The Impact of High Protein Diets on Cardiovascular Outcomes: A Systematic Review and Meta-Analysis of Prospective Cohort Studies
Abstract
1. Introduction
2. Materials and Methods
- Focused questions:
- P (population): patients free of cardiovascular disease.
- I (intervention): high protein diet.
- C (comparison): low or normal protein diet.
- O (outcome): cardiovascular disease.
- Research question: Does a high protein diet affect cardiovascular outcomes?
2.1. Search Strategy
2.2. Study Selection & Data Extraction
2.3. Quality and Risk of Bias Assessment
2.4. Data Synthesis and Analysis
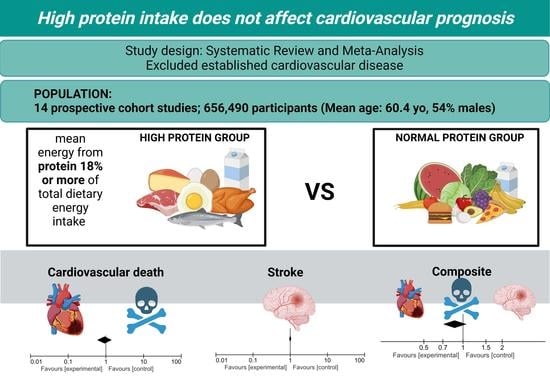
3. Results
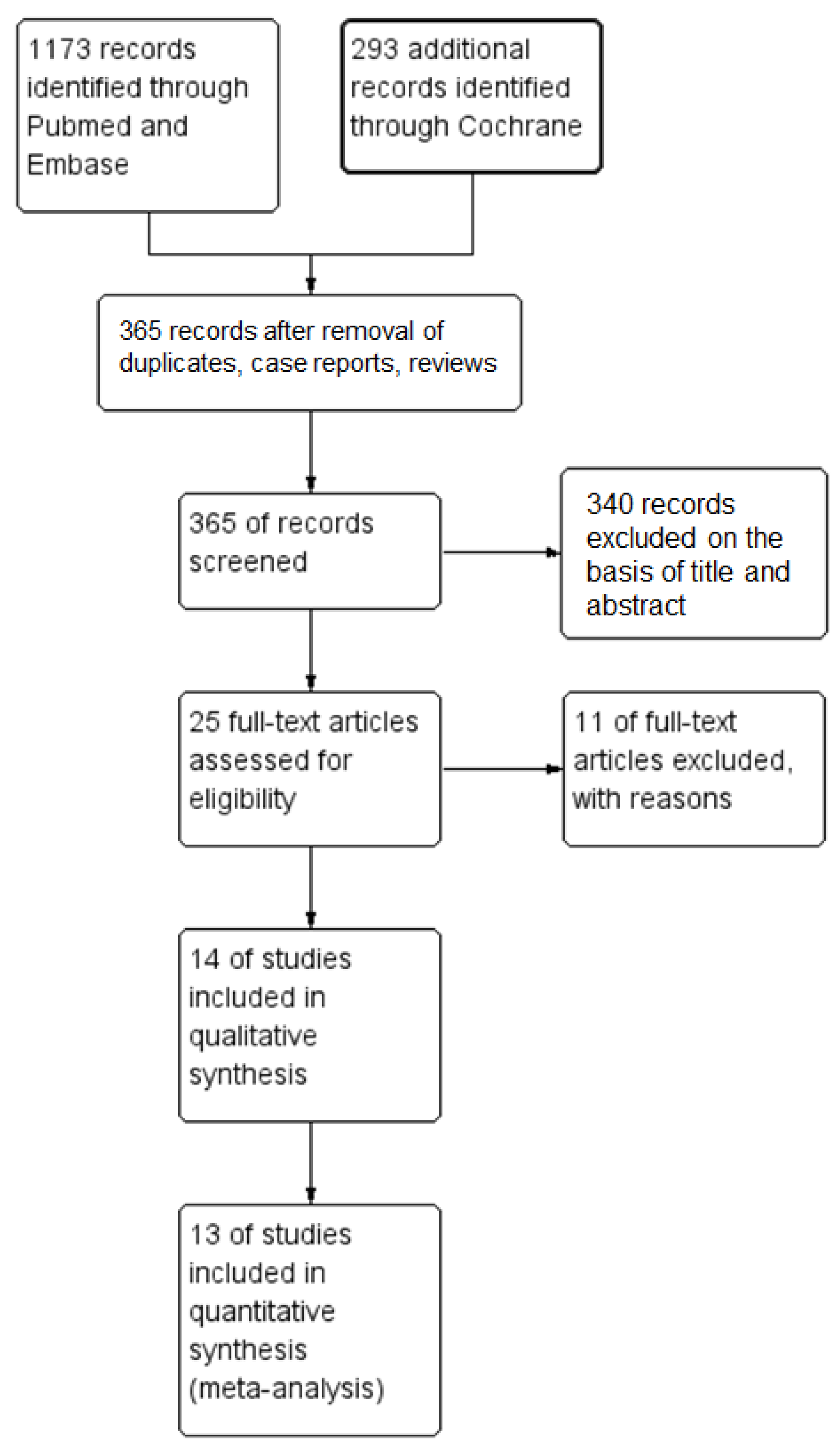
3.1. Search Results
3.2. Study Characteristics
3.3. Primary Outcomes
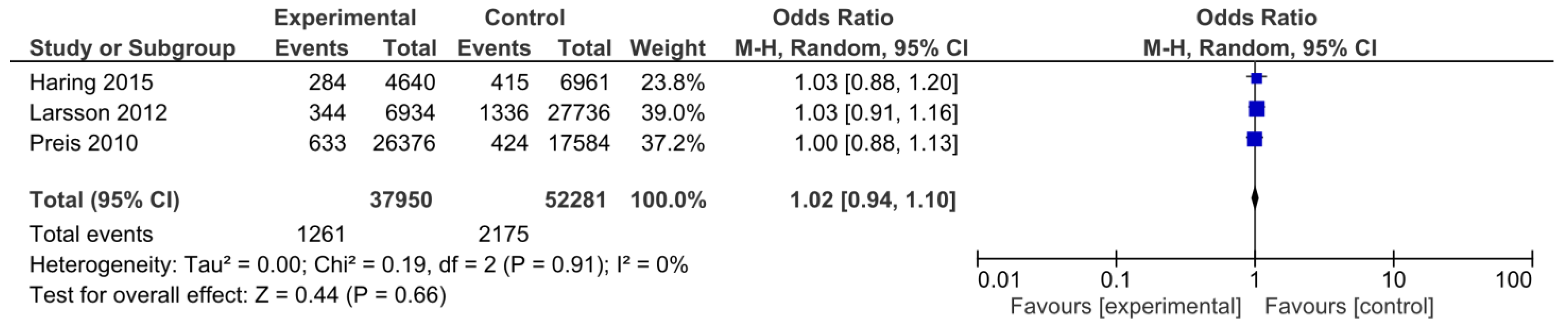
3.3.1. Cardiovascular Death
3.3.2. Stroke
3.4. Secondary Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Wang, H.; Naghavi, M.; Allen, C.; Barber, R.M.; Bhutta, Z.A.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; Coates, M.M.; et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar]
- Timmis, A.; Vardas, P.; Townsend, N.; Torbica, A.; Katus, H.; De Smedt, D.; Gale, C.P.; Maggioni, A.P.; Petersen, S.E.; Huculeci, R.; et al. European Society of Cardiology: Cardiovascular disease statistics 2021. Eur. Heart J. 2022, 43, 716–799. [Google Scholar] [PubMed]
- Swain, J.F.; McCarron, P.B.; Hamilton, E.F.; Sacks, F.M.; Appel, L.J. Characteristics of the Diet Patterns Tested in the Optimal Macronutrient Intake Trial to Prevent Heart Disease (OmniHeart): Options for a Heart-Healthy Diet. J. Am. Diet. Assoc. 2008, 108, 257–265. [Google Scholar]
- Anto, L.; Blesso, C.N. Interplay between diet, the gut microbiome, and atherosclerosis: Role of dysbiosis and microbial metabolites on inflammation and disordered lipid metabolism. J. Nutr. Biochem. 2022, 105, 108991. [Google Scholar]
- Strasser, B.; Wolters, M.; Weyh, C.; Krüger, K.; Ticinesi, A. The Effects of Lifestyle and Diet on Gut Microbiota Composition, Inflammation and Muscle Performance in Our Aging Society. Nutrients 2021, 13, 2045. [Google Scholar] [PubMed]
- Brunner, E.J.; Mosdøl, A.; Witte, D.R.; Martikainen, P.; Stafford, M.; Shipley, M.J.; Marmot, M.G. Dietary patterns and 15-y risks of major coronary events, diabetes, and mortality. Am. J. Clin. Nutr. 2008, 87, 1414–1421. [Google Scholar] [CrossRef]
- Tischmann, L.; Drummen, M.; Joris, P.; Gatta-Cherifi, B.; Raben, A.; Fogelholm, M.; Matias, I.; Cota, D.; Mensink, R.P.; Westerterp-Plantenga, M.S.; et al. Effects of a High-Protein Diet on Cardiometabolic Health, Vascular Function, and Endocannabinoids—A Preview Study. Nutrients 2020, 12, 1512. [Google Scholar] [CrossRef]
