Abstract
Milk-derived bioactive proteins have increasingly gained attention and consideration throughout the world due to their high-quality amino acids and multiple health-promoting attributes. Apparently, being at the forefront of functional foods, these bioactive proteins are also suggested as potential alternatives for the management of various complex diseases. In this review, we will focus on lactoferrin (LF) and osteopontin (OPN), two multifunctional dairy proteins, as well as to their naturally occurring bioactive LF–OPN complex. While describing their wide variety of physiological, biochemical, and nutritional functionalities, we will emphasize their specific roles in the perinatal period. Afterwards, we will evaluate their ability to control oxidative stress, inflammation, gut mucosal barrier, and intestinal microbiota in link with cardiometabolic disorders (CMD) (obesity, insulin resistance, dyslipidemia, and hypertension) and associated complications (diabetes and atherosclerosis). This review will not only attempt to highlight the mechanisms of action, but it will critically discuss the potential therapeutic applications of the underlined bioactive proteins in CMD.
1. Introduction
The scientific and medical community is increasingly realizing the benefits of the proteins present in both the human breast and bovine milk. Besides being a crucial source of nitrogen and amino acids, this milk macronutrient also consists of bioactive peptides/proteins that are progressively recognized to perform remarkable functions [,]. Recently, the sophisticated development of a milk proteomics technique revealed 1606 proteins [], of which a proportion is well established in the vast literature for its association with the development of the gastrointestinal (GI) tract, and the immune and central nervous systems. Despite this remarkable progress, efforts are still required to identify the other protein components of milk as the expression of many more than 10,000 genes was detected in mammary glands during lactation [,].
To date, few milk protein components have been largely probed in early life, but work is still needed to define their role in health and diseases later in life. Hence, the present topic is timely and worthy of exploration in complex diseases that take a heavy toll on the world’s population.
The main objective of this review is to take a critical look at two milk proteins: lactoferrin (LF) and osteopontin (OPN), which may influence the development of obesity and related disorders [], known to have reached epidemic proportions worldwide, increase morbidity and mortality, and cause a significant socioeconomic burden. Since these two proteins show a high affinity for each other and form a bioactive (LF–OPN) complex with interesting properties, we will also focus on its potential functions as suggested by several groups.
First, we will document the spectrum of general properties and functions of each of the specific milk-derived bioactive components, particularly during the perinatal period. Second, we will provide a thorough overview of their implication in relevant intestinal mechanisms (i.e., intestinal barrier, permeability, microbiota), which regulate and impact the host’s cardiometabolic health. Third, we will focus on their influence on CMD, including obesity, metabolic syndrome (MetS), type 2 diabetes, and cardiovascular diseases (CVD), with a particular emphasis on clinical studies.
2. General Lactoferrin Properties
LF is an iron-binding monomeric glycoprotein of the transferrin family, which is substantially present in human and bovine milk [], but also in lower amounts in saliva, tears, semen, and vaginal fluids []. Bovine and human LFs have features in common, including sharing the transferrin family, possessing identical iron-binding sites, and having two symmetrical homologous globular lobes (N-terminal lobe and C-terminal lobe). However, differences are also apparent, such as molecular weights (~80 and ~76 kDa, respectively), amino acid residues (689 and 691, respectively), and milk concentrations (0.02–0.35 and 1.0–3.2 mg/mL). In addition to binding nonheme iron, LF displays multiple biological activities and functions []. This multifunctional protein is highly resistant to GI digestion; promotes gut development; efficiently binds to specific membrane receptors of various tissues, including monocytes, lymphocytes, adipocytes, hepatocytes, enterocytes, and endothelial cells; and has the potential to regulate several intracellular signaling pathways following its intact absorption to the bloodstream [,]. LF is described as a pleiotropic protein with antioxidants, anti-inflammatory, antibacterial, antiviral, antifungal, anticancer, and immunomodulatory actions []. Among the several mechanisms for its beneficial impacts, intestinal microbiota seems largely involved with the potential to modulate CMD (Figure 1).
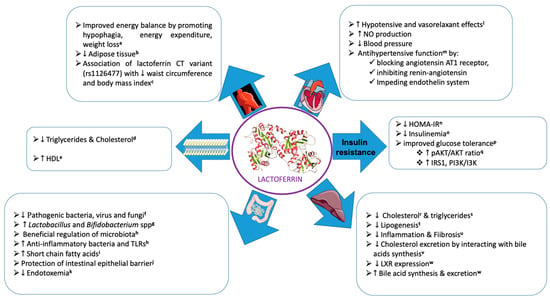
Figure 1.
Pleiotropic functions of bioactive milk lactoferrin through several mechanisms. (The images used were extracted from Servier Medical Art). Related references cited in the Figure: (a: [,,,,,]; b: [,]; c: []; d: [,]; e: []; f: [,]; g: [,]; h: []; i: [,,]; j: [,,,]; k: [,]; l: []; m: [,]; n: [,]; o: []; p: [,,]; q: [,,]; r: [,]; s: [,,]; t: [,]; u: []; v: []; w: []).
2.1. Lactoferrin and Intestinal Barrier
Early studies have shown that LF has the ability to bind to the epithelial layer of the GI mucosa of young children []. Other investigators reported the capacity of LF to protect the integrity of the gut barrier by linking to enterocyte and brush-border membranes, thus preventing the leakage and translocation of intestinal microbes to the bloodstream []. Additionally, LF may preserve the structural and functional epithelial layer by exerting protection against pathogens and toxic bacterial endotoxins that generally damage the tight junctions [,]. Importantly, LF molecules can form oligomeric complexes, which are instrumental for the maintenance of intestinal integrity, and influential in the protection against exogenous threats []. In fact, LF concomitantly stimulated intestinal epithelium growth and up-regulated the expression of jejunal tight-junction proteins such as zonula occludens and claudins in response to caloric restriction-mediated malnutrition []. The restoration of intestinal integrity, reflected by raised occludin mass and tight junction structure normalization, was caused by the inactivation of the MAPK pathway, in particular via the decrease of p38 and ERK1/2 phosphorylation []. The strengthening of the intestinal barrier was also accompanied by elevated alkaline phosphatase activity and transepithelial electrical resistance, the latter as an indicator of the strength of tight-junction proteins []. Confirmation has recently been obtained by Gao et al. [], who reported LF protective effects not only on a tight junction through MAPK signaling, but also on endocytosis, adherens, and gap junctions, thereby assuring barrier structure and function. Moreover, using cross-omics analysis of transcriptome and proteome, the same authors unraveled the association of LF with an insulin receptor, cytoplasmic FMR1 interacting protein 2, dedicator of cytokinesis and ribonucleotide reductase regulatory subunit M2 proteins, which together contributed to the biological benefits of LF in the defense of the intestinal epithelium [].
2.2. Lactoferrin and Intestinal Permeability
Generally, intact tight junction proteins impede the paracellular passage of molecules larger than ~600 kDa []. If LF undeniably preserves the intestinal barrier as reported above, it is expected that gut permeability would optimally be controlled as tight junctions constitute an integral paracellular seal []. The LF uptake resulted with lower cell permeability via the upregulation of myosin light-chain kinase expression in IEC-18 cells []. In other intestinal cell lines HT-29/B6 and T84, preincubation with LF ameliorated transepithelial resistance and attenuated cytokine-induced permeability, producing leaky gut []. In a similar way, LF was effective in reducing FITC–dextran permeability in response to bacterial-derived lipopolysaccharide (LPS) []. LF and its peptide fragments were also tested in mice with LPS-induced disruption of the intestinal tight junction structure and function []. Their intraperitoneal administration lessened permeability, inhibited colonic infiltration with polymorphonuclear leukocyte activity in colon tissue, and restored intestinal barrier integrity []. In a rat model of short bowel syndrome, bovine enteral LF supplementation increased villus height, crypt depth, and intestinal epithelial cell proliferation index, while preventing tight junction disruption and epithelium hyperpermeability []. Interestingly, engineered recombinant Lactobacillus reuteri expressing bovine LF peptide was able to reinforce the integrity of piglet’s gut barrier function as reflected by the elevation of zonula occludens and claudin proteins resulting from limited TLR4, Myd88, and myosin light-chain kinase protein expression []. Since inflammatory bowel diseases (IBD) are characterized by intestinal barrier disruption in response to epithelium injury and hyperpermeability, which augments host susceptibility to infection by pathogens [,], it seemed reasonable to determine whether LF (empowered with various biological functions) might restore gut homeostasis. This hypothesis was tested in murine IBD models (dextran sodium sulfate colitis model and TNFΔARE/+ model of ileitis), where LF recombinant administration provided a protection against mucosal injury, largely documented by the reduction of crypt architecture abrasion, goblet cell deficiency, immune cell infiltration, inflammation, and hyperpermeability []. Another group assessed the impact of direct delivery of LF using L. lactis AMJ1543 on mice with dextran sulfate sodium-induced experimental colitis. Here again, LF administration clearly improved DSS-induced colon damage, which was substantiated by tight-junction protein overexpression and intestinal permeability alleviation [].
In human, oral supplementation of 5 g LF and 50 mg indomethacin reduced the NSAID-mediated increase in small intestinal permeability, thereby providing a nutritional therapeutic tool in the treatment of hyperpermeability-associated disorders [].
2.3. Lactoferrin and Intestinal Microbiota
As reported by many research groups, LF appreciable effectiveness in preventing or blocking growth of a vast spectrum of pathogens (e.g., Staphylococcus aureus, Listeria monocytogenes, Salmonella enterica, Streptococcus, Legionella pneumophila, and Staphylococcus aureus and Escherichia coli) without affecting beneficial bacteria (e.g., Lactobacillus and Bifidobacterium) has been largely described [,]. It would even seem that LF could stimulate the proliferation of probiotic bacteria []. The exploration of the LF peptide mechanisms of action for the antibacterial effects mainly revealed (i) the sequestration of bacteria and their deprivation in iron (essential for their survival) by LF given its iron-binding ability []; (ii) the potential of LF in interacting with LPS of gram-negative bacteria, thus considerably hampering their growth [,,]; (iii) the LF competency of binding with receptors on microorganisms to obstruct the transport of nutrients, thereby restraining bacterial synthesis and metabolism [,]; (iv) the inhibition of microbial enzyme activity by LF []; (v) the LF-mediated prevention of biofilm formation by bacteria []; (vi) the capacity of LF to release antibacterial peptide lactoferricin and other bioactive peptides following hydrolysis by pepsin [,,]; and (vii) the direct antimicrobial activities exerted by LF []. It should be noted that the impact of LF is not limited to bacteria, but can also extend to antivirals [,,], antiprotozoal [], and antifungal [] activities. Another important element to remember is that additional LF functions can only be performed in synergy with other components [,].
Obviously, the multifunctional LF has the potential to shape intestinal microbiota, a common process in preterm infants, newborns, and children []. Consequently, prophylactic administration of LF was observed to prevent late-onset sepsis, necrotizing enterocolitis, and death in preterm infants compared with a placebo [,]. Moreover, treatment of mice with IBD prevented the rise in the proportions of proteobacteria, Actinobacteria, Cyanobacteria, Deferribacteres, and reversed the proportion of Firmicutes compared with those in the control group []. The amelioration of IBD-induced mouse microbiota dysbiosis in response to LF treatment comprised reduced Escherichia and Shigella, while stimulating Lactobacillus [].
2.4. Clinical Evidence for Lactoferrin Effectiveness
A significant number of clinical investigations have been carried out particularly in preterm infants to assess the LF impact on intrauterine growth restriction (IUGR). A systematic review and meta-analysis (involving 6 separate studies and 333 pregnancies) reported a diminished risk of preterm birth in women at risk in response to a prophylactic LF intake []. Nevertheless, the investigators of this study recommended the need for additional randomized trials of greater size to satisfactorily confirm the contribution of LF to improve perinatal maternal outcomes, particularly the decrease in the risk of preterm birth. Similar to infection, necrotizing enterocolitis and adverse neurodevelopment frequently lead to IUGR. Important investigations have then examined the role of LF on each of these entities, and their data were collectively analyzed by a recent meta-analysis involving 12 clinical trials totaling 5425 preterm infants []. The findings emphasized that enteral LF supplementation with or without probiotics lowered sepsis, but only the combination of LF with probiotics showed the potential to decrease GI injury. However, caution should be exercised until the results are confirmed through higher-powered clinical trials taking into account specific parameters, such as concentration, treatment duration, and the source of LF. However, it is very interesting to note the absence of adverse effects of LF administration in preterm babies [] and the reduction of hospital length of stay []. LF may as well improve maternal health (in the perinatal period) by serving as an alternative to antibiotics in treatment of vaginal infection [], and may at the same time decrease the inflammatory process and ensuing risk of preterm delivery []. It should also be underscored that various groups have examined the safety and effectiveness of LF supplementation in preventing/treating other types of infections [], dermatological conditions [], Helicobacter pylori infection [], etc. However, a few groups have reported the beneficial effect of oral LF supplementation on microbiota dysbiosis or species richness [,,,,,].
