Beneficial Effect of Vitamin D on Non-Alcoholic Fatty Liver Disease (NAFLD) Progression in the Zebrafish Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Husbandry
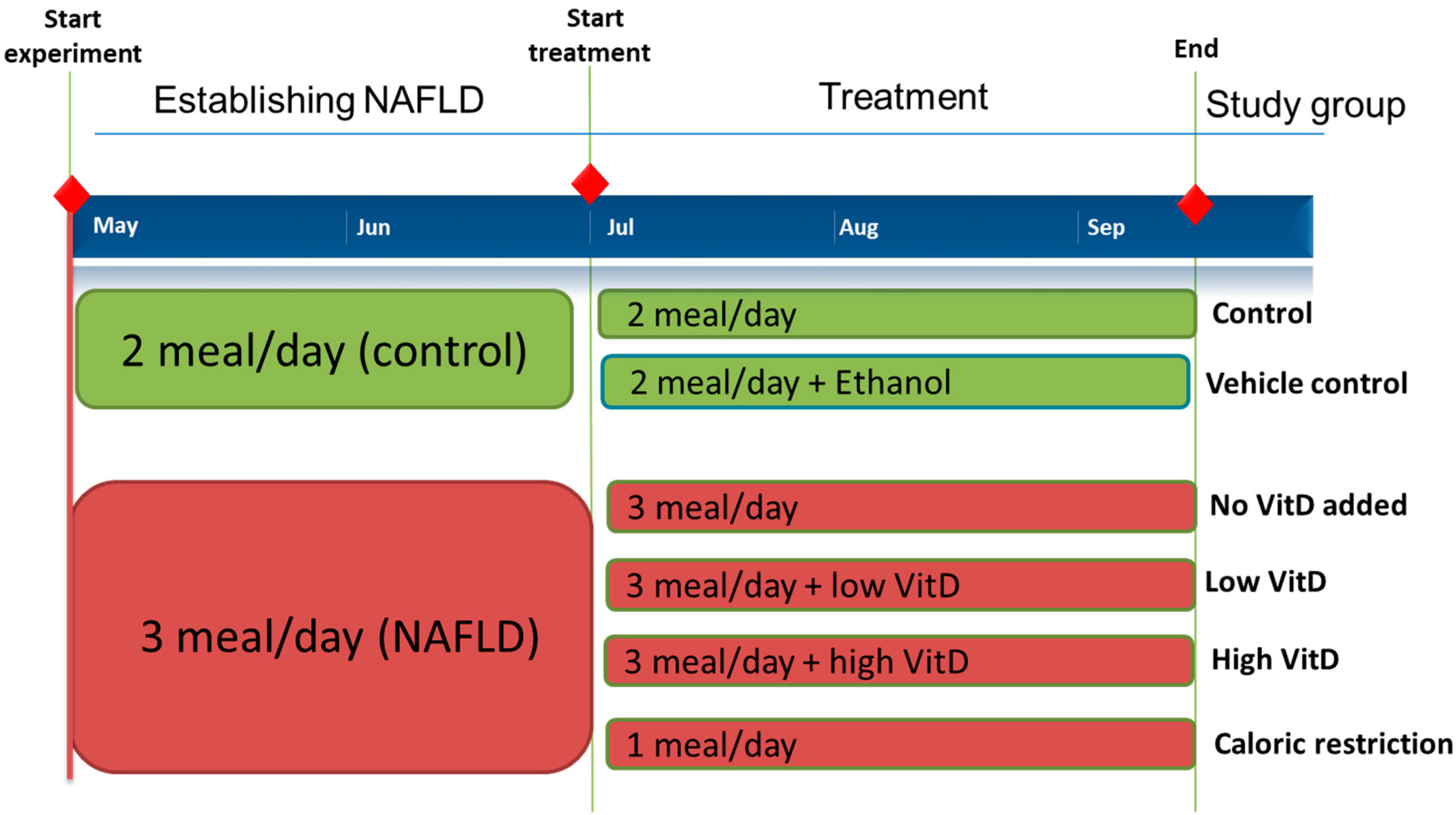
2.2. Zebrafish NAFLD Model
2.3. VitD Supplementation in NAFLD Zebrafish Food
2.4. Euthanasia and Dissection
2.5. Hematoxylin and Eosin (H&E) Staining
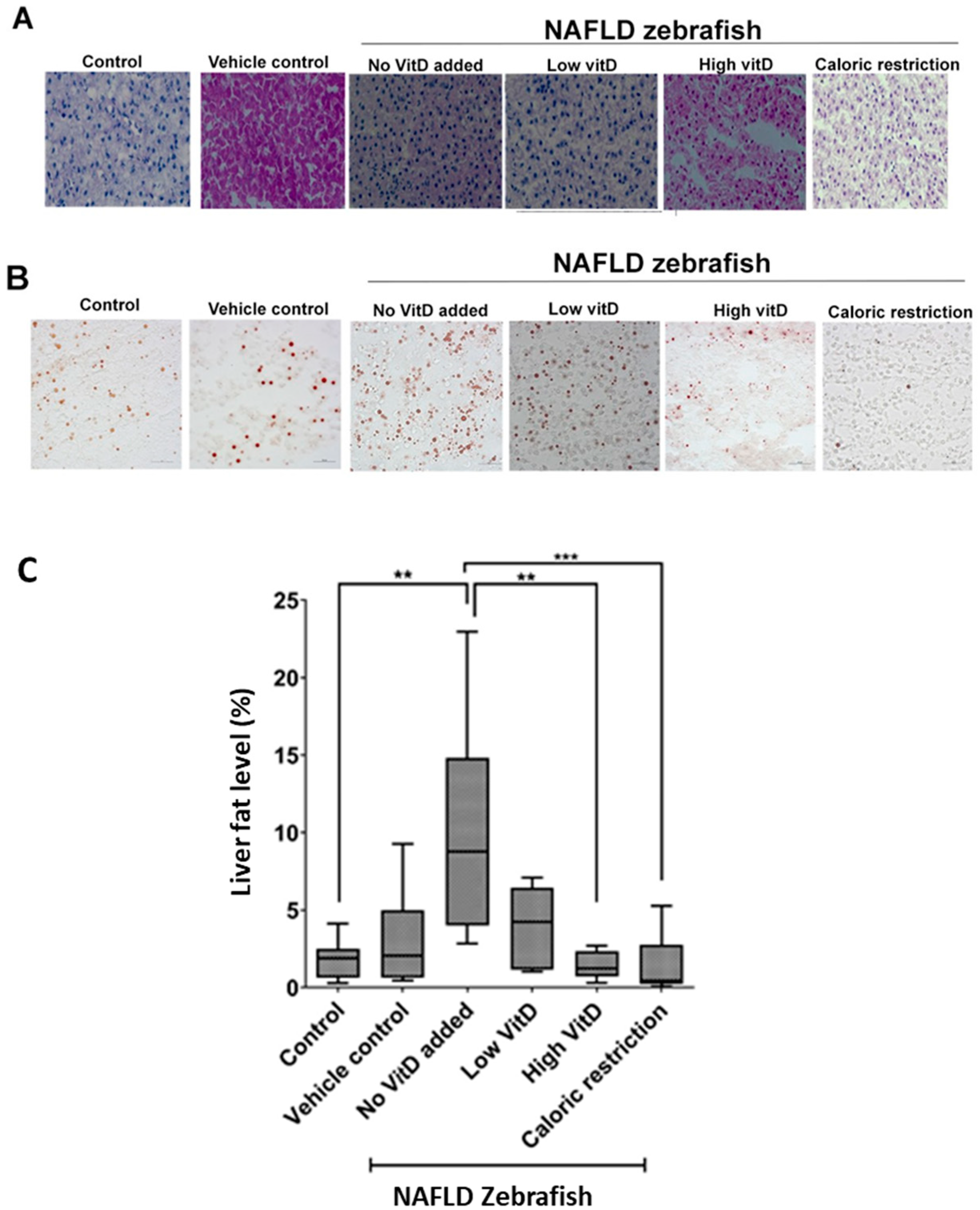
2.6. Oil Red O (ORO) Staining
2.7. RNA Extraction
2.8. RNA Sequencing and Data Analysis
2.9. Bioinformatic Analysis and Annotation
2.10. RT-qPCR and Data Analysis
2.11. Human Cell Experiments: HepG2 Cell Culture
2.12. Steatosis Induction in HepG2 Cells
2.13. Assessment of Lipid Accumulation in HepG2 Cells by Oil Red O Staining
2.14. Measurement of the Viability of HepG2 Cells following Treatment
3. Results
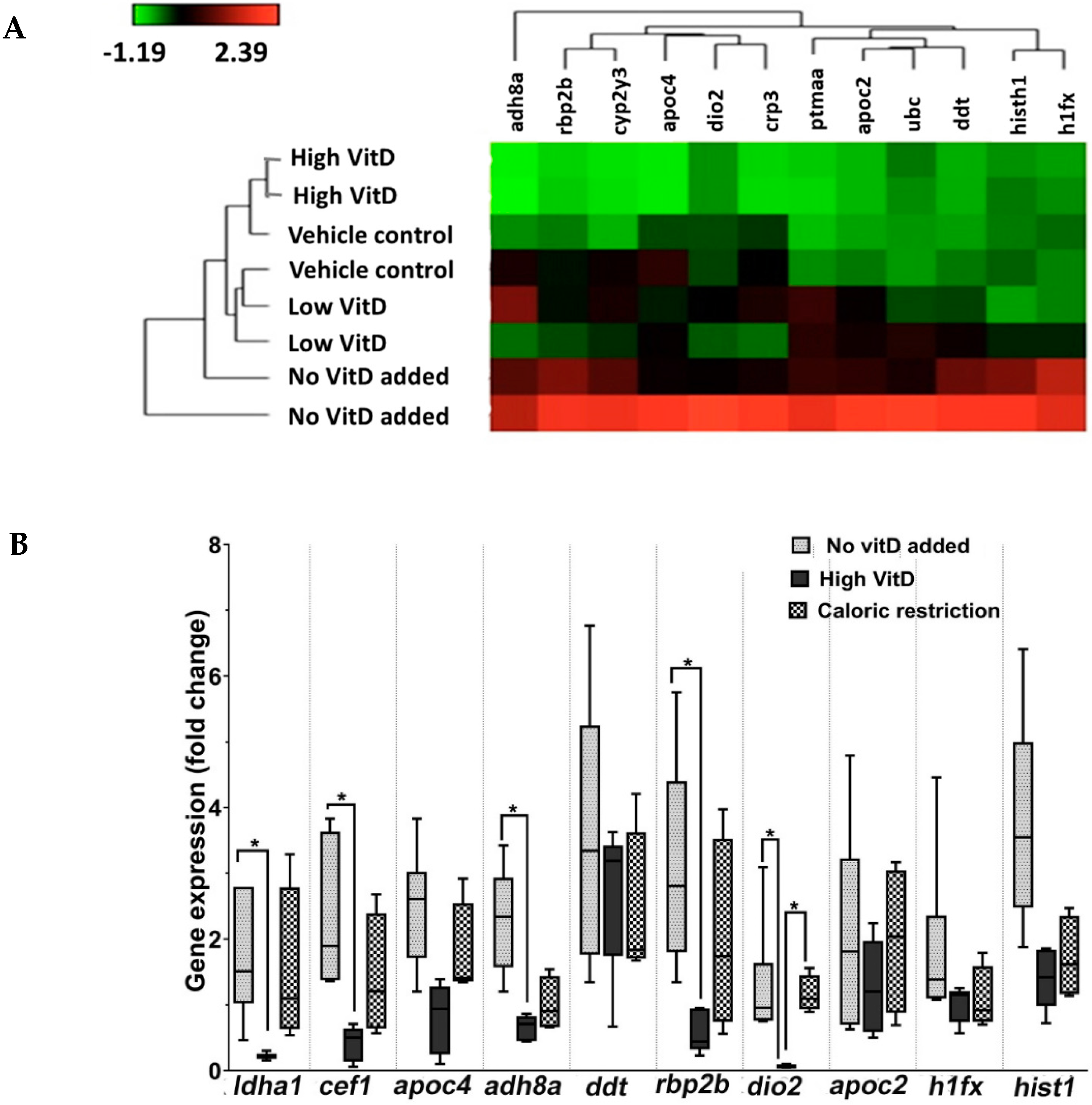
3.1. Gene Expression Analysis Revealed Activation of Pathways Involved in VitD Metabolism and NAFLD
3.2. RT-qPCR Validation of RNA Sequencing
3.3. Pathway Analysis
3.4. Human Cell Experiments
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fabbrini, E.; Magkos, F. Hepatic Steatosis as a Marker of Metabolic Dysfunction. Nutrients 2015, 7, 4995–5019. [Google Scholar] [CrossRef] [PubMed]
- Forn-Cuni, G.; Varela, M.; Fernandez-Rodriguez, C.; Figueras, A.; Novoa, B. Liver immune responses to inflammatory stimuli in a diet-induced obesity model of zebrafish. J. Endocrinol. 2015, 224, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Schwenger, K.; Allard, J.P. Clinical approaches to non-alcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 1712–1723. [Google Scholar] [CrossRef] [PubMed]
- Eliades, M.; Spyrou, E.; Agrawal, N.; Lazo, M.; Brancati, F.L.; Potter, J.J.; Koteish, A.A.; Clark, J.M.; Guallar, E.; Hernaez, R. Meta-analysis: Vitamin D and non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2013, 38, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Torgerson, S.; Hayashi, P.; Ward, J.; Schenker, S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am. J. Gastroenterol. 2003, 98, 2485–2490. [Google Scholar] [CrossRef] [PubMed]
- Neuschwander-Tetri, B.A.; Caldwell, S.H. Nonalcoholic steatohepatitis: Summary of an AASLD Single Topic Conference. Hepatology 2003, 37, 1202–1219. [Google Scholar] [CrossRef]
