Palmitoylethanolamide in the Treatment of Chronic Pain: A Systematic Review and Meta-Analysis of Double-Blind Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Study Selection
2.3. Quality and Risk of Bias Assessment
2.4. Outcomes
2.5. Analysis and Data Management
3. Results
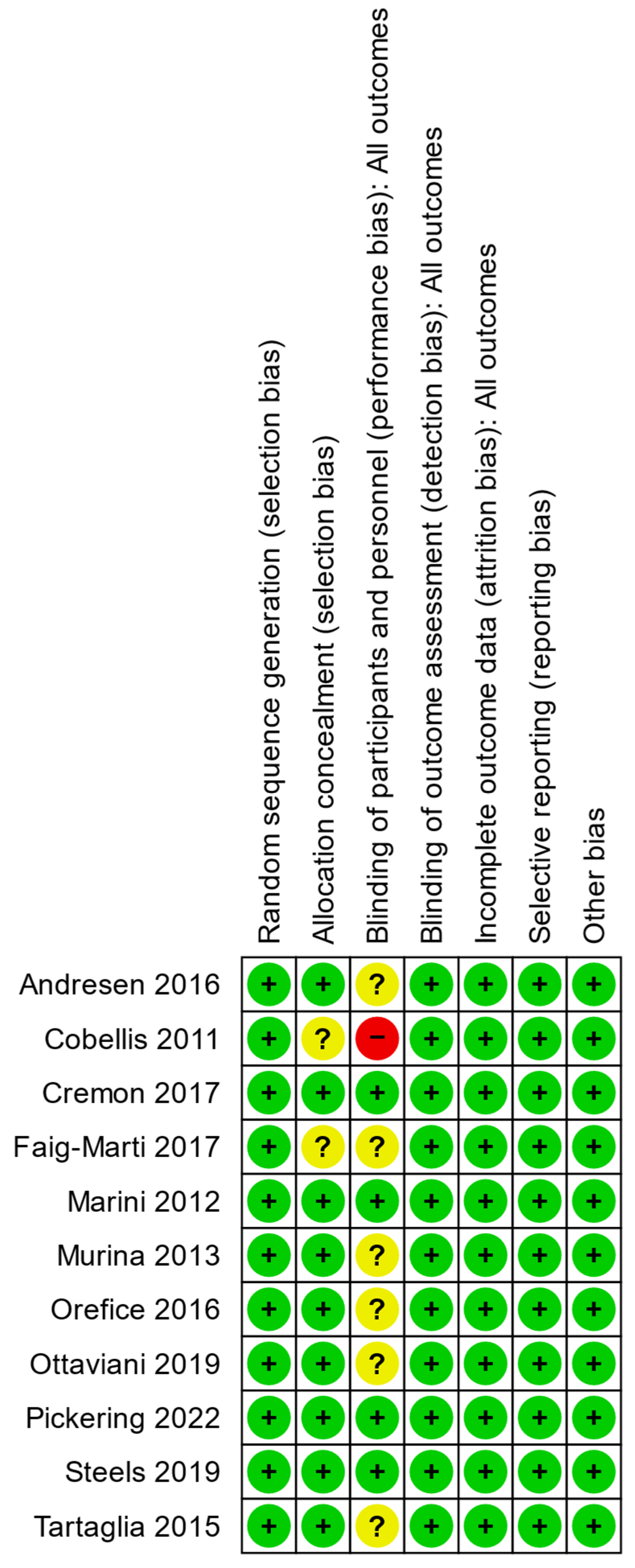
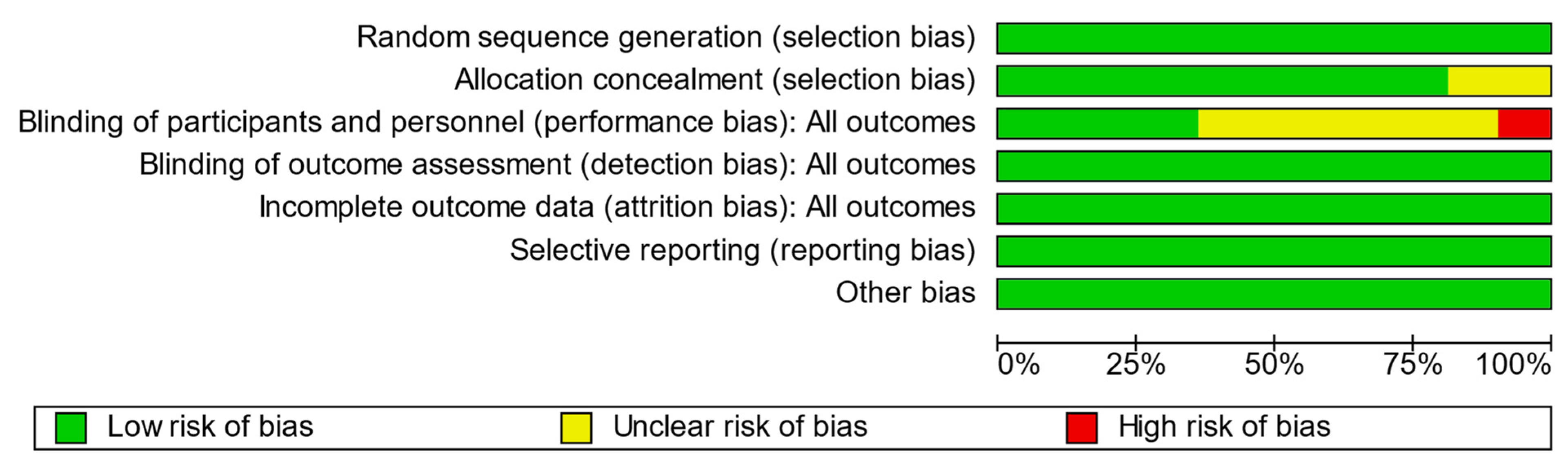
3.1. Quality and Bias Assessments
| Author, Year | Was the Study Described as Randomized? | Was the Randomization Appropriate? | Was the Study Described as Double-Blind? | Was the Blinding Appropriate? | Were the Dropouts Justified? | Was There a Clear Description of Inclusion and Exclusion Criteria? | Has the Method to Capture Adverse Events Been Described? | Has the Method of Statistical Analysis Been Described? | Jadad Score | Modified Jadad Score |
|---|---|---|---|---|---|---|---|---|---|---|
| Andresen 2016 [27] | ● | ● | ● | ○ | ● | ● | ● | ● | 4 | 7 |
| Cobellis 2011 [29] | ● | ● | ● | ○ | ○ | ● | ○ | ● | 3 | 5 |
| Cremon 2017 [25] | ● | ● | ● | ● | ● | ● | ○ | ● | 5 | 7 |
| Faig-Marti 2017 [30] | ● | ○ | ● | ○ | ● | ● | ○ | ● | 3 | 5 |
| Marini 2012 [31] | ● | ● | ● | ● | ○ | ● | ● | ● | 4 | 7 |
| Murina 2013 [32] | ● | ● | ● | ○ | ● | ● | ○ | ● | 4 | 6 |
| Orefice 2016 [28] | ● | ● | ● | ○ | ○ | ● | ● | ● | 3 | 6 |
| Ottaviani 2019 [33] | ● | ● | ● | ○ | ● | ● | ○ | ● | 4 | 6 |
| Pickering 2022 [34] | ● | ● | ● | ● | ● | ● | ● | ● | 5 | 8 |
| Steels 2019 [26] | ● | ● | ● | ● | ● | ● | ● | ● | 5 | 8 |
| Tartaglia 2017 [35] | ● | ○ | ○ | ○ | ● | ● | ○ | ● | 3 | 5 |
3.2. Study Characteristics and Outcomes
4. Discussion
4.1. Primary and Secondary Outcomes
4.2. Indication
4.3. Dosage and Timing
4.4. Duration of Treatment
4.5. Micronization
4.6. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bornemann-Cimenti, H.; Fleck, S.; Rumpold Seitlinger, G. Is deferment of the time of administration to avoid an aspirin/metamizole interaction by chronic dosing sufficient? Z. Rheumatol. 2017, 76, 552–553. [Google Scholar] [CrossRef] [PubMed]