- Ge, L.; Sadeghirad, B.; Ball, G.D.C.; da Costa, B.R.; Hitchcock, C.L.; Svendrovski, A.; Kiflen, R.; Quadri, K.; Kwon, H.Y.; Karamouzian, M.; et al. Comparison of dietary macronutrient patterns of 14 popular named dietary programmes for weight and cardiovascular risk factor reduction in adults: Systematic review and network meta-analysis of randomised trials. BMJ 2020, 369, m696. [Google Scholar] [PubMed]
- Chen, C.N.; Hsu, K.J.; Chien, K.Y.; Chen, J.J. Effects of Combined High-Protein Diet and Exercise Intervention on Cardiometabolic Health in Middle-Aged Obese Adults: A Randomized Controlled Trial. Front. Cardiovasc. Med. 2021, 8, 705282. [Google Scholar]
- Evangelista, L.S.; Jose, M.M.; Sallam, H.; Serag, H.; Golovko, G.; Khanipov, K.; Hamilton, M.A.; Fonarow, G.C. High-protein vs. standard-protein diets in overweight and obese patients with heart failure and diabetes mellitus: Findings of the Pro-HEART trial. ESC Heart Fail. 2021, 8, 1342–1348. [Google Scholar]
- Azadbakht, L.; Izadi, V.; Surkan, P.J.; Esmaillzadeh, A. Effect of a High Protein Weight Loss Diet on Weight, High-Sensitivity C-Reactive Protein, and Cardiovascular Risk among Overweight and Obese Women: A Parallel Clinical Trial. Int. J. Endocrinol. 2013, 2013, 1–8. [Google Scholar]
- Noakes, M.; Keogh, J.B.; Foster, P.R.; Clifton, P.M. Effect of an energy-restricted, high-protein, low-fat diet relative to a conventional high-carbohydrate, low-fat diet on weight loss, body composition, nutritional status, and markers of cardiovascular health in obese women. Am. J. Clin. Nutr. 2005, 81, 1298–1306. [Google Scholar] [CrossRef]
- Hu, F.B.; Stampfer, M.J.; Manson, J.E.; Rimm, E.; Colditz, G.A.; Speizer, F.E.; Hennekens, C.H.; Willett, W.C. Dietary protein and risk of ischemic heart disease in women. Am. J. Clin. Nutr. 1999, 70, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Prentice, R.L.; Huang, Y.; Kuller, L.H.; Tinker, L.F.; Horn, L.V.; Stefanick, M.L.; Sarto, G.; Ockene, J.; Johnson, K.C. Biomarker-calibrated Energy and Protein Consumption and Cardiovascular Disease Risk Among Postmenopausal Women. Epidemiology 2011, 22, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Virtamo, J.; Wolk, A. Dietary protein intake and risk of stroke in women. Atherosclerosis 2012, 224, 247–251. [Google Scholar] [CrossRef]
- Ozawa, M.; Yoshida, D.; Hata, J.; Ohara, T.; Mukai, N.; Shibata, M.; Uchida, K.; Nagata, M.; Kitazono, T.; Kiyohara, Y.; et al. Dietary Protein Intake and Stroke Risk in a General Japanese Population: The Hisayama Study. Stroke 2017, 48, 1478–1486. [Google Scholar] [PubMed]
- McKenzie, B.L.; Harris, K.; Peters, S.A.E.; Webster, J.; Woodward, M. The association of energy and macronutrient intake with all-cause mortality, cardiovascular disease and dementia: Findings from 120 963 women and men in the UK Biobank. Br. J. Nutr. 2022, 127, 1858–1867. [Google Scholar]
- Shang, X.; Scott, D.; Hodge, A.M.; English, D.R.; Giles, G.G.; Ebeling, P.R.; Sanders, K.M. Dietary protein intake and risk of type 2 diabetes: Results from the Melbourne Collaborative Cohort Study and a meta-analysis of prospective studies. Am. J. Clin. Nutr. 2016, 104, 1352–1365. [Google Scholar] [CrossRef]