- Singh, M.K.; Mobeen, A.; Chandra, A.; Joshi, S.; Ramachandran, S. A meta-analysis of comorbidities in COVID-19: Which diseases increase the susceptibility of SARS-CoV-2 infection? Comput. Biol. Med. 2021, 130, 104219. [Google Scholar] [CrossRef]
- Singh, A.; Hussain, S.; Antony, B. Non-alcoholic fatty liver disease and clinical outcomes in patients with COVID-19: A comprehensive systematic review and meta-analysis. Diabetes Metab. Syndr. 2021, 15, 813–822. [Google Scholar] [CrossRef]
- Younossi, Z.M. Non-alcoholic fatty liver disease–A global public health perspective. J. Hepatol. 2019, 70, 531–544. [Google Scholar] [CrossRef]
- Black, L.J.; Jacoby, P.; Ping-Delfos, W.C.S.; Mori, T.A.; Beilin, L.J.; Olynyk, J.K.; Ayonrinde, O.T.; Huang, R.C.; Holt, P.G.; Hart, P.H.; et al. Low serum 25-hydroxyvitamin D concentrations associate with non-alcoholic fatty liver disease in adolescents independent of adiposity. J. Gastroenterol. Hepatol. 2014, 29, 1215–1222. [Google Scholar] [CrossRef]
- Jablonski, K.L.; Jovanovich, A.; Holmen, J.; Targher, G.; McFann, K.; Kendrick, J.; Chonchol, M. Low 25-hydroxyvitamin D level is independently associated with non-alcoholic fatty liver disease. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 792–798. [Google Scholar] [PubMed]
- Kwok, R.M.; Torres, D.M.; Harrison, S.A. Vitamin D and nonalcoholic fatty liver disease (NAFLD): Is it more than just an association? Hepatology 2013, 58, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Gibson, P.S.; Quaglia, A.; Dhawan, A.; Wu, H.; Lanham-New, S.; Hart, K.H.; Fitzpatrick, E.; Moore, J.B. Vitamin D status and associated genetic polymorphisms in a cohort of UK children with non-alcoholic fatty liver disease. Pediatr. Obes. 2018, 13, 433–441. [Google Scholar]
- Fleet, J.C. The role of vitamin D in the endocrinology controlling calcium homeostasis. Mol. Cell Endocrinol. 2017, 453, 36–45. [Google Scholar] [CrossRef]
- Gal-Tanamy, M.; Bachmetov, L.; Ravid, A.; Koren, R.; Erman, A.; Tur-Kaspa, R.; Zemel, R. Vitamin D: An innate antiviral agent suppressing hepatitis C virus in human hepatocytes. Hepatology 2011, 54, 1570–1579. [Google Scholar] [CrossRef] [PubMed]