- Klivinyi, C.; Bornemann-Cimenti, H. Pain medication and long QT syndrome. Korean J. Pain 2018, 31, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Lang-Illievich, K.; Bornemann-Cimenti, H. Opioid-induced constipation: A narrative review of therapeutic options in clinical management. Korean J. Pain 2019, 32, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Wurm, W.E.; Lechner, A.; Schmidt, R.; Szilagyi, I.S.; Maier, C.; Nestler, N.; Pichler, B.; Foussek, C.; Bornemann-Cimenti, H.; Sandner-Kiesling, A. Optimising pain therapy for neurological inpatients. Fortschr. Neurol. Psychiatr. 2015, 83, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Bornemann-Cimenti, H.; Wejbora, M.; Szilagyi, I.S.; Sandner-Kiesling, A. Fentanyl for the treatment of tumor-related breakthrough pain. Dtsch. Arztebl. Int. 2013, 110, 271–277. [Google Scholar] [CrossRef]
- Kuehl, F.A., Jr.; Jacob, T.A.; Ganley, O.H.; Ormond, R.E.; Meisinger, M.A.P. The identification of N-(2-Hydroxeythyl)-Palmitamide as a naturally occurring anti-inflammatory agent. J. Am. Chem. Soc. 1957, 79, 5577–5578. [Google Scholar] [CrossRef]
- Rankin, L.; Fowler, C.J. The Basal Pharmacology of Palmitoylethanolamide. Int. J. Mol. Sci. 2020, 21, 7942. [Google Scholar] [CrossRef]
- Bachur, N.R.; Masek, K.; Melmon, K.L.; Udenfriend, S. Fatty acid amides of ethanolamine in mammalian tissues. J. Biol. Chem. 1965, 240, 1019–1024. [Google Scholar] [CrossRef]
- Masek, K.; Perlik, F.; Klima, J.; Kahlich, R. Prophylactic efficacy of N-2-hydroxyethyl palmitamide (impulsin) in acute respiratory tract infections. Eur. J. Clin. Pharmacol. 1974, 7, 415–419. [Google Scholar] [CrossRef]
- Iannotti, F.A.; Di Marzo, V.; Petrosino, S. Endocannabinoids and endocannabinoid-related mediators: Targets, metabolism and role in neurological disorders. Prog. Lipid Res. 2016, 62, 107–128. [Google Scholar] [CrossRef]
- Piomelli, D.; Tagne, A.M. Endocannabinoid-Based Therapies. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 483–507. [Google Scholar] [CrossRef]
- Lang-Illievich, K.; Klivinyi, C.; Rumpold-Seitlinger, G.; Dorn, C.; Bornemann-Cimenti, H. The Effect of Palmitoylethanolamide on Pain Intensity, Central and Peripheral Sensitization, and Pain Modulation in Healthy Volunteers—A Randomized, Double-Blinded, Placebo-Controlled Crossover Trial. Nutrients 2022, 14, 4084. [Google Scholar] [CrossRef]