- Malik, V.S.; Li, Y.; Tobias, D.K.; Pan, A.; Hu, F.B. Dietary Protein Intake and Risk of Type 2 Diabetes in US Men and Women. Am. J. Epidemiol. 2016, 183, 715–728. [Google Scholar] [CrossRef]
- Haring, B.; Selvin, E.; Liang, M.; Coresh, J.; Grams, M.E.; Petruski-Ivleva, N.; Steffen, L.M.; Rebholz, C.M. Dietary Protein Sources and Risk for Incident Chronic Kidney Disease: Results From the Atherosclerosis Risk in Communities (ARIC) Study. J. Ren. Nutr. 2017, 27, 233–242. [Google Scholar] [PubMed]
- Raikou, V.D. Protein intake, chronic renal disease progression and cardiovascular morbidity. Nutr. Health 2022, 9, 026010602211188. [Google Scholar] [CrossRef] [PubMed]
- Lagiou, P.; Sandin, S.; Lof, M.; Trichopoulos, D.; Adami, H.O.; Weiderpass, E. Low carbohydrate-high protein diet and incidence of cardiovascular diseases in Swedish women: Prospective cohort study. BMJ 2012, 44, e4026. [Google Scholar] [CrossRef]
- Chen, Z.; Glisic, M.; Song, M.; Aliahmad, H.A.; Zhang, X.; Moumdjian, A.C.; Gonzalez-Jaramillo, V.; van der Schaft, N.; Bramer, W.M.; Arfan Ikram, M.; et al. Dietary protein intake and all-cause and cause-specific mortality: Results from the Rotterdam Study and a meta-analysis of prospective cohort studies. Eur. J. Epidemiol. 2020, 35, 411–429. [Google Scholar]
- Virtanen, H.E.; Voutilainen, S.; Koskinen, T.T.; Mursu, J.; Kokko, P.; Ylilauri, M.P.; Tuomainen, T.P.; Salonen, J.T.; Virtanen, J.K. Dietary proteins and protein sources and risk of death: The Kuopio Ischaemic Heart Disease Risk Factor Study. Am. J. Clin. Nutr. 2019, 109, 1462–1471. [Google Scholar] [PubMed]
- Hernández-Alonso, P.; Salas-Salvadó, J.; Ruiz-Canela, M.; Corella, D.; Estruch, R.; Fitó, M.; Arós, F.; Gómez-Gracia, E.; Fiol, M.; Lapetra, J.; et al. High dietary protein intake is associated with an increased body weight and total death risk. Clin. Nutr. 2016, 35, 496–506. [Google Scholar]
- Trichopoulou, A.; Psaltopoulou, T.; Orfanos, P.; Hsieh, C.C.; Trichopoulos, D. Low-carbohydrate–high-protein diet and long-term survival in a general population cohort. Eur. J. Clin. Nutr. 2007, 61, 575–581. [Google Scholar] [PubMed]
- Halbesma, N.; Bakker, S.J.L.; Jansen, D.F.; Stolk, R.P.; De Zeeuw, D.; De Jong, P.E.; Gansevoort, R.T. High Protein Intake Associates with Cardiovascular Events but not with Loss of Renal Function. J. Am. Soc. Nephrol. 2009, 20, 1797–1804. [Google Scholar] [CrossRef]
- Vogtschmidt, Y.D.; Raben, A.; Faber, I.; de Wilde, C.; Lovegrove, J.A.; Givens, D.I.; Pfeiffer, A.F.H.; Soedamah-Muthu, S.S. Is protein the forgotten ingredient: Effects of higher compared to lower protein diets on cardiometabolic risk factors. A systematic review and meta-analysis of randomised controlled trials. Atherosclerosis 2021, 328, 124–135. [Google Scholar]