- Silvagno, F.; Pescarmona, G. Spotlight on vitamin D receptor, lipid metabolism and mitochondria: Some preliminary emerging issues. Mol. Cell. Endocrinol. 2017, 450, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.; Liu, L.; Lv, G.; Yang, Y.; Zhang, Y.; Yu, R.; Wang, Y.; Zhu, J. Lipid metabolism and inflammation modulated by Vitamin D in liver of diabetic rats. Lipids Health Dis. 2015, 14, 31. [Google Scholar] [CrossRef]
- Cipponeri, E.; Vitturi, N.; Mariano, V.; Boscari, F.; Galasso, S.; Crepaldi, C.; Fadini, G.P.; Vigili de Kreutzenberg, S.; Marescotti, M.C.; Iori, E.; et al. Vitamin D status and non-alcoholic fatty liver disease in patients with type 1 diabetes. J. Endocrinol. Investig. 2019, 42, 1099–1107. [Google Scholar] [CrossRef]
- Ha, Y.; Hwang, S.G.; Rim, K.S. The Association between Vitamin D Insufficiency and Nonalcoholic Fatty Liver Disease: A Population-Based Study. Nutrients 2017, 9, 806. [Google Scholar] [CrossRef]
- Jaruvongvanich, V.; Ahuja, W.; Sanguankeo, A.; Wijarnpreecha, K.; Upala, S. Vitamin D and histologic severity of nonalcoholic fatty liver disease: A systematic review and meta-analysis. Dig. Liver Dis. 2017, 49, 618–622. [Google Scholar] [CrossRef]
- Patel, Y.A.; Henao, R.; Moylan, C.A.; Guy, C.D.; Piercy, D.L.; Diehl, A.M.; Abdelmalek, M.F. Vitamin D is Not Associated with Severity in NAFLD: Results of a Paired Clinical and Gene Expression Profile Analysis. Off. J. Am. Coll. Gastroenterol.|ACG 2016, 111, 1591–1598. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, B.; Selig, T.; Li, Y.; Ovchinsky, N.; Kogan-Liberman, D.; Liszewski, M.C.; Levin, T.L.; Ewart, M.; Liu, Q.; Viswanathan, S.; et al. Relationship of Vitamin D Deficiency and Fatty Liver in Children as Defined by Multiple Imaging and Histologic Endpoints. JPGN Rep. 2021, 2, e077. [Google Scholar] [PubMed]
- Saberi, B.; Dadabhai, A.S.; Nanavati, J.; Wang, L.; Shinohara, R.T.; Mullin, G.E. Vitamin D levels do not predict the stage of hepatic fibrosis in patients with non-alcoholic fatty liver disease: A PRISMA compliant systematic review and meta-analysis of pooled data. World J. Hepatol. 2018, 10, 142–154. [Google Scholar] [PubMed]
- Wang, N.; Chen, C.; Zhao, L.; Chen, Y.; Han, B.; Xia, F.; Cheng, J.; Li, Q.; Lu, Y. Vitamin D and Nonalcoholic Fatty Liver Disease: Bi-directional Mendelian Randomization Analysis. EBioMedicine 2018, 28, 187–193. [Google Scholar] [CrossRef]