Although some information supporting a beneficial influence on the components of MetS is available, there has been no exhaustive work on CMD in humans as far as we know. An inverse relationship was apparent between circulating levels of LF, and fasting triglyceride and glucose concentrations, waist-to-hip ratio, and in direct correlation with plasma HDL-cholesterol []. Similarly, obese women displayed an inverse association between LF and adiposity despite independent positive association with insulin resistance (IR), for which the precise mechanisms for these observations have not been elucidated []. Furthermore, LF levels were positively and negatively associated with insulin sensitivity and inflammatory parameters, respectively [], an important observation since systemic low-grade inflammation commonly characterized individuals with obesity [,]. In line with these encouraging LF effects, human LF exhibited the ability to chelate LPS, and to interfere with the binding between LPS and soluble human CD14, thereby alleviating inflammation []. Interestingly, the hypolipidemic effect of LF was reported to be dependent on its binding to the LDL receptor-related protein 1 (LRP1). The investigators hypothesized that LF molecule alterations disturb the interaction with LRP1 receptors, which produces repercussions on lipid concentrations and their elimination from blood circulation. In this context, it should be noted that the Arg-rich sequence of LF N-terminus bears resemblance to apolipoprotein E structure sighted by LRP1 [].
With regard to hypertension, a major component of the MetS, the treatment of peripheral blood monocytes (from normotensive and treated hypertensive individuals) provided the evidence that LF may play a role in hypertension []. Even if no human studies are available, basic and preclinical research pointed out the ability of LF-derived peptides to modulate the renin–angiotensin, endothelin-1, and endothelial NO-dependent systems, leading to hypotensive effects [,,,,].
Finally, LF and the variants of LF receptor-related genes have been found to be related to metabolic settings in normal and pathological conditions. For example, an investigation conducted in 1749 French Canadians reported a significant difference in allele frequencies between subjects with and without MetS for the LTF rs2239692 polymorphism []. Another group of researchers documented the significant correlation between the LF rs1126477 gene variant and anthropometric parameters, allowing to propose that subjects with the CT variant of the LTF rs1126477 are endowed with a lower waist circumference compared to those with the TT variant []. On the other hand, male carriers of the G allele of the LF rs1126478 variant showed significantly elevated HDL-cholesterol and decreased fasting triglyceride concentrations []. As to blood pressure, there was a correlation between LF rs1126478 variant and hypertension [], while pointing out the relation of some SNPs in the sLF gene with the prevalence of metabolic abnormalities and CVD [,]. Despite the fascinating broad spectrum of properties and functions of LF, a molecule considered “miraculous” by some researchers, further in-depth research is required to demonstrate its benefits in large-scale clinical studies with longer follow-up.
3. General Osteopontin Properties
OPN, amply reported in human and bovine milk, is also detected in body fluids and in small amounts in a broad range of tissues in small amounts. Although OPN is encoded by a single copy gene, it is expressed in varied isoforms with different molecular weights as a result of not only alternative splicing, but also of post-translational modifications, such as glycosylation, serine/threonine phosphorylation, oxidation, tyrosine sulfation, calcium binding, and proteolytic processing, depending on the tissue [,]. The OPN glycoprotein, rich in aspartate and sialic-acid residues, displays a spectrum of molecular weights going from 25 to 75 kDa [,,]. This molecular heterogeneity may explain OPN multifunctionality and interactions with several proteins (e.g., integrins and CD44) [,,]. OPN is involved in tissue remodeling, bone morphogenesis, biomineralization, calcification, immune regulation, inflammation, cell signaling, and cytoskeleton rearrangement [,,,,]. Diminished pro-inflammatory factors have been observed in infants fed formula supplemented with OPN in comparison with infants fed regular formula [,], and a few groups have also reported antioxidant activity [,,] (Figure 2).
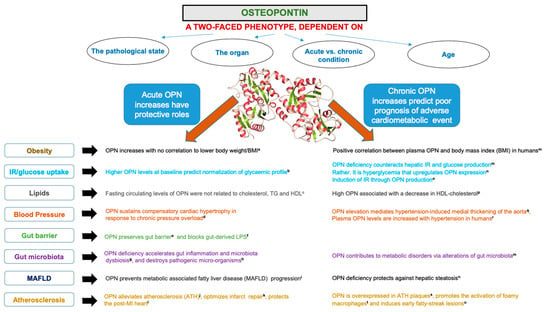
Figure 2.
Biological functions of bioactive milk osteopontin. Related references cited in the Figure: (a: [,]; b: []; c: []; d: []; e: [,,]; f: []; g: []; h: []; i: []; j: []; k: []; l: [,]; m: []; n: []; o: []; p: []; q: []; r: []; s: []; t: [,]; u: []).
3.1. Osteopontin and Intestinal Barrier
Human and bovine milk OPN have been reported to be resistant to proteolytic degradation or only partially digested in the gut []. Accordingly, integral OPN molecule or its proteolytic products with biological effects have been identified in plasma [,]. Former studies underlined the advantageous influence of OPN on the preservation of the gut barrier [] and epithelial cell survival in view of its anti-apoptotic action []. Work on animal models was able to demonstrate OPN capacity to stimulate duodenal mucosal growth, villus thickness, crypt depth, and nutrient absorption []. Even more, OPN may largely accumulate on the epithelium surface [] and exert an attenuation of epithelial injury by blunting NO output in response to macrophage activation [,]. Moreover, in vitro and in vivo experiments underscored the “guardianship” of a tight-junction complex by OPN []. Likewise, treatment of ethanol-fed mice with milk OPN alleviated disturbances of tight-junction proteins, indicating its potential to provide mucosal protection []. It is important to note, however, that very little work has been conducted to provide clear evidence of the role of OPN in gut homeostasis during childhood and adult periods.
3.2. Osteopontin and Intestinal Permeability
As ethanol intake facilitates the passage of Gram-negative bacteria from the gut to the portal blood, LPS rises in the circulation, causes inflammatory events, and provokes liver injury [,,]. Based on the demonstrated gut-protective action of OPN, Ge et al. [] examined whether OPN could maintain intestinal integrity and, consequently, prevent liver inflammation and steatosis. Their findings demonstrated that OPN, in mice with alcohol-induced liver injury, was able to protect gut integrity, while depressing liver pathogenesis. In fact, OPN enhances intestinal mucin production and blunts ethanol-mediated permeability augmentation by maintaining tight-junction integrity []. In another intestinal model, OPN prevented injury to the plasma membrane via the increased expression of tight-junction proteins, which decreased bacterial translocation and LPS deposition in the liver []. Conversely, OPN deletion in Opn−/− mice deteriorated intestinal tissue and repair [,], whereas OPN intake mitigated gut damage []. Engineered OPN-containing polymeric nanoparticles raised protection of the intestinal mucosal barrier and prevented permeability, while lessening colitis in animal models of IBD []. However, it is important to note the lack of studies on intestinal permeability in the adult period and, particularly, in the cardiometabolic field.
3.3. Osteopontin and Intestinal Microbiota
A considerable study has explored the multifaceted involvement of OPN in multiple biological processes, bone remodeling, and inflammation []. However, less is known about OPN interaction with microbiota.
As mentioned above, OPN-deficient mice showed the importance of OPN in potent development of T-helper 1 immune responses, suggesting its critical function in fighting microbial and viral infection [,]. Although not all studies are unanimous on the role of OPN in the control of intestinal inflammation, OPN knockout fast-tracked the development of spontaneous mouse colitis in response to gut microbiota dysbiosis []. Differences were noted in enteric bacterial composition, including the augmented abundance of the Clostridium cluster and the diminished abundance of the Clostridium subcluster XIVa, which earlier displayed a protective impact on murine models []. Previous investigations revealed the high effectiveness of human macrophages to phagocytose E. coli under OPN influence []. In fact, OPN favors macrophage phagocytosis, culminating in destroying bacteria and fungi []. Overall, OPN strongly bound to various bacteria via its actions as an opsonin, which stimulated macrophage phagocytosis []. The mechanism for the preservation of gut barrier and liver homeostasis in animal-administered alcohol could account for the beneficial modification of intestinal microbiota, thereby leading to the production of tryptophan metabolites and short-chain fatty acids. Thus, OPN acts as a multifunctional cytokine that can exert both pro- and anti-inflammatory effects, depending on physiological and pathophysiological conditions. This duality may suggest divergent intestinal microbiota regulation.
3.4. Clinical Evidence for Osteopontin Effectiveness
As mentioned previously, clinical trials provided the evidence that supplementation of infant formulas with bovine OPN was safe and well absorbed [], lowered inflammatory cytokines, and displayed potency in decreasing the prevalence of fever [,]. In a preclinical investigation using the pig model, OPN administration led to slight ameliorations in gut structure and systemic immunity []. Furthermore, OPN supplementation could prevent necrotizing enterocolitis in preterm piglets [].
The influence of weight loss was assessed on OPN status in children and adolescents, who were on a diet and exercise program []. The results revealed a positive relationship between decreased endogenous OPN and diminished body mass index, which confirms the data of a study documenting OPN elevation in overweight and obese subjects []. Interestingly, higher OPN levels were detected in the adipose tissue of morbidly obese patients in association with macrophage infiltration [,]. Likewise, mouse obesity resulting from a high-fat diet regimen was characterized by high plasma OPN concentrations and enhanced OPN expression in macrophages recruited into adipose tissue []. On the other hand, other reports pointed out the lack of correlation between plasma OPN and waist circumference or body mass index [,,]. This is in line with adipocyte cultures, which exhibited modest effects after their exposure to OPN []. Similarly, the induction of OPN and its receptor CD44 in the liver was locally related to IR and liver damage []. It is possible that OPN overexpression in hepatic natural killer cells led to endoplasmic reticulum stress and JNK hyperactivation, thereby impairing insulin signaling in the liver []. Various groups also highlighted the association of OPN with diabetes, metabolic associated fatty liver disease, stroke, and carotid atherosclerosis [,,,,].
OPN may act as an immune system enhancer, promoting infant and child development. Furthermore, OPN exhibits the capacity to protect the intestinal mucosal barrier while maintaining the ecological balance of gut microbiota, thereby preserving cardiometabolic health. Nevertheless, if OPN has been instrumental for various physiological processes, it has been associated with a broad range of pathophysiological conditions, especially in the adult stage. OPN was considered as a central mediator of atherosclerotic plaque development and arterial calcification [,,,]. In addition, in subjects with coronary artery disease and peripheral artery disease, elevated OPN concentrations could predict future cardiovascular death and long-term adverse outcomes, respectively [,]. However, not only were other groups unable to detect any association between circulating OPN and coronary artery disease degree or severity [], but inconsistencies and contradictions were reported. In addition, beneficial actions of OPN have been highlighted, including cytoprotective impact on cardiac endothelial cells along with their survival in stress conditions [,], and inhibition of their apoptosis [,]. In keeping with favorable OPN findings, OPN-knockout mice revealed defective myocardial angiogenic response post-myocardial infarction, thereby culminating in detrimental left ventricle remodeling [], which clearly indicates OPN function in restoring myocardial capillarization in infarcted myocardium. In the same line, OPN has recently been found to promote infarct repair via the amelioration of scar formation and cardiac function []; and, on the other hand, OPN knockout mice exhibited vulnerability in developing post-myocardial infarction left-ventricular-chamber dilatation [].
Indeed, tissue infiltration of macrophages as observed in obesity is dependent on the expression of OPN, which promotes monocyte chemotaxis and motility. Recently, Nomiyama et al. [] demonstrated that mice after a high-fat diet exhibited increased circulating OPN levels. Obese mice lacking OPN showed improved insulin sensitivity and decreased macrophage infiltration into adipose tissue. These experiments add OPN to a long list of pro-inflammatory pathways involved in the development of IR.