- D’Amico, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. ALIAmides Update: Palmitoylethanolamide and Its Formulations on Management of Peripheral Neuropathic Pain. Int. J. Mol. Sci. 2020, 21, 5330. [Google Scholar] [CrossRef]
- Scuteri, D.; Guida, F.; Boccella, S.; Palazzo, E.; Maione, S.; Rodríguez-Landa, J.F.; Martínez-Mota, L.; Tonin, P.; Bagetta, G.; Corasaniti, M.T. Effects of Palmitoylethanolamide (PEA) on Nociceptive, Musculoskeletal and Neuropathic Pain: Systematic Review and Meta-Analysis of Clinical Evidence. Pharmaceutics 2022, 14, 1672. [Google Scholar] [CrossRef]
- Davis, M.P.; Behm, B.; Mehta, Z.; Fernandez, C. The potential benefits of palmitoylethanolamide in palliation: A qualitative systematic review. Am. J. Hosp. Palliat. Med. 2019, 36, 1134–1154. [Google Scholar] [CrossRef]
- Artukoglu, B.B.; Beyer, C.; Zuloff-Shani, A.; Brener, E.; Bloch, M.H. Efficacy of Palmitoylethanolamide for Pain: A Meta-Analysis. Pain Physician 2017, 20, 353–362. [Google Scholar]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Szilagyi, I.S.; Bornemann-Cimenti, H. Gender Distribution of Authorship in Pain Publications Is More Balanced than in Other Scientific Fields. Pain Med. 2018, 19, 2104–2105. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 1–10. [Google Scholar] [CrossRef]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Sterne, J.A.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 28, 366. [Google Scholar] [CrossRef] [PubMed]
- Gerstle, D.S.; All, A.C.; Wallace, D.C. Quality of life and chronic nonmalignant pain. Pain Manag. Nurs. Off. J. Am. Soc. Pain Manag. Nurses 2001, 2, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Marcus, D.A.; Scharff, L.; Mercer, S.; Turk, D.C. Nonpharmacological treatment for migraine: Incremental utility of physical therapy with relaxation and thermal biofeedback. Cephalalgia Int. J. Headache 1998, 18, 266–272. [Google Scholar] [CrossRef] [PubMed]
- The Cochrane Collaboration Review Manager (RevMan Web). Available online: https://revman.cochrane.org/ (accessed on 7 March 2023).