- Barchetta, I.; Cimini, F.A.; Cavallo, M.G. Vitamin D and Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD): An Update. Nutrients 2020, 12, 3302. [Google Scholar] [CrossRef]
- Loomba, R.; Cortez-Pinto, H. Exercise and improvement of NAFLD: Practical recommendations. J. Hepatol. 2015, 63, 10–12. [Google Scholar] [CrossRef]
- Whitsett, M.; VanWagner, L.B. Physical activity as a treatment of non-alcoholic fatty liver disease: A systematic review. World J. Hepatol 2015, 7, 2041–2052. [Google Scholar] [CrossRef]
- Hao, Y.P.; Ma, X.J.; Luo, Y.Q.; Ni, J.; Dou, J.X.; Hu, Y.Q.; Zhu, J.A.; Bao, Y.Q.; Jia, W.P. Serum vitamin D is associated with non-alcoholic fatty liver disease in Chinese males with normal weight and liver enzymes. Acta Pharmacol. Sin. 2014, 35, 1150–1156. [Google Scholar] [CrossRef]
- Hariri, M.; Zohdi, S. Effect of Vitamin D on Non-Alcoholic Fatty Liver Disease: A Systematic Review of Randomized Controlled Clinical Trials. Int. J. Prev. Med. 2019, 10, 14. [Google Scholar] [CrossRef]
- Singh, S.; Venkatesh, S.K.; Loomba, R.; Wang, Z.; Sirlin, C.; Chen, J.; Yin, M.; Miller, F.H.; Low, R.N.; Hassanein, T.; et al. Magnetic resonance elastography for staging liver fibrosis in non-alcoholic fatty liver disease: A diagnostic accuracy systematic review and individual participant data pooled analysis. Eur. Radiol. 2016, 26, 1431–1440. [Google Scholar]
- Cortes, M.; Chen, M.J.; Stachura, D.L.; Liu, S.Y.; Kwan, W.; Wright, F.; Vo, L.T.; Theodore, L.N.; Esain, V.; Frost, I.M.; et al. Developmental Vitamin D Availability Impacts Hematopoietic Stem Cell Production. Cell Rep. 2016, 17, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, J.R.; Jobin, C. Think small: Zebrafish as a model system of human pathology. J. Biomed. Biotechnol. 2012, 2012, 817341. [Google Scholar] [CrossRef] [PubMed]
- Lieschke, G.J.; Currie, P.D. Animal models of human disease: Zebrafish swim into view. Nat. Rev. Genet. 2007, 8, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Kuwashiro, S.; Terai, S.; Oishi, T.; Fujisawa, K.; Matsumoto, T.; Nishina, H.; Sakaida, I. Telmisartan improves nonalcoholic steatohepatitis in medaka (Oryzias latipes) by reducing macrophage infiltration and fat accumulation. Cell Tissue Res. 2011, 344, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Terai, S.; Oishi, T.; Kuwashiro, S.; Fujisawa, K.; Yamamoto, N.; Fujita, Y.; Hamamoto, Y.; Furutani-Seiki, M.; Nishina, H.; et al. Medaka as a model for human nonalcoholic steatohepatitis. Dis. Model. Mech. 2010, 3, 431–440. [Google Scholar] [CrossRef]