- Santesso, N.; Akl, E.A.; Bianchi, M.; Mente, A.; Mustafa, R.; Heels-Ansdell, D.; Schünemann, H.J. Effects of higher- versus lower-protein diets on health outcomes: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2012, 66, 780–788. [Google Scholar]
- Wycherley, T.P.; Moran, L.J.; Clifton, P.M.; Noakes, M.; Brinkworth, G.D. Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2012, 96, 1281–1298. [Google Scholar]
- Schwingshackl, L.; Hoffmann, G. Long-term effects of low-fat diets either low or high in protein on cardiovascular and metabolic risk factors: A systematic review and meta-analysis. Nutr. J. 2013, 12, 48. [Google Scholar] [PubMed]
- Zhang, X.W.; Yang, Z.; Li, M.; Li, K.; Deng, Y.Q.; Tang, Z.Y. Association between dietary protein intake and risk of stroke: A meta-analysis of prospective studies. Int. J. Cardiol. 2016, 223, 548–551. [Google Scholar] [PubMed]
- Qi, X.X.; Shen, P. Associations of dietary protein intake with all-cause, cardiovascular disease, and cancer mortality: A systematic review and meta-analysis of cohort studies. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1094–1105. [Google Scholar] [CrossRef] [PubMed]
- Naghshi, S.; Sadeghi, O.; Willett, W.C.; Esmaillzadeh, A. Dietary intake of total, animal, and plant proteins and risk of all cause, cardiovascular, and cancer mortality: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2020, 370, m2412. [Google Scholar] [PubMed]
- Joint FAO/WHO/UNU Expert Consultation on Protein and Amino Acid Requirements in Human Nutrition 2002: Geneva S, Food and Agriculture Organization of the United Nations, World Health Organization, United Nations University. Protein and amino acid requirements in human nutrition: Report of a joint FAO/WHO/UNU expert consultation. 2007. Available online: https://apps.who.int/iris/handle/10665/43411 (accessed on 2 February 2023).
- Deutz, N.E.P.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznariç, Z.; Nair, K.S.; et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-Based Recommendations for Optimal Dietary Protein Intake in Older People: A Position Paper From the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar]
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids. J. Am. Diet. Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture; U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025, 3rd ed.December 2020. Available online: https://www.dietaryguidelines.gov/ (accessed on 3 February 2023).
- Recommendations, N.N. Part 2: Energy, fat and fatty acids, carbohydrates, protein, alcohol, fluid and water balance and physical activity. In Nordic Nutrition Recommendations 2012; Nordic Council of Ministers: Copenhagen, Denmark, 2014. [Google Scholar]
- Spaaij, C.J.K.; Pijls, L.T.J. New dietary reference intakes in the Netherlands for energy, proteins, fats and digestible carbohydrates. Eur. J. Clin. Nutr. 2004, 58, 191–194. [Google Scholar] [CrossRef]