The same discords apply to the role of OPN in CMD. If OPN was associated in the development of adipose tissue inflammation and IR [,], obesity, and hepatic steatosis [,], there was an unaltered fatty acid oxidation and synthesis between wild-type and OPN knockout livers in obesity (along with the downregulation of Forkhead box O1 and PPAR gamma co-activator 1α), which usually enhance mitochondrion genesis, lipid degradation, and insulin sensitivity []. Other reports underline that OPN deficiency in old mice led to hepatic steatosis and hypertriglyceridemia in relationship with IR []. Similarly, liver fibrosis was noted in OPN-knockout mice under high-fat feeding in association with inflammation, DNA damage, and IR []. The hepatic protective role of OPN was emphasized in alcoholic hepatitis [,] and in transgenic mice, which markedly lowered hepatic steatosis, balloon cell degeneration, lipid peroxidation, inflammation, and plasma alanine aminotransferase [].
3.5. Attempts to Explain the Areas of Uncertainty and Divergence Observed in the Numerous Studies
The first observation that can be made is that surprisingly levels of OPN have not been established in healthy people, and, furthermore, the cut-off values are quite arbitrary in most scientific reports, which may have an impact on the interpretation of the findings. What further complicates the picture is that investigators measured total OPN expression, without taking into account the three OPN isoforms resulting from human OPN splicing (OPN-a, OPN-b, and OPN-c) []. To this should be added divergences in experimental models and the methodology for determining OPN, thereby explicating the huge heterogeneity of the outcomes. Probably also, the limited number of specimens/animals, the small size of the cohorts, and the fluctuating follow-up duration must have significantly favored the great variability of the results. Other important drawbacks include the cause-effect relationship between OPN and clinical outcomes, as well as the interference of different pharmaceutical agents that the patients were already on at the time the treatment when OPN was added. Finally, the discordant effects of OPN may be attributed to its large groups of variants that happen as a consequence of transcriptional, posttranscriptional, and post translational modifications; and include phosphorylation, proteolytic cleavage, sialylation, and transglutaminase cross-linking. These OPN variants may be specific to the type of organs, tissues, and cells. It is also worth noting that CD44, the receptor of OPN, also presents various isoforms that probably are tissue-specific in accordance with their OPN ligands of a polymorphic nature []. Clearly, studies to date have not taken into account the specificities of intra-tissue or cellular OPN and CD44. Definitely, additional work is needed to elucidate these exciting aspects.
4. General Lactoferrin–Osteopontin Properties
The multifunctional LF and OPN molecules have a high affinity for each other due to their opposite charge [], and form a complex in native human and bovine milk []. Even in vitro, purified LF and OPN proteins can form an LF–OPN complex with high affinity, driven by electrostatic forces []. The LF–OPN complex binds to LF and/or OPN receptors on the cell membrane of intestinal epithelial cells, thus exerting similar or stronger effects on the intestine compared to individual proteins of this complex []. In fact, the LF–OPN complex exhibited a higher ability to stimulate proliferation and differentiation of intestinal cells than LF and OPN separately []. The LF–OPN complex is resistant to GI digestion, crosses the intestinal barrier without degradation, and reaches in intact from the bloodstream [,]. In addition, the LF–OPN complex triggered an enhanced secretion of the IL-18 intermediate between LF and OPN [], which protects the intestinal epithelium from inflammation [] (Table 1).

Table 1.
Characteristics of lactoferrin, osteopontin, and lactoferrin–osteopontin complex.
4.1. LF–OPN Complex, Intestinal Barrier, and Microbiota
Despite the intense efforts that have been deployed on each of the LF and OPN proteins in relation to the intestinal barrier and gut microbiota, there is virtually no work on the influence of the LF–OPN complex. Astonishingly, limited evidences are reported by the scientific literature concerning the impact of the complex. In this context, the exposure of Caco-2 cells to the LF–OPN complex resulted in a marked secretory stimulation of IL-18 []. This cytokine is known to produce a protective effect against enterocyte inflammation and colitis [,], which is supported by the susceptibility of IL-18 knockout mice and IL-18 receptor knockout mice to colitis []. Both IL-18 and IL-18r seem necessary for intestinal barrier integrity, epithelium homeostasis, mucosal repair mechanisms, and appropriate host interaction with the gut microbiota [,]. However, it is essential to obtain direct proof of concept by studying the effect of the LF–OPN complex on barrier function, especially in view of the contradictions reported by other studies [,]. There is also a need to delineate the mechanisms of the antibacterial activity and function (as noted for enteropathogenic Escherichia coli) in response to the LF–OPN complex [].
4.2. Clinical Evidence for LF–OPN Complex Effectiveness in Cardiometabolic Diseases
In spite of their praiseworthy features of LF and OPN individually, to our knowledge, no study has yet been reported examining their combinative effects on CMD. Therefore, additional investigations are required to tackle the following issues: Does the LF–OPN complex alleviate OxS and inflammation, which contribute to IR, a factor promoting the clinical manifestations of MetS components? Would the LF–OPN complex be capable of blunting obesity, dyslipidemia, metabolic endotoxemia, and gut–liver axis pathogenesis (responsible for the development and progression of metabolic associated fatty liver disease)? Is the metabolic health improvement mediated by intestinal barrier function and a gut microbiota-dependent mechanism?
5. Conclusions
Intense research is underway to develop new therapeutic strategies in the treatment and management of CMD. Attention is specially paid to new therapeutic strategies that target their causal factors. Currently, a growing attention is devoted to human breast and bovine milk proteins, which are regarded as indispensable health-promoting foods. This review has outlined the current knowledge of two multifunctional bioactive milk proteins, LF and OPN, which can bind together to form a powerful LF–OPN complex with more beneficial effects. Considerable emphasis has initially been placed on the physiological, biochemical, and nutritional functionalities of these biologically active compounds, while revealing their advantageous effects on the development of preterm babies, neonates, and infants. Antioxidant, anti-inflammatory, immunomodulatory, antibacterial, antifungal, and antiviral activities explain the highly protective and growth-promoting mechanisms of these milk-derived proteins. In a second step, an updated overview was provided on how the latter shape intestinal homeostasis through the reinforcement of intestinal mucosal barrier and gut microbiota, two robust guardians of the cardiometabolic health. Finally, a critical analysis of preclinical and clinical studies was presented documenting the influence of each bioactive protein on CMD. Particular attention should be devoted to the administered dose of LF and OPN. By examining the literature, one can quickly notice that the doses of lactoferrin or osteopontin are very variable in the different preclinical and clinical trials. Some groups administered them orally, intraperitoneally, or systemically. Others delivered them in a single dose or gave them over several days with or without consideration of body weight. These differences make comparisons difficult and explain the discrepancies in results. In addition, the relationship between the dose administered and blood concentration is very poorly documented in clinical trials. This is a set of important points that require the attention of the scientific community.
Author Contributions
Conceptualization, E.L. and S.S.; methodology, E.L. and S.S.; resources, E.L.; writing—original draft preparation, E.L., E.D. and S.S.; writing—review and editing, E.L., V.M., S.T.É.B., N.D., E.D. and S.S.; visualization, E.L. and S.S.; supervision, E.L.; funding acquisition, E.L. All authors have read and agreed to the published version of the manuscript.
Funding
The current work was supported by a research grant from the Dairy Farmers of Canada (E8497) and the JA DeSève Research Chair in Nutrition (E.L.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Haschke, F.; Haiden, N.; Thakkar, S.K. Nutritive and Bioactive Proteins in Breastmilk. Ann. Nutr. Metab. 2016, 69 (Suppl. 2), 17–26. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Meletharayil, G.; Kapoor, R.; Abbaspourrad, A. Bioactives in bovine milk: Chemistry, technology, and applications. Nutr. Rev. 2021, 79, 48–69. [Google Scholar] [CrossRef] [PubMed]
- Beck, K.L.; Weber, D.; Phinney, B.S.; Smilowitz, J.T.; Hinde, K.; Lonnerdal, B.; Korf, I.; Lemay, D.G. Comparative Proteomics of Human and Macaque Milk Reveals Species-Specific Nutrition during Postnatal Development. J. Proteome Res. 2015, 14, 2143–2157. [Google Scholar] [CrossRef] [PubMed]
- Harhay, G.P.; Smith, T.P.; Alexander, L.J.; Haudenschild, C.D.; Keele, J.W.; Matukumalli, L.K.; Schroeder, S.G.; Van Tassell, C.P.; Gresham, C.R.; Bridges, S.M.; et al. An atlas of bovine gene expression reveals novel distinctive tissue characteristics and evidence for improving genome annotation. Genome Biol. 2010, 11, R102. [Google Scholar] [CrossRef]
- Lemay, D.G.; Hovey, R.C.; Hartono, S.R.; Hinde, K.; Smilowitz, J.T.; Ventimiglia, F.; Schmidt, K.A.; Lee, J.W.; Islas-Trejo, A.; Silva, P.I.; et al. Sequencing the transcriptome of milk production: Milk trumps mammary tissue. BMC Genom. 2013, 14, 872. [Google Scholar] [CrossRef]