- Oishi, T.; Terai, S.; Kuwashiro, S.; Fujisawa, K.; Matsumoto, T.; Nishina, H.; Sakaida, I. Ezetimibe reduces fatty acid quantity in liver and decreased inflammatory cell infiltration and improved NASH in medaka model. Biochem. Biophys. Res. Commun. 2012, 422, 22–27. [Google Scholar] [CrossRef]
- Oka, T.; Nishimura, Y.; Zang, L.; Hirano, M.; Shimada, Y.; Wang, Z.; Umemoto, N.; Kuroyanagi, J.; Nishimura, N.; Tanaka, T. Diet-induced obesity in zebrafish shares common pathophysiological pathways with mammalian obesity. BMC Physiol. 2010, 10, 21. [Google Scholar] [CrossRef]
- Ghaddar, B.; Veeren, B.; Rondeau, P.; Bringart, M.; Lefebvre d’Hellencourt, C.; Meilhac, O.; Bascands, J.-L.; Diotel, N. Impaired brain homeostasis and neurogenesis in diet-induced overweight zebrafish: A preventive role from A. borbonica extract. Sci. Rep. 2020, 10, 14496. [Google Scholar] [CrossRef]
- Chen, B.; Zheng, Y.-M.; Zhang, J.-P. Comparative Study of Different Diets-Induced NAFLD Models of Zebrafish. Front. Endocrinol. 2018, 9, 366. [Google Scholar] [CrossRef]
- Fraser, D.R. Chapter 2-Evolutionary Biology: Mysteries of Vitamin D in Fish. In Vitamin D, 4th ed.; Feldman, D., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 13–27. [Google Scholar]
- Knuth, M.M.; Mahapatra, D.; Jima, D.; Wan, B.D.; Hammock, B.; Law, M.; Kullman, S.K. Vitamin D deficiency serves as a precursor to stunted growth and central adiposity in zebrafish. Sci. Rep. 2020, 10, 16032. [Google Scholar] [CrossRef]
- Dasarathy, J.; Varghese, R.; Feldman, A.; Khiyami, A.; McCullough, A.J.; Dasarathy, S. Patients with Nonalcoholic Fatty Liver Disease Have a Low Response Rate to Vitamin D Supplementation. J. Nutr. 2017, 147, 1938–1946. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Sun, J.; Wang, X.; Zhang, T.; Zhao, M.; Li, H. Low vitamin D status is associated with coronavirus disease 2019 outcomes: A systematic review and meta-analysis. Int. J. Infect. Dis. 2021, 104, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Saxena, D.; Mavalankar, D. Vitamin D supplementation, COVID-19 and disease severity: A meta-analysis. QJM 2021, 114, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Lansiaux, É.; Pébaÿ, P.P.; Picard, J.-L.; Forget, J. COVID-19 and vit-d: Disease mortality negatively correlates with sunlight exposure. Spat. Spatio-Temporal Epidemiol. 2020, 35, 100362. [Google Scholar] [CrossRef] [PubMed]