- Naude, C.E.; Brand, A.; Schoonees, A.; Nguyen, K.A.; Chaplin, M.; Volmink, J. Low-carbohydrate versus balanced-carbohydrate diets for reducing weight and cardiovascular risk. Cochrane Public Health Group, Cochrane Heart Group, editors. Cochrane Database Syst. Rev. 2022, 1–450. [Google Scholar]
- Clifton, P.M.; Condo, D.; Keogh, J.B. Long term weight maintenance after advice to consume low carbohydrate, higher protein diets—A systematic review and meta analysis. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 224–235. [Google Scholar]
- Furlan, A.D.; Pennick, V.; Bombardier, C.; van Tulder, M. 2009 Updated Method Guidelines for Systematic Reviews in the Cochrane Back Review Group. Spine 2009, 34, 1929–1941. [Google Scholar] [CrossRef]
- PRISMA-P Group; Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Stewart, L.A.; Shekelle, P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F. Meta-analysis of Observational Studies in EpidemiologyA Proposal for Reporting. JAMA 2000, 283, 2008. [Google Scholar] [CrossRef]
- Centre for Reviews and Dissemination. CRD’s Guidance for Undertaking Reviews in Healthcare, 3rd ed.; York Publ. Services: New York, NY, USA, 2009; 281p. [Google Scholar]
- Chan, R.; Leung, J.; Woo, J. High Protein Intake Is Associated with Lower Risk of All-Cause Mortality in Community-Dwelling Chinese Older Men and Women. J. Nutr. Health Aging 2019, 23, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, M.; Mente, A.; Zhang, X.; Swaminathan, S.; Li, W.; Mohan, V.; Kumar, R.; Wentzel-Viljoen, E.; Rosengren, A.; Amma, L.I.; et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): A prospective cohort study. Lancet 2017, 390, 2050–2062. [Google Scholar] [CrossRef] [PubMed]
- Haring, B.; Misialek, J.R.; Rebholz, C.M.; Petruski-Ivleva, N.; Gottesman, R.F.; Mosley, T.H.; Alonso, A. Association of Dietary Protein Consumption With Incident Silent Cerebral Infarcts and Stroke: The Atherosclerosis Risk in Communities (ARIC) Study. Stroke 2015, 46, 3443–3450. [Google Scholar] [CrossRef]
- Liu, S.; Willett, W.C.; Stampfer, M.J.; Hu, F.B.; Franz, M.; Sampson, L.; Hennekens, C.H.; Manson, J.E. A prospective study of dietary glycemic load, carbohydrate intake, and risk of coronary heart disease in US women. Am. J. Clin. Nutr. 2000, 71, 1455–1461. [Google Scholar] [CrossRef]
- Kelemen, L.E. Associations of Dietary Protein with Disease and Mortality in a Prospective Study of Postmenopausal Women. Am. J. Epidemiol. 2005, 161, 239–249. [Google Scholar] [CrossRef]
- Hu, F.B.; Rimm, E.B.; Stampfer, M.J.; Ascherio, A.; Spiegelman, D.; Willett, W.C. Prospective study of major dietary patterns and risk of coronary heart disease in men. Am. J. Clin. Nutr. 2000, 72, 912–921. [Google Scholar] [CrossRef]
- Preis, S.R.; Stampfer, M.J.; Spiegelman, D.; Willett, W.C.; Rimm, E.B. Dietary protein and risk of ischemic heart disease in middle-aged men. Am. J. Clin. Nutr. 2010, 92, 1265–1272. [Google Scholar] [CrossRef]