- Schack, L.; Lange, A.; Kelsen, J.; Agnholt, J.; Christensen, B.; Petersen, T.E.; Sorensen, E.S. Considerable variation in the concentration of osteopontin in human milk, bovine milk, and infant formulas. J. Dairy Sci. 2009, 92, 5378–5385. [Google Scholar] [CrossRef]
- Ward, P.P.; Uribe-Luna, S.; Conneely, O.M. Lactoferrin and host defense. Biochem. Cell Biol. 2002, 80, 95–102. [Google Scholar] [CrossRef]
- Farnaud, S.; Evans, R.W. Lactoferrin—A multifunctional protein with antimicrobial properties. Mol. Immunol. 2003, 40, 395–405. [Google Scholar] [CrossRef]
- Ward, P.P.; Paz, E.; Conneely, O.M. Multifunctional roles of lactoferrin: A critical overview. Cell. Mol. Life Sci. 2005, 62, 2540–2548. [Google Scholar] [CrossRef]
- Conneely, O.M. Antiinflammatory activities of lactoferrin. J. Am. Coll. Nutr. 2001, 20, 389S–395S; discussion 396S–397S. [Google Scholar] [CrossRef]
- Li, Y.C.; Hsieh, C.C. Lactoferrin dampens high-fructose corn syrup-induced hepatic manifestations of the metabolic syndrome in a murine model. PLoS ONE 2014, 9, e97341. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.S.; Lin, C.F.; Chen, P.W. Transcriptome analysis of Lactobacillus rhamnosus GG strain treated with prebiotic—Bovine lactoferrin under a cold environment. J. Food Drug. Anal. 2021, 29, 402–418. [Google Scholar] [CrossRef] [PubMed]
- Mayeur, S.; Veilleux, A.; Pouliot, Y.; Lamarche, B.; Beaulieu, J.F.; Hould, F.S.; Richard, D.; Tchernof, A.; Levy, E. Plasma Lactoferrin Levels Positively Correlate with Insulin Resistance despite an Inverse Association with Total Adiposity in Lean and Severely Obese Patients. PLoS ONE 2016, 11, e0166138. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Zapata, R.C.; Pezeshki, A.; Chelikani, P.K. Dietary lactalbumin and lactoferrin interact with inulin to modulate energy balance in obese rats. Obesity 2017, 25, 1050–1060. [Google Scholar] [CrossRef]
- Singh, A.; Zapata, R.C.; Pezeshki, A.; Knight, C.G.; Tuor, U.I.; Chelikani, P.K. Whey Protein and Its Components Lactalbumin and Lactoferrin Affect Energy Balance and Protect against Stroke Onset and Renal Damage in Salt-Loaded, High-Fat Fed Male Spontaneously Hypertensive Stroke-Prone Rats. J. Nutr. 2020, 150, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Ren, F.; Lv, J.; Zhang, H.; Guo, H. Lactoferrin attenuates high-fat diet-induced hepatic steatosis and lipid metabolic dysfunctions by suppressing hepatic lipogenesis and down-regulating inflammation in C57BL/6J mice. Food Funct. 2018, 9, 4328–4339. [Google Scholar] [CrossRef]
- Morishita, S.; Ono, T.; Fujisaki, C.; Ishihara, Y.; Murakoshi, M.; Kato, H.; Hosokawa, M.; Miyashita, K.; Sugiyama, K.; Nishino, H. Bovine lactoferrin reduces visceral fat and liver triglycerides in ICR mice. J. Oleo Sci. 2013, 62, 97–103. [Google Scholar] [CrossRef]
- Jamka, M.; Kaczmarek, N.; Madry, E.; Krzyzanowska-Jankowska, P.; Bajerska, J.; Kregielska-Narozna, M.; Bogdanski, P.; Walkowiak, J. Metabolic Health in Obese Subjects-Is There a Link to Lactoferrin and Lactoferrin Receptor-Related Gene Polymorphisms? Nutrients 2020, 12, 2843. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Ortega, F.J.; Bassols, J.; Ricart, W.; Fernandez-Real, J.M. Decreased circulating lactoferrin in insulin resistance and altered glucose tolerance as a possible marker of neutrophil dysfunction in type 2 diabetes. J. Clin. Endocrinol. Metab. 2009, 94, 4036–4044. [Google Scholar] [CrossRef]
- Chen, P.W.; Liu, Z.S.; Kuo, T.C.; Hsieh, M.C.; Li, Z.W. Prebiotic effects of bovine lactoferrin on specific probiotic bacteria. Biometals 2017, 30, 237–248. [Google Scholar] [CrossRef]
- Sienkiewicz, M.; Jaskiewicz, A.; Tarasiuk, A.; Fichna, J. Lactoferrin: An overview of its main functions, immunomodulatory and antimicrobial role, and clinical significance. Crit. Rev. Food Sci. Nutr. 2022, 62, 6016–6033. [Google Scholar] [CrossRef] [PubMed]
- Grzywacz, K.; Butcher, J.; Romain, G.; Li, J.; Stintzi, A. The impact of probiotics and lactoferrin supplementation on piglet gastrointestinal microbial communities. Biometals 2019, 32, 533–543. [Google Scholar] [CrossRef]
- Belles, A.; Aguirre-Ramirez, D.; Abad, I.; Parras-Molto, M.; Sanchez, L.; Grasa, L. Lactoferrin modulates gut microbiota and Toll-like receptors (TLRs) in mice with dysbiosis induced by antibiotics. Food Funct. 2022, 13, 5854–5869. [Google Scholar] [CrossRef] [PubMed]
- Connell, S.; Kawashima, M.; Nakamura, S.; Imada, T.; Yamamoto, H.; Tsubota, K.; Fukuda, S. Lactoferrin Ameliorates Dry Eye Disease Potentially through Enhancement of Short-Chain Fatty Acid Production by Gut Microbiota in Mice. Int. J. Mol. Sci. 2021, 22, 12384. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Zhao, F.; Wang, J.; Zhu, W. Early-life lactoferrin intervention modulates the colonic microbiota, colonic microbial metabolites and intestinal function in suckling piglets. Appl. Microbiol. Biotechnol. 2020, 104, 6185–6197. [Google Scholar] [CrossRef]
- Juskiewicz, J.; Rawicka, A.; Fotschki, B.; Majewski, M.; Zdunczyk, Z. Influence of Supplementation of Lactoferrin, Melittin and Cecropin A to Rat Diet on Changes in Faecal Ammonia Concentrations, Short-Chain Fatty Acid Concentrations and Activities of Bacterial Enzymes. Animals 2021, 11, 1203. [Google Scholar] [CrossRef]
- Hu, P.; Zong, Q.; Zhao, Y.; Gu, H.; Liu, Y.; Gu, F.; Liu, H.Y.; Ahmed, A.A.; Bao, W.; Cai, D. Lactoferrin Attenuates Intestinal Barrier Dysfunction and Inflammation by Modulating the MAPK Pathway and Gut Microbes in Mice. J. Nutr. 2022, 152, 2451–2460. [Google Scholar] [CrossRef]
- Liu, N.; Feng, G.; Zhang, X.; Hu, Q.; Sun, S.; Sun, J.; Sun, Y.; Wang, R.; Zhang, Y.; Wang, P.; et al. The Functional Role of Lactoferrin in Intestine Mucosal Immune System and Inflammatory Bowel Disease. Front. Nutr. 2021, 8, 759507. [Google Scholar] [CrossRef]
- Wang, W.; Cheng, Z.; Wang, X.; An, Q.; Huang, K.; Dai, Y.; Meng, Q.; Zhang, Y. Lactoferrin, a Critical Player in Neonate Intestinal Development: RHLF may be a Good Choice in Formula. J. Agric. Food Chem. 2021, 69, 8726–8736. [Google Scholar] [CrossRef]
- Liu, C.; Peng, Q.; Wei, L.; Li, Z.; Zhang, X.; Wu, Y.; Wang, J.; Zheng, X.; Wen, Y.; Zheng, R.; et al. Deficiency of Lactoferrin aggravates lipopolysaccharide-induced acute inflammation via recruitment macrophage in mice. Biometals 2022, 1–14. [Google Scholar] [CrossRef]
- Valenti, P.; Antonini, G. Lactoferrin: An important host defence against microbial and viral attack. Cell. Mol. Life Sci. 2005, 62, 2576–2587. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, K.; Takeuchi, T.; Ozaki, T.; Shimizu, H.; Ando, K.; Miyamoto, A.; Harada, E. Bovine lactoferrin has a nitric oxide-dependent hypotensive effect in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R359–R365. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Musoles, R.; Lopez-Diez, J.J.; Torregrosa, G.; Valles, S.; Alborch, E.; Manzanares, P.; Salom, J.B. Lactoferricin B-derived peptides with inhibitory effects on ECE-dependent vasoconstriction. Peptides 2010, 31, 1926–1933. [Google Scholar] [CrossRef]
- Manzanares, P.; Salom, J.B.; Garcia-Tejedor, A.; Fernandez-Musoles, R.; Ruiz-Gimenez, P.; Gimeno-Alcaniz, J.V. Unraveling the mechanisms of action of lactoferrin-derived antihypertensive peptides: ACE inhibition and beyond. Food Funct. 2015, 6, 2440–2452. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Li, D.; Chen, J.; Li, Y.H.; Zhang, Z.; Hidayat, K.; Wan, Z.; Xu, J.Y.; Qin, L.Q. Lactoferrin improves hepatic insulin resistance and pancreatic dysfunction in high-fat diet and streptozotocin-induced diabetic mice. Nutr. Res. 2022, 103, 47–58. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Ortega, F.J.; Ricart, W.; Fernandez-Real, J.M. Lactoferrin increases (172Thr)AMPK phosphorylation and insulin-induced (p473Ser)AKT while impairing adipocyte differentiation. Int. J. Obes. 2009, 33, 991–1000. [Google Scholar] [CrossRef]
- Takeuchi, T.; Shimizu, H.; Ando, K.; Harada, E. Bovine lactoferrin reduces plasma triacylglycerol and NEFA accompanied by decreased hepatic cholesterol and triacylglycerol contents in rodents. Br. J. Nutr. 2004, 91, 533–538. [Google Scholar] [CrossRef]
- Guo, C.; Xue, H.; Guo, T.; Zhang, W.; Xuan, W.Q.; Ren, Y.T.; Wang, D.; Chen, Y.H.; Meng, Y.H.; Gao, H.L.; et al. Recombinant human lactoferrin attenuates the progression of hepatosteatosis and hepatocellular death by regulating iron and lipid homeostasis in ob/ob mice. Food Funct. 2020, 11, 7183–7196. [Google Scholar] [CrossRef]
- Nakamura, K.; Morishita, S.; Ono, T.; Murakoshi, M.; Sugiyama, K.; Kato, H.; Ikeda, I.; Nishino, H. Lactoferrin interacts with bile acids and increases fecal cholesterol excretion in rats. Biochem. Cell Biol. 2017, 95, 142–147. [Google Scholar] [CrossRef]
- Ling, C.J.; Xu, J.Y.; Li, Y.H.; Tong, X.; Yang, H.H.; Yang, J.; Yuan, L.X.; Qin, L.Q. Lactoferrin promotes bile acid metabolism and reduces hepatic cholesterol deposition by inhibiting the farnesoid X receptor (FXR)-mediated enterohepatic axis. Food Funct. 2019, 10, 7299–7307. [Google Scholar] [CrossRef]
- Itell, H.L.; Berenz, A.; Mangan, R.J.; Permar, S.R.; Kaufman, D.A. Systemic and mucosal levels of lactoferrin in very low birth weight infants supplemented with bovine lactoferrin. Biochem. Cell Biol. 2021, 99, 25–34. [Google Scholar] [CrossRef]
- Moriguchi, J.; Kato, R.; Nakagawa, M.; Hirotani, Y.; Ijiri, Y.; Tanaka, K. Effects of lipopolysaccharide on intestinal P-glycoprotein expression and activity. Eur. J. Pharmacol. 2007, 565, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Fink, M.P.; Yang, R.; Delude, R.L. Increased iNOS activity is essential for intestinal epithelial tight junction dysfunction in endotoxemic mice. Shock 2004, 21, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Drolia, R.; Bhunia, A.K. Crossing the Intestinal Barrier via Listeria Adhesion Protein and Internalin A. Trends Microbiol. 2019, 27, 408–425. [Google Scholar] [CrossRef] [PubMed]
- Garas, L.C.; Feltrin, C.; Hamilton, M.K.; Hagey, J.V.; Murray, J.D.; Bertolini, L.R.; Bertolini, M.; Raybould, H.E.; Maga, E.A. Milk with and without lactoferrin can influence intestinal damage in a pig model of malnutrition. Food Funct. 2016, 7, 665–678. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, X.X.; Liu, Y.; Xi, E.Z.; An, J.J.; Tabys, D.; Liu, N. The In Vitro Protective Role of Bovine Lactoferrin on Intestinal Epithelial Barrier. Molecules 2019, 24, 148. [Google Scholar] [CrossRef]