- Shochat, C.; Wang, Z.; Mo, C.; Nelson, S.; Donaka, R.; Huang, J.; Karasik, D.; Brotto, M. Deletion of SREBF1, a functional bone-muscle pleiotropic gene, alters bone density and lipid signaling in zebrafish. Endocrinology 2020, 162, bqaa189. [Google Scholar] [CrossRef] [PubMed]
- Pierens, S.L.; Fraser, D.R. The origin and metabolism of vitamin D in rainbow trout. J. Steroid Biochem. Mol. Biol. 2015, 145, 58–64, reprinted in J. Steroid Biochem. Mol. Biol. 2015, 148, 298–304. [Google Scholar] [CrossRef]
- Fleming, A.; Sato, M.; Goldsmith, P. High-throughput in vivo screening for bone anabolic compounds with zebrafish. J. Biomol. Screen. 2005, 10, 823–831. [Google Scholar] [CrossRef]
- Naslund, J. A simple non-invasive method for measuring gross brain size in small live fish with semi-transparent heads. PeerJ 2014, 2, e586. [Google Scholar] [CrossRef]
- Mehlem, A.; Hagberg, C.E.; Muhl, L.; Eriksson, U.; Falkevall, A. Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat. Protoc. 2013, 8, 1149–1154. [Google Scholar] [CrossRef]
- McDonald, J.H. Handbook of Biological Statistics; Sparky House Publishing: Baltimore, MD, USA, 2014. [Google Scholar]
- RNA Reagents. Available online: https://www.neb.com/products/rna-reagents/rna-reagents#tabselect1 (accessed on 1 March 2023).
- Chen, J.; Bardes, E.E.; Aronow, B.J.; Jegga, A.G. ToppGene Suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res. 2009, 37, W305–W311. [Google Scholar] [CrossRef]
- Pike, J.W.; Meyer, M.B. Regulation of Mouse Cyp24a1 Expression via Promoter-Proximal and Downstream-Distal Enhancers Highlights New Concepts of 1,25-Dihydroxyvitamin D(3) Action. Arch. Biochem. Biophys. 2012, 523, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [PubMed]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Dalbeni, A. Treatments for NAFLD: State of Art. Int. J. Mol. Sci. 2021, 22, 2350. [Google Scholar] [PubMed]
