschner, M. Manganese Is Essential for Neuronal Health. Annu. Rev. Nutr. 2015, 35, 71–108. [Google Scholar] [CrossRef] [PubMed]
- Roth, J.; Ponzoni, S.; Aschner, M. Manganese homeostasis and transport. Met. Ions Life Sci. 2013, 12, 169–201. [Google Scholar]
- Yoon, M.; Nong, A.; Clewell, H.J.; Taylor, M.D.; Dorman, D.C.; Andersen, M.E. Evaluating placental transfer and tissue concentrations of manganese in the pregnant rat and fetuses after inhalation exposures with a PBPK model. Toxicol. Sci. 2009, 112, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.G.; Simpson, C.D.; Stover, B.; Sheppard, L.; Checkoway, H.; Racette, B.A.; Seixas, N.S. Blood manganese as an exposure biomarker: State of the evidence. J. Occup. Environ. Hyg. 2014, 11, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Hoet, P.; Vanmarcke, E.; Geens, T.; Deumer, G.; Haufroid, V.; Roels, H.A. Manganese in plasma: A promising biomarker of exposure to Mn in welders. A pilot study. Toxicol. Lett. 2012, 213, 69–74. [Google Scholar] [CrossRef]
- Ashrap, P.; Watkins, D.J.; Mukherjee, B.; Boss, J.; Richards, M.J.; Rosario, Z.; Vélez-Vega, C.M.; Alshawabkeh, A.; Cordero, J.F.; Meeker, J.D. Predictors of urinary and blood Metal(loid) concentrations among pregnant women in Northern Puerto Rico. Environ. Res. 2020, 183, 109178. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.E.; Cheong, H.K.; Ha, E.H.; Kim, B.N.; Ha, M.; Kim, Y.; Hong, Y.C.; Park, H.; Oh, S.Y. Maternal Blood Manganese and Early Neurodevelopment: The Mothers and Children’s Environmental Health (MOCEH) Study. Environ. Health Perspect. 2015, 123, 717–722. [Google Scholar] [CrossRef]
- Gong, L.; Yang, Q.; Liu, C.-W.; Wang, X.; Zeng, H.-L. Assessment of 12 Essential and Toxic Elements in Whole Blood of Pregnant and Non-pregnant Women Living in Wuhan of China. Biol. Trace Elem. Res. 2021, 199, 2121–2130. [Google Scholar] [CrossRef]
- Yamamoto, M.; Sakurai, K.; Eguchi, A.; Yamazaki, S.; Nakayama, S.F.; Isobe, T.; Takeuchi, A.; Sato, T.; Hata, A.; Mori, C.; et al. Association between blood manganese level during pregnancy and birth size: The Japan environment and children’s study (JECS). Environ. Res. 2019, 172, 117–126. [Google Scholar] [CrossRef]
- Vigeh, M.; Yokoyama, K.; Ramezanzadeh, F.; Dahaghin, M.; Fakhriazad, E.; Seyedaghamiri, Z.; Araki, S. Blood manganese concentrations and intrauterine growth restriction. Reprod. Toxicol. 2008, 25, 219–223. [Google Scholar] [CrossRef]
- Bocca, B.; Ruggieri, F.; Pino, A.; Rovira, J.; Calamandrei, G.; Mirabella, F.; Martínez, M.Á.; Domingo, J.L.; Alimonti, A.; Schuhmacher, M. Human biomonitoring to evaluate exposure to toxic and essential trace elements during pregnancy. Part, A. concentrations in maternal blood, urine and cord blood. Environ. Res. 2019, 177, 108599. [Google Scholar] [CrossRef]
- Caspersen, I.H.; Thomsen, C.; Haug, L.S.; Knutsen, H.K.; Brantsæter, A.L.; Papadopoulou, E.; Erlund, I.; Lundh, T.; Alexander, J.; Meltzer, H.M. Patterns and dietary determinants of essential and toxic elements in blood measured in mid-pregnancy: The Norwegian Environmental Biobank. Sci. Total Environ. 2019, 671, 299–308. [Google Scholar] [CrossRef]
- Irwinda, R.; Wibowo, N.; Putri, A.S. The Concentration of Micronutrients and Heavy Metals in Maternal Serum, Placenta, and Cord Blood: A Cross-Sectional Study in Preterm Birth. J. Pregnancy 2019, 2019, 5062365. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Ali, I.; Rust, P.; Kundi, M.; Ekmekcioglu, C. Selenium, Zinc, and Manganese Status in Pregnant Women and Its Relation to Maternal and Child Complications. Nutrients 2020, 12, 725. [Google Scholar] [CrossRef]
- Liang, C.M.; Wu, X.Y.; Huang, K.; Yan, S.Q.; Li, Z.J.; Xia, X.; Pan, W.J.; Sheng, J.; Tao, Y.R.; Xiang, H.Y.; et al. Trace element profiles in pregnant women’s sera and umbilical cord sera and influencing factors: Repeated measurements. Chemosphere 2019, 218, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yan, L.; Li, H.; Li, X.; Liu, Y.; Liu, J. Patterns and Determinants of Essential and Toxic Elements in Chinese Women at Mid-Pregnancy, Late Pregnancy, and Lactation. Nutrients 2021, 13, 668. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Piao, J.; Mao, D.; Li, Y.; Li, W.; Yang, L.; Yang, X. Reference Values of 14 Serum Trace Elements for Pregnant Chinese Women: A Cross-Sectional Study in the China Nutrition and Health Survey 2010–2012. Nutrients 2017, 9, 309. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-S.; Liao, K.-W.; Chang, C.-H.; Chien, L.-C.; Mao, I.-F.; Tsai, Y.-A.; Chen, M.-L. The critical fetal stage for maternal manganese exposure. Environ. Res. 2015, 137, 215–221. [Google Scholar] [CrossRef]
- Alacios, C. The role of nutrients in bone health, from A to Z. Crit. Rev. Food Sci. Nutr. 2006, 46, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Couceiro, J.; Matos, I.; Mendes, J.J.; Baptista, P.V.; Fernandes, A.R.; Quintas, A. Inflammatory factors, genetic variants, and predisposition for preterm birth. Clin. Genet. 2021, 100, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Lien, Y.-C.; Zhang, Z.; Cheng, Y.; Polyak, E.; Sillers, L.; Falk, M.J.; Ischiropoulos, H.; Parry, S.; Simmons, R.A. Human Placental Transcriptome Reveals Critical Alterations in Inflammation and Energy Metabolism with Fetal Sex Differences in Spontaneous Preterm Birth. Int. J. Mol. Sci. 2021, 22, 7899. [Google Scholar] [CrossRef]
- Wang, P.; Pan, J.; Tian, X.; Dong, X.; Ju, W.; Wang, Y.; Zhong, N. Transcriptomics-determined chemokine-cytokine pathway presents a common pathogenic mechanism in pregnancy loss and spontaneous preterm birth. Am. J. Reprod. Immunol. 2021, 86, e13398. [Google Scholar] [CrossRef] [PubMed]
- Phillippe, M. Telomeres, oxidative stress, and timing for spontaneous term and preterm labor. Am. J. Obstet. Gynecol. 2022, 227, 148–162. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Yan, H.; Liu, Y.; Zhang, L.; Li, Z.; Ye, R. Research progress on the effect of essential trace elements on premature delivery. Chin. J. Reprod. Health 2020, 31, 174–176+183. (In Chinese) [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).