- Preis, S.R.; Stampfer, M.J.; Spiegelman, D.; Willett, W.C.; Rimm, E.B. Lack of association between dietary protein intake and risk of stroke among middle-aged men. Am. J. Clin. Nutr. 2010, 91, 39–45. [Google Scholar] [CrossRef]
- Guallar-Castillón, P.; Rodríguez-Artalejo, F.; Tormo, M.J.; Sánchez, M.J.; Rodríguez, L.; Quirós, J.R.; Navarro, C.; Molina, E.; Martínez, C.; Marín, P.; et al. Major dietary patterns and risk of coronary heart disease in middle-aged persons from a Mediterranean country: The EPIC-Spain cohort study. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 192–199. [Google Scholar] [CrossRef]
- Song, M.; Fung, T.T.; Hu, F.B.; Willett, W.C.; Longo, V.D.; Chan, A.T.; Giovannucci, E.L. Association of Animal and Plant Protein Intake With All-Cause and Cause-Specific Mortality. JAMA Intern. Med. 2016, 176, 1453. [Google Scholar] [CrossRef]
- Zhang, X.; Sergin, I.; Evans, T.D.; Jeong, S.J.; Rodriguez-Velez, A.; Kapoor, D.; Chen, S.; Song, E.; Holloway, K.B.; Crowley, J.R.; et al. High-protein diets increase cardiovascular risk by activating macrophage mTOR to suppress mitophagy. Nat. Metab. 2020, 2, 110–125. [Google Scholar] [CrossRef]
- Kurihara, A.; Okamura, T.; Sugiyama, D.; Higashiyama, A.; Watanabe, M.; Okuda, N.; Kadota, A.; Miyagawa, N.; Fujiyoshi, A.; Yoshita, K.; et al. Vegetable Protein Intake was Inversely Associated with Cardiovascular Mortality in a 15-Year Follow-Up Study of the General Japanese Population. J. Atheroscler. Thromb. 2019, 26, 198–206. [Google Scholar] [CrossRef]
- Nilsson, L.M.; Winkvist, A.; Eliasson, M.; Jansson, J.H.; Hallmans, G.; Johansson, I.; Lindahl, B.; Lenner, P.; Guelpen, B.V. Low-carbohydrate, high-protein score and mortality in a northern Swedish population-based cohort. Eur. J. Clin. Nutr. 2012, 66, 694–700. [Google Scholar] [CrossRef]
- Iso, H. Fat and Protein Intakes and Risk of Intraparenchymal Hemorrhage among Middle-aged Japanese. Am. J. Epidemiol. 2003, 157, 32–39. [Google Scholar] [CrossRef]
- Li, S.S.; Blanco Mejia, S.; Lytvyn, L.; Stewart, S.E.; Viguiliouk, E.; Ha, V.; de Souza, R.J.; Leiter, L.A.; Kendall, C.W.C.; Jenkins, D.J.A.; et al. Effect of Plant Protein on Blood Lipids: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. JAHA 2017, 6, e006659. [Google Scholar] [CrossRef]
- Budhathoki, S.; Sawada, N.; Iwasaki, M.; Yamaji, T.; Goto, A.; Kotemori, A.; Ishihara, J.; Takachi, R.; Charvat, H.; Mizoue, T.; et al. Association of Animal and Plant Protein Intake With All-Cause and Cause-Specific Mortality in a Japanese Cohort. JAMA Intern. Med. 2019, 179, 1509. [Google Scholar] [CrossRef]
- Glenn, A.J.; Viguiliouk, E.; Seider, M.; Boucher, B.A.; Khan, T.A.; Blanco Mejia, S.; Jenkins, D.J.A.; Kahleova, H.; Rahelic, D.; Salas-Salvado, J.; et al. Relation of Vegetarian Dietary Patterns With Major Cardiovascular Outcomes: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Front. Nutr. 2019, 6, 80. [Google Scholar] [CrossRef] [PubMed]