- Pydyn, N.; Miękus, K.; Jura, J.; Kotlinowski, J. New therapeutic strategies in nonalcoholic fatty liver disease: A focus on promising drugs for nonalcoholic steatohepatitis. Pharmacol. Rep. 2020, 72, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Keane, J.T.; Elangovan, H.; Stokes, R.A.; Gunton, J.E. Vitamin D and the Liver-Correlation or Cause? Nutrients 2018, 10, 496. [Google Scholar] [CrossRef]
- García-Ruiz, I.; Solís-Muñoz, P.; Fernández-Moreira, D.; Muñoz-Yagüe, T.; Solís-Herruzo, J.A. In vitro treatment of HepG2 cells with saturated fatty acids reproduces mitochondrial dysfunction found in nonalcoholic steatohepatitis. Dis. Model. Mech. 2015, 8, 183–191. [Google Scholar]
- Reimers, M.J.; Hahn, M.E.; Tanguay, R.L. Two zebrafish alcohol dehydrogenases share common ancestry with mammalian class I, II, IV, and V alcohol dehydrogenase genes but have distinct functional characteristics. J. Biol. Chem. 2004, 279, 38303–38312. [Google Scholar] [CrossRef]
- Clemente, M.G.; Mandato, C.; Poeta, M.; Vajro, P. Pediatric non-alcoholic fatty liver disease: Recent solutions, unresolved issues, and future research directions. World J. Gastroenterol. 2016, 22, 8078–8093. [Google Scholar] [CrossRef]
- Seth, A.; Stemple, D.L.; Barroso, I. The emerging use of zebrafish to model metabolic disease. Dis. Model. Mech. 2013, 6, 1080–1088. [Google Scholar]
- Landgraf, K.; Schuster, S.; Meusel, A.; Garten, A.; Riemer, T.; Schleinitz, D.; Kiess, W.; Körner, A. Short-term overfeeding of zebrafish with normal or high-fat diet as a model for the development of metabolically healthy versus unhealthy obesity. BMC Physiol. 2017, 17, 4. [Google Scholar] [CrossRef] [PubMed]
- Zon, L.I.; Peterson, R.T. In vivo drug discovery in the zebrafish. Nat. Rev. Drug Discov. 2005, 4, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Rennekamp, A.J.; Peterson, R.T. 15 years of zebrafish chemical screening. Curr. Opin. Chem. Biol. 2015, 24, 58–70. [Google Scholar] [CrossRef]
- Peng, X.; Shang, G.; Wang, W.; Chen, X.; Lou, Q.; Zhai, G.; Li, D.; Du, Z.; Ye, Y.; Jin, X.; et al. Fatty Acid Oxidation in Zebrafish Adipose Tissue Is Promoted by 1α,25(OH)2D3. Cell Rep. 2017, 19, 1444–1455. [Google Scholar] [CrossRef] [PubMed]
- The Panel on Additives and Products or Substances used in Animal Feed; Rychen, G.; Aquilina, G.; Azimonti, G.; Bampidis, V.; Bastos, M.d.L.; Bories, G.; Chesson, A.; Cocconcelli, P.S.; Flachowsky, G.; et al. Safety of vitamin D3 addition to feeding stuffs for fish. EFSA J. 2017, 15, e04713. [Google Scholar]