- Cremon, C.; Stanghellini, V.; Barbaro, M.R.; Cogliandro, R.F.; Bellacosa, L.; Santos, J.; Vicario, M.; Pigrau, M.; Alonso Cotoner, C.; Lobo, B.; et al. Randomised clinical trial: The analgesic properties of dietary supplementation with palmitoylethanolamide and polydatin in irritable bowel syndrome. Aliment. Pharm. Ther. 2017, 45, 909–922. [Google Scholar] [CrossRef]
- Steels, E.; Venkatesh, R.; Steels, E.; Vitetta, G.; Vitetta, L. A double-blind randomized placebo controlled study assessing safety, tolerability and efficacy of palmitoylethanolamide for symptoms of knee osteoarthritis. Inflammopharmacology 2019, 27, 475–485. [Google Scholar] [CrossRef]
- Andresen, S.R.; Bing, J.; Hansen, R.M.; Biering-Sorensen, F.; Johannesen, I.L.; Hagen, E.M.; Rice, A.S.C.; Nielsen, J.F.; Bach, F.W.; Finnerup, N.B. Ultramicronized palmitoylethanolamide in spinal cord injury neuropathic pain: A randomized, double-blind, placebo-controlled trial. Pain 2016, 157, 2097–2103. [Google Scholar] [CrossRef]
- Orefice, N.S.; Alhouayek, M.; Carotenuto, A.; Montella, S.; Barbato, F.; Comelli, A.; Calignano, A.; Muccioli, G.G.; Orefice, G. Oral palmitoylethanolamide treatment is associated with reduced cutaneous adverse effects of interferon-β1a and circulating proinflammatory cytokines in relapsing–remitting multiple sclerosis. Neurotherapeutics 2016, 13, 428–438. [Google Scholar] [CrossRef]
- Cobellis, L.; Castaldi, M.A.; Giordano, V.; Trabucco, E.; De Franciscis, P.; Torella, M.; Colacurci, N. Effectiveness of the association micronized N-Palmitoylethanolamine (PEA)-transpolydatin in the treatment of chronic pelvic pain related to endometriosis after laparoscopic assessment: A pilot study. Eur. J. Obs. Gynecol. Reprod. Biol. 2011, 158, 82–86. [Google Scholar] [CrossRef]
- Faig-Marti, J.; Martinez-Catassus, A. Use of palmitoylethanolamide in carpal tunnel syndrome: A prospective randomized study. J. Orthop. Traumatol. 2017, 18, 451–455. [Google Scholar] [CrossRef]
- Marini, I.; Lavinia Bartolucci, M.; Bortolotti, F.; Rosaria Gatto, M.; Alessandri Bonetti, G. Palmitoylethanolamide versus a nonsteroidal anti-inflammatory drug in the treatment of temporomandibular joint inflammatory pain. J. Orofac. Pain 2012, 26, 99. [Google Scholar]
- Murina, F.; Graziottin, A.; Felice, R.; Radici, G.; Tognocchi, C. Vestibulodynia: Synergy between palmitoylethanolamide+ transpolydatin and transcutaneous electrical nerve stimulation. J. Low. Genit. Tract. Dis. 2013, 17, 111–116. [Google Scholar] [CrossRef]
- Ottaviani, G.; Rupel, K.; Gobbo, M.; Poropat, A.; Zoi, V.; Faraon, M.; Di Lenarda, R.; Biasotto, M. Efficacy of ultramicronized palmitoylethanolamide in burning mouth syndrome-affected patients: A preliminary randomized double-blind controlled trial. Clin. Oral. Investig. 2019, 23, 2743–2750. [Google Scholar] [CrossRef]