- Ramdath, D.; Padhi, E.; Sarfaraz, S.; Renwick, S.; Duncan, A. Beyond the Cholesterol-Lowering Effect of Soy Protein: A Review of the Effects of Dietary Soy and Its Constituents on Risk Factors for Cardiovascular Disease. Nutrients 2017, 9, 324. [Google Scholar] [CrossRef] [PubMed]
- Wirunsawanya, K.; Upala, S.; Jaruvongvanich, V.; Sanguankeo, A. Whey Protein Supplementation Improves Body Composition and Cardiovascular Risk Factors in Overweight and Obese Patients: A Systematic Review and Meta-Analysis. J. Am. Coll. Nutr. 2018, 37, 60–70. [Google Scholar] [CrossRef] [PubMed]


| Group A | and | Group B |
|---|---|---|
| Protein diet OR | Cardiovascular disease OR | |
| Protein intake OR | CVD OR | |
| Dietary protein consumption OR | Cardiovascular morbidity OR | |
| High protein score OR | Cardiovascular mortality OR | |
| Carbohydrate diet OR | Cardiovascular events OR | |
| Carbohydrate intake OR | Myocardial infarction OR | |
| Dietary carbohydrate consumption OR | Ischemic heart disease OR | |
| Fat intake OR | Ischemic heart disease | |
| Fat diet OR | Coronary artery disease OR | |
| Dietary fat consumption OR | Coronary heart disease OR | |
| Dietary pattern OR | Stroke OR | |
| Macronutrients intake OR | Cerebral infarcts OR | |
| Energy consumption | Intraparenchymal hemorrhage |
| Study | Country | Type of Study | Population | Years of Enrollment | Age (y) | Follow-Up (y) | Exposure | Exposure Assessment | Exposure Follow-Up | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Chan et al., 2019 [49] | China | Prospective cohort | 2262 men and women | 2001–2003 | 65 and over | 13.8 | Quantity and source of protein intake | 280-item FFQ | Every 1 year | Death from all causes, cancer, and CVD |
| Chen et al., 2019 [23] | Netherlands | Prospective cohort | 7786 men and women | 1989–2008 | 63.7 ± 8.7 | 13 (8.3–19.1) | Quantity and source of protein intake | 170-item FFQ, 389-item FFQ | Every 3–5 years | All-cause and cause-specific mortality |
| Dehghan et al., 2017 [50] | International | Prospective cohort | 135,335 men and women | 2003–2013 | 50.3 ± 10 | 7.4 (5.3–9.3) | Dietary intake of fats and carbohydrates | FFQ | Every 3 years | Total mortality and major cardiovascular events (fatal CVD, non-fatal MI, stroke, and heart failure), MI, stroke, CVD mortality, and non-CVD mortality. |
| Haring et al., 2015 [51] | US | Prospective cohort | 11,601 men and women | 1987–1989 | 45–64 | 22.7 | Dietary protein intake | 66-item FFQ | 6 years from baseline | Non-fatal stroke |
| Hernández-Alonso et al., 2015 [25] | Spain | Prospective cohort | 7216 men and women | 2003–2009 | men 55–80 women 60–80 | 4.8 | Quantity and source of protein intake | 137-item FFQ | Cardiovascular events (i.e., MI, stroke, or death from cardiovascular causes), and death by cardiovascular, cancer, and all-cause | |