- Kazmierczak, N.; Grygorcewicz, B.; Roszak, M.; Bochentyn, B.; Piechowicz, L. Comparative Assessment of Bacteriophage and Antibiotic Activity against Multidrug-Resistant Staphylococcus aureus Biofilms. Int. J. Mol. Sci. 2022, 23, 1274. [Google Scholar] [CrossRef]
- Shen, L.; Weber, C.R.; Raleigh, D.R.; Yu, D.; Turner, J.R. Tight junction pore and leak pathways: A dynamic duo. Annu. Rev. Physiol. 2011, 73, 283–309. [Google Scholar] [CrossRef]
- Duffey, M.E.; Hainau, B.; Ho, S.; Bentzel, C.J. Regulation of epithelial tight junction permeability by cyclic AMP. Nature 1981, 294, 451–453. [Google Scholar] [CrossRef]
- Bein, A.; Zilbershtein, A.; Golosovsky, M.; Davidov, D.; Schwartz, B. LPS Induces Hyper-Permeability of Intestinal Epithelial Cells. J. Cell. Physiol. 2017, 232, 381–390. [Google Scholar] [CrossRef]
- Hering, N.A.; Luettig, J.; Krug, S.M.; Wiegand, S.; Gross, G.; van Tol, E.A.; Schulzke, J.D.; Rosenthal, R. Lactoferrin protects against intestinal inflammation and bacteria-induced barrier dysfunction in vitro. Ann. N. Y. Acad. Sci. 2017, 1405, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Hirotani, Y.; Ikeda, K.; Kato, R.; Myotoku, M.; Umeda, T.; Ijiri, Y.; Tanaka, K. Protective effects of lactoferrin against intestinal mucosal damage induced by lipopolysaccharide in human intestinal Caco-2 cells. Yakugaku Zasshi 2008, 128, 1363–1368. [Google Scholar] [CrossRef] [PubMed]
- Zong, X.; Hu, W.; Song, D.; Li, Z.; Du, H.; Lu, Z.; Wang, Y. Porcine lactoferrin-derived peptide LFP-20 protects intestinal barrier by maintaining tight junction complex and modulating inflammatory response. Biochem. Pharmacol. 2016, 104, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, J.; Wu, W.; Shi, J.; Zhong, Y.; van Tol, E.A.; Tang, Q.; Cai, W. Enteral supplementation of bovine lactoferrin improves gut barrier function in rats after massive bowel resection. Br. J. Nutr. 2014, 112, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Song, L.; Wang, X.; Xu, Y.; Liu, Z.; Zhao, D.; Wang, S.; Fan, X.; Wang, Z.; Gao, C.; et al. A bovine lactoferricin-lactoferrampin-encoding Lactobacillus reuteri CO21 regulates the intestinal mucosal immunity and enhances the protection of piglets against enterotoxigenic Escherichia coli K88 challenge. Gut Microbes 2021, 13, 1956281. [Google Scholar] [CrossRef]
- Arnott, I.D.; Kingstone, K.; Ghosh, S. Abnormal intestinal permeability predicts relapse in inactive Crohn disease. Scand. J. Gastroenterol. 2000, 35, 1163–1169. [Google Scholar] [CrossRef]
- Michielan, A.; D’Inca, R. Intestinal Permeability in Inflammatory Bowel Disease: Pathogenesis, Clinical Evaluation, and Therapy of Leaky Gut. Mediat. Inflamm. 2015, 2015, 628157. [Google Scholar] [CrossRef]
- MacManus, C.F.; Collins, C.B.; Nguyen, T.T.; Alfano, R.W.; Jedlicka, P.; de Zoeten, E.F. VEN-120, a Recombinant Human Lactoferrin, Promotes a Regulatory T Cell [Treg] Phenotype and Drives Resolution of Inflammation in Distinct Murine Models of Inflammatory Bowel Disease. J. Crohn’s Colitis 2017, 11, 1101–1112. [Google Scholar] [CrossRef]
- Song, L.; Xie, W.; Liu, Z.; Guo, D.; Zhao, D.; Qiao, X.; Wang, L.; Zhou, H.; Cui, W.; Jiang, Y.; et al. Oral delivery of a Lactococcus lactis strain secreting bovine lactoferricin-lactoferrampin alleviates the development of acute colitis in mice. Appl. Microbiol. Biotechnol. 2019, 103, 6169–6186. [Google Scholar] [CrossRef]
- Troost, F.J.; Saris, W.H.; Brummer, R.J. Recombinant human lactoferrin ingestion attenuates indomethacin-induced enteropathy in vivo in healthy volunteers. Eur. J. Clin. Nutr. 2003, 57, 1579–1585. [Google Scholar] [CrossRef]
- Tian, H.; Maddox, I.S.; Ferguson, L.R.; Shu, Q. Influence of bovine lactoferrin on selected probiotic bacteria and intestinal pathogens. Biometals 2010, 23, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Woodman, T.; Strunk, T.; Patole, S.; Hartmann, B.; Simmer, K.; Currie, A. Effects of lactoferrin on neonatal pathogens and Bifidobacterium breve in human breast milk. PLoS ONE 2018, 13, e0201819. [Google Scholar] [CrossRef] [PubMed]
- De Sa Almeida, J.S.; de Oliveira Marre, A.T.; Teixeira, F.L.; Boente, R.F.; Domingues, R.; de Paula, G.R.; Lobo, L.A. Lactoferrin and lactoferricin B reduce adhesion and biofilm formation in the intestinal symbionts Bacteroides fragilis and Bacteroides thetaiotaomicron. Anaerobe 2020, 64, 102232. [Google Scholar] [CrossRef]
- Nairz, M.; Schroll, A.; Sonnweber, T.; Weiss, G. The struggle for iron—A metal at the host-pathogen interface. Cell. Microbiol. 2010, 12, 1691–1702. [Google Scholar] [CrossRef]
- Elass-Rochard, E.; Roseanu, A.; Legrand, D.; Trif, M.; Salmon, V.; Motas, C.; Montreuil, J.; Spik, G. Lactoferrin-lipopolysaccharide interaction: Involvement of the 28–34 loop region of human lactoferrin in the high-affinity binding to Escherichia coli 055B5 lipopolysaccharide. Biochem. J. 1995, 312 Pt 3, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, K.; Jurgens, G.; Muller, M.; Fukuoka, S.; Koch, M.H. Biophysical characterization of lipopolysaccharide and lipid A inactivation by lactoferrin. Biol. Chem. 2001, 382, 1215–1225. [Google Scholar] [CrossRef]
- Appelmelk, B.J.; An, Y.Q.; Geerts, M.; Thijs, B.G.; de Boer, H.A.; MacLaren, D.M.; de Graaff, J.; Nuijens, J.H. Lactoferrin is a lipid A-binding protein. Infect. Immun. 1994, 62, 2628–2632. [Google Scholar] [CrossRef]
- Ostan, N.K.H.; Moraes, T.F.; Schryvers, A.B. Lactoferrin receptors in Gram-negative bacteria: An evolutionary perspective. Biochem. Cell Biol. 2021, 99, 102–108. [Google Scholar] [CrossRef]
- Singh, P.K.; Parsek, M.R.; Greenberg, E.P.; Welsh, M.J. A component of innate immunity prevents bacterial biofilm development. Nature 2002, 417, 552–555. [Google Scholar] [CrossRef]
- Brock, J.H. Lactoferrin—50 years on. Biochem. Cell Biol. 2012, 90, 245–251. [Google Scholar] [CrossRef]
- Bruni, N.; Capucchio, M.T.; Biasibetti, E.; Pessione, E.; Cirrincione, S.; Giraudo, L.; Corona, A.; Dosio, F. Antimicrobial Activity of Lactoferrin-Related Peptides and Applications in Human and Veterinary Medicine. Molecules 2016, 21, 752. [Google Scholar] [CrossRef] [PubMed]
- De Bortoli, N.; Leonardi, G.; Ciancia, E.; Merlo, A.; Bellini, M.; Costa, F.; Mumolo, M.G.; Ricchiuti, A.; Cristiani, F.; Santi, S.; et al. Helicobacter pylori eradication: A randomized prospective study of triple therapy versus triple therapy plus lactoferrin and probiotics. Am. J. Gastroenterol. 2007, 102, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, Y.; He, J.; Zhu, W. Antibacterial properties of lactoferrin: A bibliometric analysis from 2000 to early 2022. Front. Microbiol. 2022, 13, 947102. [Google Scholar] [CrossRef] [PubMed]
- Shestakov, A.; Jenssen, H.; Nordstrom, I.; Eriksson, K. Lactoferricin but not lactoferrin inhibit herpes simplex virus type 2 infection in mice. Antivir. Res. 2012, 93, 340–345. [Google Scholar] [CrossRef]
- Andersen, J.H.; Jenssen, H.; Gutteberg, T.J. Lactoferrin and lactoferricin inhibit Herpes simplex 1 and 2 infection and exhibit synergy when combined with acyclovir. Antivir. Res. 2003, 58, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.B.; Cheung, R.C.; Wong, J.H.; Wang, Y.; Ip, D.T.; Wan, D.C.; Xia, J. Antiviral activities of whey proteins. Appl. Microbiol. Biotechnol. 2015, 99, 6997–7008. [Google Scholar] [CrossRef]
- Omata, Y.; Satake, M.; Maeda, R.; Saito, A.; Shimazaki, K.; Yamauchi, K.; Uzuka, Y.; Tanabe, S.; Sarashina, T.; Mikami, T. Reduction of the infectivity of Toxoplasma gondii and Eimeria stiedai sporozoites by treatment with bovine lactoferricin. J. Vet. Med. Sci. 2001, 63, 187–190. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Abe, S.; Takakura, N. Potential usefulness of bovine lactoferrrin for adjunctive immunotherapy for mucosal Candida infections. Biometals 2004, 17, 245–248. [Google Scholar] [CrossRef]
- Fernandes, K.E.; Weeks, K.; Carter, D.A. Lactoferrin Is Broadly Active against Yeasts and Highly Synergistic with Amphotericin B. Antimicrob. Agents Chemother. 2020, 64, E02284-19. [Google Scholar] [CrossRef]
- Montone, A.M.I.; Papaianni, M.; Malvano, F.; Capuano, F.; Capparelli, R.; Albanese, D. Lactoferrin, Quercetin, and Hydroxyapatite Act Synergistically against Pseudomonas fluorescens. Int. J. Mol. Sci. 2021, 22, 9247. [Google Scholar] [CrossRef]
- Ochoa, T.J.; Pezo, A.; Cruz, K.; Chea-Woo, E.; Cleary, T.G. Clinical studies of lactoferrin in children. Biochem. Cell Biol. 2012, 90, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Lowe, D.G.; Chang, M.S.; Hellmiss, R.; Chen, E.; Singh, S.; Garbers, D.L.; Goeddel, D.V. Human atrial natriuretic peptide receptor defines a new paradigm for second messenger signal transduction. EMBO J. 1989, 8, 1377–1384. [Google Scholar] [CrossRef]
- Gao, Y.; Hou, L.; Lu, C.; Wang, Q.; Pan, B.; Wang, Q.; Tian, J.; Ge, L. Enteral Lactoferrin Supplementation for Preventing Sepsis and Necrotizing Enterocolitis in Preterm Infants: A Meta-Analysis With Trial Sequential Analysis of Randomized Controlled Trials. Front. Pharmacol. 2020, 11, 1186. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, A.; Buca, D.; Tinari, S.; Oronzii, L.; Lucidi, A.; Sebastiano, F.D.; Liberati, M.; D’Antonio, F. Role of lactoferrin in preventing preterm birth and pregnancy complications: A systematic review and meta-analysis. Minerva Obstet. Gynecol. 2022; online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Pammi, M.; Suresh, G. Enteral lactoferrin supplementation for prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Database Syst. Rev. 2020, 3, CD007137. [Google Scholar] [CrossRef]
- Kaufman, D.A.; Berenz, A.; Itell, H.L.; Conaway, M.; Blackman, A.; Nataro, J.P.; Permar, S.R. Dose escalation study of bovine lactoferrin in preterm infants: Getting the dose right. Biochem. Cell Biol. 2021, 99, 7–13. [Google Scholar] [CrossRef]
- Dobryk, D.; Dobryk, O.; Dobryanskyy, D. The Effect of Enteral Lactoferrin Supplementation in Prevention of Morbidity Associated with Immature Digestive Tract in Premature Infants: Prospective Cohort Study. Georgian Med. News 2022, 323, 94–101. Available online: https://europepmc.org/article/med/35271478 (accessed on 7 January 2023).