- Holden, L.A.; Brown, K.H. Baseline mRNA expression differs widely between common laboratory strains of zebrafish. Sci. Rep. 2018, 8, 4780. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, W.R. Nonobese Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2017, 15, 474–485. [Google Scholar] [CrossRef]
- Leibold, S.; Hammerschmidt, M. Long-term hyperphagia and caloric restriction caused by low- or high-density husbandry have differential effects on zebrafish postembryonic development, somatic growth, fat accumulation and reproduction. PLoS ONE 2015, 10, e0120776. [Google Scholar] [CrossRef]
- Chen, A.; Han, Y.; Poss, K.D. Regulation of zebrafish fin regeneration by vitamin D signaling. Dev. Dyn. 2021, 250, 1330–1339. [Google Scholar] [CrossRef]
- Marcinowska-Suchowierska, E.; Kupisz-Urbańska, M.; Łukaszkiewicz, J.; Płudowski, P.; Jones, G. Vitamin D Toxicity-A Clinical Perspective. Front. Endocrinol. 2018, 9, 550. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grinberg, L.; Dabbah Assadi, F.; Baum, G.; Zemel, R.; Tur-Kaspa, R.; Shochat, C.; Karasik, D.; Karpuj, M.V. Beneficial Effect of Vitamin D on Non-Alcoholic Fatty Liver Disease (NAFLD) Progression in the Zebrafish Model. Nutrients 2023, 15, 1362. https://doi.org/10.3390/nu15061362
Grinberg L, Dabbah Assadi F, Baum G, Zemel R, Tur-Kaspa R, Shochat C, Karasik D, Karpuj MV. Beneficial Effect of Vitamin D on Non-Alcoholic Fatty Liver Disease (NAFLD) Progression in the Zebrafish Model. Nutrients. 2023; 15(6):1362. https://doi.org/10.3390/nu15061362
Chicago/Turabian StyleGrinberg, Lihi, Fadwa Dabbah Assadi, Gideon Baum, Romy Zemel, Ran Tur-Kaspa, Chen Shochat, David Karasik, and Marcela V. Karpuj. 2023. "Beneficial Effect of Vitamin D on Non-Alcoholic Fatty Liver Disease (NAFLD) Progression in the Zebrafish Model" Nutrients 15, no. 6: 1362. https://doi.org/10.3390/nu15061362
APA StyleGrinberg, L., Dabbah Assadi, F., Baum, G., Zemel, R., Tur-Kaspa, R., Shochat, C., Karasik, D., & Karpuj, M. V. (2023). Beneficial Effect of Vitamin D on Non-Alcoholic Fatty Liver Disease (NAFLD) Progression in the Zebrafish Model. Nutrients, 15(6), 1362. https://doi.org/10.3390/nu15061362