| Liu et al., 2000 [52] | US | Prospective cohort | 75,521 women | 1984 | 38–63 | 10 | dietary glycemic load, carbohydrate content, and frequency of intake of individual foods | 126-item FFQ | every 2 years | Fatal CHD and nonfatal MI |
| Larsson et al., 2012 [15] | Sweeden | Prospective cohort | 34,670 women | 1997 | 61.4 (49–83) | 10.4 | Quantity and source of protein intake | 96-item FFQ | Non-fatal stroke | |
| Kelemen et al., 2004 [53] | US | Prospective cohort | 29,017 women | 1986 | 75.8 | 15 | Quantity and source of protein intake | FFQ | At 2, 5, and 7 years | Mortality from all causes, cancer, and CVD |
| Hu et al., 1999 [13] | US | Prospective cohort | 80,082 women | 1976 | 45.8 (34–59) | 14 | Quantity and source of protein intake | 61-item FFQ, 116-item FFQ | Every 2 years | Fatal CHD and nonfatal MI |
| Hu et al., 2000 [54] | US | Prospective cohort | 44,875 men | 1986 | 53.8 (40–75) | 8 | Prudent and Western dietary pattern score | 131-FFQ | Every 2 years | Fatal CHD and nonfatal MI |
| Preis et al., 2010 (2 studies) [55,56] | US | Prospective cohort | 43,960 men | 1986 | 40–75 | 18 | Quantity and source of protein intake | 131-FFQ | Every 4 years | Fatal CHD and nonfatal MI, non-fatal stroke |
| Guallar-Castillon et al., 2010 [57] | Spain | Prospective cohort | 40,757 men and women | 1992–1996 | 29–69 | 11 | Westernized and Evolved Mediterranean dietary pattern score | computerized dietary history | CHD events (fatal and non-fatal acute MI or angina requiring revascularization). | |
| Song 2016 [58] | US | Prospective cohort | 131,342 men and women | 1976–1986 | 30–75 | 32 | Animal versus plant protein | FFQ | Every 4 years | All-cause and cause-specific mortality |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantzouranis, E.; Kakargia, E.; Kakargias, F.; Lazaros, G.; Tsioufis, K. The Impact of High Protein Diets on Cardiovascular Outcomes: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Nutrients 2023, 15, 1372. https://doi.org/10.3390/nu15061372
Mantzouranis E, Kakargia E, Kakargias F, Lazaros G, Tsioufis K. The Impact of High Protein Diets on Cardiovascular Outcomes: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Nutrients. 2023; 15(6):1372. https://doi.org/10.3390/nu15061372
Chicago/Turabian StyleMantzouranis, Emmanouil, Eleftheria Kakargia, Fotis Kakargias, George Lazaros, and Konstantinos Tsioufis. 2023. "The Impact of High Protein Diets on Cardiovascular Outcomes: A Systematic Review and Meta-Analysis of Prospective Cohort Studies" Nutrients 15, no. 6: 1372. https://doi.org/10.3390/nu15061372
APA StyleMantzouranis, E., Kakargia, E., Kakargias, F., Lazaros, G., & Tsioufis, K. (2023). The Impact of High Protein Diets on Cardiovascular Outcomes: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Nutrients, 15(6), 1372. https://doi.org/10.3390/nu15061372