- Miranda, M.; Saccone, G.; Ammendola, A.; Salzano, E.; Iannicelli, M.; De Rosa, R.; Nazzaro, G.; Locci, M. Vaginal lactoferrin in prevention of preterm birth in women with bacterial vaginosis. J. Matern. Fetal Neonatal Med. 2021, 34, 3704–3708. [Google Scholar] [CrossRef]
- Locci, M.; Nazzaro, G.; Miranda, M.; Salzano, E.; Montagnani, S.; Castaldo, C.; De Placido, G. Vaginal lactoferrin in asymptomatic patients at low risk for pre-term labour for shortened cervix: Cervical length and interleukin-6 changes. J. Obstet. Gynaecol. 2013, 33, 144–148. [Google Scholar] [CrossRef]
- Sinopoli, A.; Isonne, C.; Santoro, M.M.; Baccolini, V. The effects of orally administered lactoferrin in the prevention and management of viral infections: A systematic review. Rev. Med. Virol. 2022, 32, e2261. [Google Scholar] [CrossRef]
- Hassoun, L.A.; Sivamani, R.K. A systematic review of lactoferrin use in dermatology. Crit. Rev. Food Sci. Nutr. 2017, 57, 3632–3639. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, A.; Nagpal, J. Meta-analysis: Efficacy of bovine lactoferrin in Helicobacter pylori eradication. Aliment. Pharmacol. Ther. 2009, 29, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Grzywacz, K.; Butcher, J.; Li, J.; Barrington, K.; Mohamed, I.; Stintzi, A. Bovine Lactoferrin Supplementation Does Not Disrupt Microbiota Development in Preterm Infants Receiving Probiotics. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Sortino, O.; Hullsiek, K.H.; Richards, E.; Rupert, A.; Schminke, A.; Tetekpor, N.; Quinones, M.; Prosser, R.; Schacker, T.; Sereti, I.; et al. The Effects of Recombinant Human Lactoferrin on Immune Activation and the Intestinal Microbiome Among Persons Living with Human Immunodeficiency Virus and Receiving Antiretroviral Therapy. J. Infect. Dis. 2019, 219, 1963–1968. [Google Scholar] [CrossRef]
- Dix, C.; Wright, O. Bioavailability of a Novel Form of Microencapsulated Bovine Lactoferrin and Its Effect on Inflammatory Markers and the Gut Microbiome: A Pilot Study. Nutrients 2018, 10, 1115. [Google Scholar] [CrossRef]
- Sherman, M.P.; Sherman, J.; Arcinue, R.; Niklas, V. Randomized Control Trial of Human Recombinant Lactoferrin: A Substudy Reveals Effects on the Fecal Microbiome of Very Low Birth Weight Infants. J. Pediatr. 2016, 173, S37–S42. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Ortega, F.J.; Bassols, J.; Castro, A.; Ricart, W.; Fernandez-Real, J.M. Association of circulating lactoferrin concentration and 2 nonsynonymous LTF gene polymorphisms with dyslipidemia in men depends on glucose-tolerance status. Clin. Chem. 2008, 54, 301–309. [Google Scholar] [CrossRef]
- Sjostrom, L. Review of the key results from the Swedish Obese Subjects (SOS) trial—A prospective controlled intervention study of bariatric surgery. J. Intern. Med. 2013, 273, 219–234. [Google Scholar] [CrossRef]
- Illan-Gomez, F.; Gonzalvez-Ortega, M.; Orea-Soler, I.; Alcaraz-Tafalla, M.S.; Aragon-Alonso, A.; Pascual-Diaz, M.; Perez-Paredes, M.; Lozano-Almela, M.L. Obesity and inflammation: Change in adiponectin, C-reactive protein, tumour necrosis factor-alpha and interleukin-6 after bariatric surgery. Obes. Surg. 2012, 22, 950–955. [Google Scholar] [CrossRef]
- Baveye, S.; Elass, E.; Fernig, D.G.; Blanquart, C.; Mazurier, J.; Legrand, D. Human lactoferrin interacts with soluble CD14 and inhibits expression of endothelial adhesion molecules, E-selectin and ICAM-1, induced by the CD14-lipopolysaccharide complex. Infect. Immun. 2000, 68, 6519–6525. [Google Scholar] [CrossRef]
- Ziere, G.J.; Bijsterbosch, M.K.; van Berkel, T.J. Removal of 14 N-terminal amino acids of lactoferrin enhances its affinity for parenchymal liver cells and potentiates the inhibition of beta-very low density lipoprotein binding. J. Biol. Chem. 1993, 268, 27069–27075. [Google Scholar] [CrossRef]
- Alexander, M.R.; Norlander, A.E.; Elijovich, F.; Atreya, R.V.; Gaye, A.; Gnecco, J.S.; Laffer, C.L.; Galindo, C.L.; Madhur, M.S. Human monocyte transcriptional profiling identifies IL-18 receptor accessory protein and lactoferrin as novel immune targets in hypertension. Br. J. Pharmacol. 2019, 176, 2015–2027. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Musoles, R.; Castello-Ruiz, M.; Arce, C.; Manzanares, P.; Ivorra, M.D.; Salom, J.B. Antihypertensive mechanism of lactoferrin-derived peptides: Angiotensin receptor blocking effect. J. Agric. Food Chem. 2014, 62, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Tejedor, A.; Sanchez-Rivera, L.; Castello-Ruiz, M.; Recio, I.; Salom, J.B.; Manzanares, P. Novel antihypertensive lactoferrin-derived peptides produced by Kluyveromyces marxianus: Gastrointestinal stability profile and in vivo angiotensin I-converting enzyme (ACE) inhibition. J. Agric. Food Chem. 2014, 62, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- Marcil, V.; Mayeur, S.; Lamarche, B.; England, J.; Henderson, M.; Delvin, E.; Amre, D.; Levy, E. Cardiometabolic risk factors and lactoferrin: Polymorphisms and plasma levels in French-Canadian children. Pediatr. Res. 2017, 82, 741–748. [Google Scholar] [CrossRef]
- Videm, V.; Dahl, H.; Walberg, L.E.; Wiseth, R. Functional polymorphisms in the LTF gene and risk of coronary artery stenosis. Hum. Immunol. 2012, 73, 554–559. [Google Scholar] [CrossRef]
- Sodek, J.; Ganss, B.; McKee, M.D. Osteopontin. Crit. Rev. Oral Biol. Med. 2000, 11, 279–303. [Google Scholar] [CrossRef]
- Johnson, G.A.; Burghardt, R.C.; Bazer, F.W.; Spencer, T.E. Osteopontin: Roles in implantation and placentation. Biol. Reprod. 2003, 69, 1458–1471. [Google Scholar] [CrossRef]
- Mirza, M.; Shaughnessy, E.; Hurley, J.K.; Vanpatten, K.A.; Pestano, G.A.; He, B.; Weber, G.F. Osteopontin-c is a selective marker of breast cancer. Int. J. Cancer 2008, 122, 889–897. [Google Scholar] [CrossRef]
- Gimba, E.R.; Tilli, T.M. Human osteopontin splicing isoforms: Known roles, potential clinical applications and activated signaling pathways. Cancer Lett. 2013, 331, 11–17. [Google Scholar] [CrossRef]
- Anborgh, P.H.; Mutrie, J.C.; Tuck, A.B.; Chambers, A.F. Pre- and post-translational regulation of osteopontin in cancer. J. Cell Commun. Signal. 2011, 5, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Shou, P.; Zhang, L.; Xu, C.; Zheng, C.; Han, Y.; Li, W.; Huang, Y.; Zhang, X.; Shao, C.; et al. An osteopontin-integrin interaction plays a critical role in directing adipogenesis and osteogenesis by mesenchymal stem cells. Stem Cells 2014, 32, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Shinohara, M.L. Intracellular osteopontin (iOPN) and immunity. Immunol. Res. 2011, 49, 160–172. [Google Scholar] [CrossRef]
- Lok, Z.S.Y.; Lyle, A.N. Osteopontin in Vascular Disease. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 613–622. [Google Scholar] [CrossRef]
- Ashkar, S.; Weber, G.F.; Panoutsakopoulou, V.; Sanchirico, M.E.; Jansson, M.; Zawaideh, S.; Rittling, S.R.; Denhardt, D.T.; Glimcher, M.J.; Cantor, H. Eta-1 (osteopontin): An early component of type-1 (cell-mediated) immunity. Science 2000, 287, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Denhardt, D.T.; Noda, M.; O’Regan, A.W.; Pavlin, D.; Berman, J.S. Osteopontin as a means to cope with environmental insults: Regulation of inflammation, tissue remodeling, and cell survival. J. Clin. Investig. 2001, 107, 1055–1061. [Google Scholar] [CrossRef]
- Nomiyama, T.; Perez-Tilve, D.; Ogawa, D.; Gizard, F.; Zhao, Y.; Heywood, E.B.; Jones, K.L.; Kawamori, R.; Cassis, L.A.; Tschop, M.H.; et al. Osteopontin mediates obesity-induced adipose tissue macrophage infiltration and insulin resistance in mice. J. Clin. Investig. 2007, 117, 2877–2888. [Google Scholar] [CrossRef]
- Collins, A.R.; Schnee, J.; Wang, W.; Kim, S.; Fishbein, M.C.; Bruemmer, D.; Law, R.E.; Nicholas, S.; Ross, R.S.; Hsueh, W.A. Osteopontin modulates angiotensin II-induced fibrosis in the intact murine heart. J. Am. Coll. Cardiol. 2004, 43, 1698–1705. [Google Scholar] [CrossRef]
- Scatena, M.; Liaw, L.; Giachelli, C.M. Osteopontin: A multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2302–2309. [Google Scholar] [CrossRef]
- Kainonen, E.; Rautava, S.; Isolauri, E. Immunological programming by breast milk creates an anti-inflammatory cytokine milieu in breast-fed infants compared to formula-fed infants. Br. J. Nutr. 2013, 109, 1962–1970. [Google Scholar] [CrossRef]
- Lonnerdal, B.; Kvistgaard, A.S.; Peerson, J.M.; Donovan, S.M.; Peng, Y.M. Growth, Nutrition, and Cytokine Response of Breast-fed Infants and Infants Fed Formula With Added Bovine Osteopontin. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Guo, H.; Mi, Z.; Grusby, M.J.; Kuo, P.C. Osteopontin induces ubiquitin-dependent degradation of STAT1 in RAW264.7 murine macrophages. J. Immunol. 2007, 178, 1870–1881. [Google Scholar] [CrossRef]
- Wai, P.Y.; Guo, L.; Gao, C.; Mi, Z.; Guo, H.; Kuo, P.C. Osteopontin inhibits macrophage nitric oxide synthesis to enhance tumor proliferation. Surgery 2006, 140, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Rollo, E.E.; Laskin, D.L.; Denhardt, D.T. Osteopontin inhibits nitric oxide production and cytotoxicity by activated RAW264.7 macrophages. J. Leukoc. Biol. 1996, 60, 397–404. [Google Scholar] [CrossRef]
- Lancha, A.; Moncada, R.; Valenti, V.; Rodriguez, A.; Catalan, V.; Becerril, S.; Ramirez, B.; Mendez-Gimenez, L.; Gil, M.J.; Rotellar, F.; et al. Comparative effects of gastric bypass and sleeve gastrectomy on plasma osteopontin concentrations in humans. Surg. Endosc. 2014, 28, 2412–2420. [Google Scholar] [CrossRef] [PubMed]
- Schaller, G.; Aso, Y.; Schernthaner, G.H.; Kopp, H.P.; Inukai, T.; Kriwanek, S.; Schernthaner, G. Increase of osteopontin plasma concentrations after bariatric surgery independent from inflammation and insulin resistance. Obes. Surg. 2009, 19, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Caserza, L.; Casula, M.; Elia, E.; Bonaventura, A.; Liberale, L.; Bertolotto, M.; Artom, N.; Minetti, S.; Contini, P.; Verzola, D.; et al. Serum osteopontin predicts glycaemic profile improvement in metabolic syndrome: A pilot study. Eur. J. Clin. Investig. 2021, 51, e13403. [Google Scholar] [CrossRef]
- Wang, C.; He, M.; Peng, J.; Li, S.; Long, M.; Chen, W.; Liu, D.; Yang, G.; Zhang, L. Increased plasma osteopontin levels are associated with nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus. Cytokine 2020, 125, 154837. [Google Scholar] [CrossRef]
- Xie, Z.; Singh, M.; Singh, K. Osteopontin modulates myocardial hypertrophy in response to chronic pressure overload in mice. Hypertension 2004, 44, 826–831. [Google Scholar] [CrossRef]
- Das, S.; Song, Z.; Han, H.; Ge, X.; Desert, R.; Athavale, D.; Babu Komakula, S.S.; Magdaleno, F.; Chen, W.; Lantvit, D.; et al. Intestinal Osteopontin Protects From Alcohol-induced Liver Injury by Preserving the Gut Microbiome and the Intestinal Barrier Function. Cell. Mol. Gastroenterol. Hepatol. 2022, 14, 813–839. [Google Scholar] [CrossRef]
- Nakase, H. OPeNing the Epithelial Barrier: Osteopontin Preserves Gut Barrier Function During Intestinal Inflammation. Dig. Dis. Sci. 2019, 64, 294–296. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Lang, Y.; Shen, Q.; Xu, L.; Cheng, N.; Chu, Y.; Lyu, H.; Chen, F. Osteopontin-loaded PLGA nanoparticles enhance the intestinal mucosal barrier and alleviate inflammation via the NF-kappaB signaling pathway. Colloids Surf. B Biointerfaces 2020, 190, 110952. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Leung, T.M.; Arriazu, E.; Lu, Y.; Urtasun, R.; Christensen, B.; Fiel, M.I.; Mochida, S.; Sorensen, E.S.; Nieto, N. Osteopontin binding to lipopolysaccharide lowers tumor necrosis factor-alpha and prevents early alcohol-induced liver injury in mice. Hepatology 2014, 59, 1600–1616. [Google Scholar] [CrossRef]
- Toyonaga, T.; Nakase, H.; Ueno, S.; Matsuura, M.; Yoshino, T.; Honzawa, Y.; Itou, A.; Namba, K.; Minami, N.; Yamada, S.; et al. Osteopontin Deficiency Accelerates Spontaneous Colitis in Mice with Disrupted Gut Microbiota and Macrophage Phagocytic Activity. PLoS ONE 2015, 10, e0135552. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Moriwaki, Y.; Arikawa, T.; Chen, Y.H.; Oh, Y.J.; Oliver, T.; Shinohara, M.L. Cutting edge: Critical role of intracellular osteopontin in antifungal innate immune responses. J. Immunol. 2011, 186, 19–23. [Google Scholar] [CrossRef]
- Gomez-Santos, B.; Saenz de Urturi, D.; Nunez-Garcia, M.; Gonzalez-Romero, F.; Buque, X.; Aurrekoetxea, I.; Gutierrez de Juan, V.; Gonzalez-Rellan, M.J.; Garcia-Monzon, C.; Gonzalez-Rodriguez, A.; et al. Liver osteopontin is required to prevent the progression of age-related nonalcoholic fatty liver disease. Aging Cell 2020, 19, e13183. [Google Scholar] [CrossRef]
- Morishita, S.; Kawaguchi, H.; Ono, T.; Miura, N.; Murakoshi, M.; Sugiyama, K.; Kato, H.; Tanimoto, A.; Nishino, H. Enteric lactoferrin attenuates the development of high-fat and high-cholesterol diet-induced hypercholesterolemia and atherosclerosis in Microminipigs. Biosci. Biotechnol. Biochem. 2016, 80, 295–303. [Google Scholar] [CrossRef]
- Rotem, I.; Konfino, T.; Caller, T.; Schary, Y.; Shaihov-Teper, O.; Palevski, D.; Lewis, N.; Lendengolts, D.; Naftali-Shani, N.; Leor, J. Osteopontin promotes infarct repair. Basic Res. Cardiol. 2022, 117, 51. [Google Scholar] [CrossRef]
- Shirakawa, K.; Endo, J.; Kataoka, M.; Katsumata, Y.; Yoshida, N.; Yamamoto, T.; Isobe, S.; Moriyama, H.; Goto, S.; Kitakata, H.; et al. IL (Interleukin)-10-STAT3-Galectin-3 Axis Is Essential for Osteopontin-Producing Reparative Macrophage Polarization After Myocardial Infarction. Circulation 2018, 138, 2021–2035. [Google Scholar] [CrossRef]
- Trueblood, N.A.; Xie, Z.; Communal, C.; Sam, F.; Ngoy, S.; Liaw, L.; Jenkins, A.W.; Wang, J.; Sawyer, D.B.; Bing, O.H.; et al. Exaggerated left ventricular dilation and reduced collagen deposition after myocardial infarction in mice lacking osteopontin. Circ. Res. 2001, 88, 1080–1087. [Google Scholar] [CrossRef]
- Chen, J.; Zeng, P.; Gong, L.; Zhang, X.; Ling, Z.; Bi, K.; Shi, F.; Wang, K.; Zhang, Q.; Jiang, J.; et al. Osteopontin Exacerbates High-Fat Diet-Induced Metabolic Disorders in a Microbiome-Dependent Manner. mBio 2022, 13, e0253122. [Google Scholar] [CrossRef]
- Kiefer, F.W.; Neschen, S.; Pfau, B.; Legerer, B.; Neuhofer, A.; Kahle, M.; Hrabe de Angelis, M.; Schlederer, M.; Mair, M.; Kenner, L.; et al. Osteopontin deficiency protects against obesity-induced hepatic steatosis and attenuates glucose production in mice. Diabetologia 2011, 54, 2132–2142. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wu, D.; Zhang, L.; Lin, C.; Liao, J.; Xie, R.; Li, Z.; Wu, S.; Liu, A.; Hu, W.; et al. NK cells induce hepatic ER stress to promote insulin resistance in obesity through osteopontin production. J. Leukoc. Biol. 2020, 107, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Filardi, T.; Carnevale, V.; Massoud, R.; Russo, C.; Nieddu, L.; Tavaglione, F.; Turinese, I.; Lenzi, A.; Romagnoli, E.; Morano, S. High serum osteopontin levels are associated with prevalent fractures and worse lipid profile in post-menopausal women with type 2 diabetes. J. Endocrinol. Investig. 2019, 42, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Caesar, C.; Lyle, A.N.; Joseph, G.; Weiss, D.; Alameddine, F.M.F.; Lassegue, B.; Griendling, K.K.; Taylor, W.R. Cyclic Strain and Hypertension Increase Osteopontin Expression in the Aorta. Cell. Mol. Bioeng. 2017, 10, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Kurata, M.; Okura, T.; Watanabe, S.; Fukuoka, T.; Higaki, J. Osteopontin and carotid atherosclerosis in patients with essential hypertension. Clin. Sci. 2006, 111, 319–324. [Google Scholar] [CrossRef]
- Bidder, M.; Shao, J.S.; Charlton-Kachigian, N.; Loewy, A.P.; Semenkovich, C.F.; Towler, D.A. Osteopontin transcription in aortic vascular smooth muscle cells is controlled by glucose-regulated upstream stimulatory factor and activator protein-1 activities. J. Biol. Chem. 2002, 277, 44485–44496. [Google Scholar] [CrossRef]
- Giachelli, C.M.; Lombardi, D.; Johnson, R.J.; Murry, C.E.; Almeida, M. Evidence for a role of osteopontin in macrophage infiltration in response to pathological stimuli in vivo. Am. J. Pathol. 1998, 152, 353–358. [Google Scholar]
- Isoda, K.; Kamezawa, Y.; Ayaori, M.; Kusuhara, M.; Tada, N.; Ohsuzu, F. Osteopontin transgenic mice fed a high-cholesterol diet develop early fatty-streak lesions. Circulation 2003, 107, 679–681. [Google Scholar] [CrossRef]
- Chiba, S.; Okamoto, H.; Kon, S.; Kimura, C.; Murakami, M.; Inobe, M.; Matsui, Y.; Sugawara, T.; Shimizu, T.; Uede, T.; et al. Development of atherosclerosis in osteopontin transgenic mice. Heart Vessels 2002, 16, 111–117. [Google Scholar] [CrossRef]
- Lonnerdal, B. Human Milk: Bioactive Proteins/Peptides and Functional Properties. Nestle Nutr. Inst. Workshop Ser. 2016, 86, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Rittling, S.R.; Wejse, P.L.; Yagiz, K.; Warot, G.A.; Hui, T. Suppression of tumour growth by orally administered osteopontin is accompanied by alterations in tumour blood vessels. Br. J. Cancer 2014, 110, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.P.; Ellen, R.P.; Sorensen, E.S.; Goldberg, H.A.; Zohar, R.; Sodek, J. Osteopontin attenuation of dextran sulfate sodium-induced colitis in mice. Lab. Investig. 2009, 89, 1169–1181. [Google Scholar] [CrossRef]
- Gassler, N.; Autschbach, F.; Gauer, S.; Bohn, J.; Sido, B.; Otto, H.F.; Geiger, H.; Obermuller, N. Expression of osteopontin (Eta-1) in Crohn disease of the terminal ileum. Scand. J. Gastroenterol. 2002, 37, 1286–1295. [Google Scholar] [CrossRef] [PubMed]
- O’Regan, A.; Berman, J.S. Osteopontin: A key cytokine in cell-mediated and granulomatous inflammation. Int. J. Exp. Pathol. 2000, 81, 373–390. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Lonnerdal, B. Evaluation of Bioactivities of Bovine Milk Osteopontin Using a Knockout Mouse Model. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 125–131. [Google Scholar] [CrossRef]
- Brown, L.F.; Berse, B.; Van de Water, L.; Papadopoulos-Sergiou, A.; Perruzzi, C.A.; Manseau, E.J.; Dvorak, H.F.; Senger, D.R. Expression and distribution of osteopontin in human tissues: Widespread association with luminal epithelial surfaces. Mol. Biol. Cell 1992, 3, 1169–1180. [Google Scholar] [CrossRef]
- Atkins, K.; Berry, J.E.; Zhang, W.Z.; Harris, J.F.; Chambers, A.F.; Simpson, R.U.; Somerman, M.J. Coordinate expression of OPN and associated receptors during monocyte/macrophage differentiation of HL-60 cells. J. Cell. Physiol. 1998, 175, 229–237. [Google Scholar] [CrossRef]
- Woo, S.H.; Lee, S.H.; Park, J.W.; Go, D.M.; Kim, D.Y. Osteopontin Protects Colonic Mucosa from Dextran Sodium Sulfate-Induced Acute Colitis in Mice by Regulating Junctional Distribution of Occludin. Dig. Dis. Sci. 2019, 64, 421–431. [Google Scholar] [CrossRef]
- Keshavarzian, A.; Choudhary, S.; Holmes, E.W.; Yong, S.; Banan, A.; Jakate, S.; Fields, J.Z. Preventing gut leakiness by oats supplementation ameliorates alcohol-induced liver damage in rats. J. Pharmacol. Exp. Ther. 2001, 299, 442–448. [Google Scholar]
- Szabo, G.; Bala, S. Alcoholic liver disease and the gut-liver axis. World J. Gastroenterol. 2010, 16, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Mathurin, P.; Deng, Q.G.; Keshavarzian, A.; Choudhary, S.; Holmes, E.W.; Tsukamoto, H. Exacerbation of alcoholic liver injury by enteral endotoxin in rats. Hepatology 2000, 32, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Lu, Y.; Leung, T.M.; Sorensen, E.S.; Nieto, N. Milk osteopontin, a nutritional approach to prevent alcohol-induced liver injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G929–G939. [Google Scholar] [CrossRef]
- Da Silva, A.P.; Pollett, A.; Rittling, S.R.; Denhardt, D.T.; Sodek, J.; Zohar, R. Exacerbated tissue destruction in DSS-induced acute colitis of OPN-null mice is associated with downregulation of TNF-alpha expression and non-programmed cell death. J. Cell. Physiol. 2006, 208, 629–639. [Google Scholar] [CrossRef]
- Icer, M.A.; Gezmen-Karadag, M. The multiple functions and mechanisms of osteopontin. Clin. Biochem. 2018, 59, 17–24. [Google Scholar] [CrossRef]
- Rollo, E.E.; Hempson, S.J.; Bansal, A.; Tsao, E.; Habib, I.; Rittling, S.R.; Denhardt, D.T.; Mackow, E.R.; Shaw, R.D. The cytokine osteopontin modulates the severity of rotavirus diarrhea. J. Virol. 2005, 79, 3509–3516. [Google Scholar] [CrossRef] [PubMed]
- Nau, G.J.; Liaw, L.; Chupp, G.L.; Berman, J.S.; Hogan, B.L.; Young, R.A. Attenuated host resistance against Mycobacterium bovis BCG infection in mice lacking osteopontin. Infect. Immun. 1999, 67, 4223–4230. [Google Scholar] [CrossRef]
- Atarashi, K.; Tanoue, T.; Oshima, K.; Suda, W.; Nagano, Y.; Nishikawa, H.; Fukuda, S.; Saito, T.; Narushima, S.; Hase, K.; et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013, 500, 232–236. [Google Scholar] [CrossRef]
- Heilmann, K.; Hoffmann, U.; Witte, E.; Loddenkemper, C.; Sina, C.; Schreiber, S.; Hayford, C.; Holzlohner, P.; Wolk, K.; Tchatchou, E.; et al. Osteopontin as two-sided mediator of intestinal inflammation. J. Cell. Mol. Med. 2009, 13, 1162–1174. [Google Scholar] [CrossRef]
- Schack, L.; Stapulionis, R.; Christensen, B.; Kofod-Olsen, E.; Skov Sorensen, U.B.; Vorup-Jensen, T.; Sorensen, E.S.; Hollsberg, P. Osteopontin enhances phagocytosis through a novel osteopontin receptor, the alphaXbeta2 integrin. J. Immunol. 2009, 182, 6943–6950. [Google Scholar] [CrossRef]
- Efsa Panel on Nutrition, N.F.; Food, A.; Turck, D.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; et al. Safety of bovine milk osteopontin as a Novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2022, 20, e07137. [Google Scholar] [CrossRef] [PubMed]
- West, C.E.; Kvistgaard, A.S.; Peerson, J.M.; Donovan, S.M.; Peng, Y.M.; Lonnerdal, B. Effects of osteopontin-enriched formula on lymphocyte subsets in the first 6 months of life: A randomized controlled trial. Pediatr. Res. 2017, 82, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Aasmul-Olsen, K.; Henriksen, N.L.; Nguyen, D.N.; Heckmann, A.B.; Thymann, T.; Sangild, P.T.; Bering, S.B. Milk Osteopontin for Gut, Immunity and Brain Development in Preterm Pigs. Nutrients 2021, 13, 2675. [Google Scholar] [CrossRef] [PubMed]
- Moller, H.K.; Thymann, T.; Fink, L.N.; Frokiaer, H.; Kvistgaard, A.S.; Sangild, P.T. Bovine colostrum is superior to enriched formulas in stimulating intestinal function and necrotising enterocolitis resistance in preterm pigs. Br. J. Nutr. 2011, 105, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Karampatsou, S.I.; Paltoglou, G.; Genitsaridi, S.M.; Kassari, P.; Charmandari, E. The Effect of a Comprehensive Life-Style Intervention Program of Diet and Exercise on Four Bone-Derived Proteins, FGF-23, Osteopontin, NGAL and Sclerostin, in Overweight or Obese Children and Adolescents. Nutrients 2022, 14, 3772. [Google Scholar] [CrossRef]
- Aztatzi-Aguilar, O.G.; Sierra-Vargas, M.P.; Ortega-Romero, M.; Jimenez-Corona, A.E. Osteopontin’s relationship with malnutrition and oxidative stress in adolescents. A pilot study. PLoS ONE 2021, 16, e0249057. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Bertola, A.; Deveaux, V.; Bonnafous, S.; Rousseau, D.; Anty, R.; Wakkach, A.; Dahman, M.; Tordjman, J.; Clement, K.; McQuaid, S.E.; et al. Elevated expression of osteopontin may be related to adipose tissue macrophage accumulation and liver steatosis in morbid obesity. Diabetes 2009, 58, 125–133. [Google Scholar] [CrossRef]
- Gomez-Ambrosi, J.; Catalan, V.; Ramirez, B.; Rodriguez, A.; Colina, I.; Silva, C.; Rotellar, F.; Mugueta, C.; Gil, M.J.; Cienfuegos, J.A.; et al. Plasma osteopontin levels and expression in adipose tissue are increased in obesity. J. Clin. Endocrinol. Metab. 2007, 92, 3719–3727. [Google Scholar] [CrossRef]
- Toossi, P.; Sadat Amini, S.H.; Sadat Amini, M.S.; Partovi Kia, M.; Enamzade, R.; Kazeminejad, A.; Esmaeily Radvar, S.; Younespour, S. Assessment of serum levels of osteopontin, selenium and prolactin in patients with psoriasis compared with healthy controls, and their association with psoriasis severity. Clin. Exp. Dermatol. 2015, 40, 741–746. [Google Scholar] [CrossRef]
- Kadry, D.; Hegazy, R.A.; Rashed, L. Osteopontin and adiponectin: How far are they related in the complexity of psoriasis? Arch. Dermatol. Res. 2013, 305, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Fitter, S.; Zannettino, A.C.W. Osteopontin in the pathophysiology of obesity: Is Opn a fat cell foe? Obes. Res. Clin. Pract. 2018, 12, 249–250. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, M.; Yokote, K.; Nishimura, M.; Shigematsu, T.; Hasegawa, T.; Kon, S.; Uede, T.; Matsumoto, T.; Saito, Y.; Mori, S. Enhanced expression of osteopontin in human diabetic artery and analysis of its functional role in accelerated atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 624–628. [Google Scholar] [CrossRef]
- Carbone, F.; Adami, G.; Liberale, L.; Bonaventura, A.; Bertolotto, M.; Andraghetti, G.; Scopinaro, N.; Camerini, G.B.; Papadia, F.S.; Cordera, R.; et al. Serum levels of osteopontin predict diabetes remission after bariatric surgery. Diabetes Metab. 2019, 45, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Carbone, F.; Rigamonti, F.; Burger, F.; Roth, A.; Bertolotto, M.; Spinella, G.; Pane, B.; Palombo, D.; Pende, A.; Bonaventura, A.; et al. Serum levels of osteopontin predict major adverse cardiovascular events in patients with severe carotid artery stenosis. Int. J. Cardiol. 2018, 255, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Carbone, F.; Vuilleumier, N.; Burger, F.; Roversi, G.; Tamborino, C.; Casetta, I.; Seraceni, S.; Trentini, A.; Padroni, M.; Dallegri, F.; et al. Serum osteopontin levels are upregulated and predict disability after an ischaemic stroke. Eur. J. Clin. Investig. 2015, 45, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Glass, O.; Henao, R.; Patel, K.; Guy, C.D.; Gruss, H.J.; Syn, W.K.; Moylan, C.A.; Streilein, R.; Hall, R.; Mae Diehl, A.; et al. Serum Interleukin-8, Osteopontin, and Monocyte Chemoattractant Protein 1 Are Associated With Hepatic Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Hepatol. Commun. 2018, 2, 1344–1355. [Google Scholar] [CrossRef] [PubMed]
- Wolak, T. Osteopontin—A multi-modal marker and mediator in atherosclerotic vascular disease. Atherosclerosis 2014, 236, 327–337. [Google Scholar] [CrossRef]
- Cho, H.J.; Cho, H.J.; Kim, H.S. Osteopontin: A multifunctional protein at the crossroads of inflammation, atherosclerosis, and vascular calcification. Curr. Atheroscler. Rep. 2009, 11, 206–213. [Google Scholar] [CrossRef]
- Steitz, S.A.; Speer, M.Y.; Curinga, G.; Yang, H.Y.; Haynes, P.; Aebersold, R.; Schinke, T.; Karsenty, G.; Giachelli, C.M. Smooth muscle cell phenotypic transition associated with calcification: Upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ. Res. 2001, 89, 1147–1154. [Google Scholar] [CrossRef]
- Giachelli, C.M.; Bae, N.; Almeida, M.; Denhardt, D.T.; Alpers, C.E.; Schwartz, S.M. Osteopontin is elevated during neointima formation in rat arteries and is a novel component of human atherosclerotic plaques. J. Clin. Investig. 1993, 92, 1686–1696. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.F.; Wu, S.; Juang, J.J.; Chiang, F.T.; Hsu, L.A.; Teng, M.S.; Cheng, S.T.; Huang, H.L.; Ko, Y.L. Osteoprotegerin and osteopontin levels, but not gene polymorphisms, predict mortality in cardiovascular diseases. Biomark. Med. 2019, 13, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Abdalrhim, A.D.; Marroush, T.S.; Austin, E.E.; Gersh, B.J.; Solak, N.; Rizvi, S.A.; Bailey, K.R.; Kullo, I.J. Plasma Osteopontin Levels and Adverse Cardiovascular Outcomes in the PEACE Trial. PLoS ONE 2016, 11, e0156965. [Google Scholar] [CrossRef]
- Kadoglou, N.P.E.; Kapetanios, D.; Korakas, E.; Valsami, G.; Tentolouris, N.; Papanas, N.; Lambadiari, V.; Karkos, C. Association of serum levels of osteopontin and osteoprotegerin with adverse outcomes after endovascular revascularisation in peripheral artery disease. Cardiovasc. Diabetol. 2022, 21, 171. [Google Scholar] [CrossRef] [PubMed]
- Scatena, M.; Almeida, M.; Chaisson, M.L.; Fausto, N.; Nicosia, R.F.; Giachelli, C.M. NF-kappaB mediates alphavbeta3 integrin-induced endothelial cell survival. J. Cell Biol. 1998, 141, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Peng, L.; Fan, K.; Wang, H.; Wei, R.; Ji, G.; Cai, J.; Lu, B.; Li, B.; Zhang, D.; et al. Osteopontin induces angiogenesis through activation of PI3K/AKT and ERK1/2 in endothelial cells. Oncogene 2009, 28, 3412–3422. [Google Scholar] [CrossRef]
- Khan, S.A.; Lopez-Chua, C.A.; Zhang, J.; Fisher, L.W.; Sorensen, E.S.; Denhardt, D.T. Soluble osteopontin inhibits apoptosis of adherent endothelial cells deprived of growth factors. J. Cell. Biochem. 2002, 85, 728–736. [Google Scholar] [CrossRef]
- Zhao, X.; Johnson, J.N.; Singh, K.; Singh, M. Impairment of myocardial angiogenic response in the absence of osteopontin. Microcirculation 2007, 14, 233–240. [Google Scholar] [CrossRef]
- Shirakawa, K.; Endo, J.; Kataoka, M.; Katsumata, Y.; Anzai, A.; Moriyama, H.; Kitakata, H.; Hiraide, T.; Ko, S.; Goto, S.; et al. MerTK Expression and ERK Activation Are Essential for the Functional Maturation of Osteopontin-Producing Reparative Macrophages After Myocardial Infarction. J. Am. Heart Assoc. 2020, 9, e017071. [Google Scholar] [CrossRef]
- Kiefer, F.W.; Zeyda, M.; Gollinger, K.; Pfau, B.; Neuhofer, A.; Weichhart, T.; Saemann, M.D.; Geyeregger, R.; Schlederer, M.; Kenner, L.; et al. Neutralization of osteopontin inhibits obesity-induced inflammation and insulin resistance. Diabetes 2010, 59, 935–946. [Google Scholar] [CrossRef]
- Sahai, A.; Malladi, P.; Pan, X.; Paul, R.; Melin-Aldana, H.; Green, R.M.; Whitington, P.F. Obese and diabetic db/db mice develop marked liver fibrosis in a model of nonalcoholic steatohepatitis: Role of short-form leptin receptors and osteopontin. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G1035–G1043. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, F.W.; Zeyda, M.; Todoric, J.; Huber, J.; Geyeregger, R.; Weichhart, T.; Aszmann, O.; Ludvik, B.; Silberhumer, G.R.; Prager, G.; et al. Osteopontin expression in human and murine obesity: Extensive local up-regulation in adipose tissue but minimal systemic alterations. Endocrinology 2008, 149, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- Magdaleno, F.; Ge, X.; Fey, H.; Lu, Y.; Gaskell, H.; Blajszczak, C.C.; Aloman, C.; Fiel, M.I.; Nieto, N. Osteopontin deletion drives hematopoietic stem cell mobilization to the liver and increases hepatic iron contributing to alcoholic liver disease. Hepatol. Commun. 2018, 2, 84–98. [Google Scholar] [CrossRef]
- Lazaro, R.; Wu, R.; Lee, S.; Zhu, N.L.; Chen, C.L.; French, S.W.; Xu, J.; Machida, K.; Tsukamoto, H. Osteopontin deficiency does not prevent but promotes alcoholic neutrophilic hepatitis in mice. Hepatology 2015, 61, 129–140. [Google Scholar] [CrossRef]
- Lee, G.S.; Salazar, H.F.; Joseph, G.; Lok, Z.S.Y.; Caroti, C.M.; Weiss, D.; Taylor, W.R.; Lyle, A.N. Osteopontin isoforms differentially promote arteriogenesis in response to ischemia via macrophage accumulation and survival. Lab. Investig. 2019, 99, 331–345. [Google Scholar] [CrossRef]
- Senbanjo, L.T.; Chellaiah, M.A. CD44: A Multifunctional Cell Surface Adhesion Receptor Is a Regulator of Progression and Metastasis of Cancer Cells. Front. Cell Dev. Biol. 2017, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Yamniuk, A.P.; Burling, H.; Vogel, H.J. Thermodynamic characterization of the interactions between the immunoregulatory proteins osteopontin and lactoferrin. Mol. Immunol. 2009, 46, 2395–2402. [Google Scholar] [CrossRef] [PubMed]
- Mehra, R.; Marnila, P.; Korhonen, H. Milk immunoglobulins for health promotion. Int. Dairy J. 2006, 16, 1262–1271. [Google Scholar] [CrossRef]
- Jiang, R.; Liu, L.; Du, X.; Lonnerdal, B. Evaluation of Bioactivities of the Bovine Milk Lactoferrin-Osteopontin Complex in Infant Formulas. J. Agric. Food Chem. 2020, 68, 6104–6111. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, R.; Lonnerdal, B. Assessment of bioactivities of the human milk lactoferrin-osteopontin complex in vitro. J. Nutr. Biochem. 2019, 69, 10–18. [Google Scholar] [CrossRef]
- Davidson, L.A.; Lonnerdal, B. Persistence of human milk proteins in the breast-fed infant. Acta. Paediatr. 1987, 76, 733–740. [Google Scholar] [CrossRef]
- Reuter, B.K.; Pizarro, T.T. Commentary: The role of the IL-18 system and other members of the IL-1R/TLR superfamily in innate mucosal immunity and the pathogenesis of inflammatory bowel disease: Friend or foe? Eur. J. Immunol. 2004, 34, 2347–2355. [Google Scholar] [CrossRef] [PubMed]
- Dupaul-Chicoine, J.; Yeretssian, G.; Doiron, K.; Bergstrom, K.S.; McIntire, C.R.; LeBlanc, P.M.; Meunier, C.; Turbide, C.; Gros, P.; Beauchemin, N.; et al. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity 2010, 32, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Salcedo, R.; Worschech, A.; Cardone, M.; Jones, Y.; Gyulai, Z.; Dai, R.M.; Wang, E.; Ma, W.; Haines, D.; O’HUigin, C.; et al. MyD88-mediated signaling prevents development of adenocarcinomas of the colon: Role of interleukin 18. J. Exp. Med. 2010, 207, 1625–1636. [Google Scholar] [CrossRef] [PubMed]
- Vaishnava, S.; Behrendt, C.L.; Ismail, A.S.; Eckmann, L.; Hooper, L.V. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc. Natl. Acad. Sci. USA 2008, 105, 20858–20863. [Google Scholar] [CrossRef]
- Oficjalska, K.; Raverdeau, M.; Aviello, G.; Wade, S.C.; Hickey, A.; Sheehan, K.M.; Corr, S.C.; Kay, E.W.; O’Neill, L.A.; Mills, K.H.; et al. Protective role for caspase-11 during acute experimental murine colitis. J. Immunol. 2015, 194, 1252–1260. [Google Scholar] [CrossRef]
- Nowarski, R.; Jackson, R.; Gagliani, N.; de Zoete, M.R.; Palm, N.W.; Bailis, W.; Low, J.S.; Harman, C.C.; Graham, M.; Elinav, E.; et al. Epithelial IL-18 Equilibrium Controls Barrier Function in Colitis. Cell 2015, 163, 1444–1456. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).