- Pickering, E.; Steels, E.L.; Steadman, K.J.; Rao, A.; Vitetta, L. A randomized controlled trial assessing the safety and efficacy of palmitoylethanolamide for treating diabetic-related peripheral neuropathic pain. Inflammopharmacology 2022, 30, 2063–2077. [Google Scholar] [CrossRef]
- Tartaglia, E.; Armentano, M.; Giugliano, B.; Sena, T.; Giuliano, P.; Loffredo, C.; Mastrantonio, P. Effectiveness of the Association N-Palmitoylethanolamine and Transpolydatin in the Treatment of Primary Dysmenorrhea. J. Pediatr. Adolesc. Gynecol. 2015, 28, 447–450. [Google Scholar] [CrossRef]
- Jung, J.I.; Lee, H.S.; Jeon, Y.E.; Kim, S.M.; Hong, S.H.; Moon, J.M.; Lim, C.Y.; Kim, Y.H.; Kim, E.J. Anti-inflammatory activity of palmitoylethanolamide ameliorates osteoarthritis induced by monosodium iodoacetate in Sprague-Dawley rats. Inflammopharmacology 2021, 29, 1475–1486. [Google Scholar] [CrossRef]
- Paladini, A.; Fusco, M.; Cenacchi, T.; Schievano, C.; Piroli, A.; Varrassi, G. Palmitoylethanolamide, a Special Food for Medical Purposes, in the Treatment of Chronic Pain: A Pooled Data Meta-analysis. Pain Physician 2016, 19, 11–24. [Google Scholar]
- Petrosino, S.; Schiano Moriello, A. Palmitoylethanolamide: A Nutritional Approach to Keep Neuroinflammation within Physiological Boundaries-A Systematic Review. Int. J. Mol. Sci 2020, 21, 9526. [Google Scholar] [CrossRef]
- Petrosino, S.; Schiano Moriello, A.; Cerrato, S.; Fusco, M.; Puigdemont, A.; De Petrocellis, L.; Di Marzo, V. The anti-inflammatory mediator palmitoylethanolamide enhances the levels of 2-arachidonoyl-glycerol and potentiates its actions at TRPV1 cation channels. Br. J. Pharmacol. 2016, 173, 1154–1162. [Google Scholar] [CrossRef]
- Petrosino, S.; Di Marzo, V. The pharmacology of palmitoylethanolamide and first data on the therapeutic efficacy of some of its new formulations. Br. J. Pharmacol. 2017, 174, 1349–1365. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Bruschetta, G.; Cordaro, M.; Crupi, R.; Siracusa, R.; Esposito, E.; Cuzzocrea, S. Micronized/ultramicronized palmitoylethanolamide displays superior oral efficacy compared to nonmicronized palmitoylethanolamide in a rat model of inflammatory pain. J. Neuroinflamm. 2014, 11, 136. [Google Scholar] [CrossRef]
- Kriek, R. Palmitoylethanolamide: Problems regarding micronization, ultra-micronization and additives. Inflammopharmacology 2014, 22, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Cepeda, M.S.; Africano, J.M.; Polo, R.; Alcala, R.; Carr, D.B. What decline in pain intensity is meaningful to patients with acute pain? Pain 2003, 105, 151–157. [Google Scholar] [CrossRef] [PubMed]


| Objective | Search Terms |
|---|---|
| Substance | PEA OR palmitoylethanolamide OR n palmitoyl ethanol amine OR um-pea OR palmidrol OR Impulsin |
| Population | human OR female OR male OR proband OR patient OR volunteer |
| Indication | pain OR chronic pain OR acute pain OR neuropathic pain OR nociceptive pain OR allodynia OR analgesia OR arthralgia OR brachialgia OR causalgia OR cephalalgia OR cephalic OR cervicodynia OR colic OR eudynia OR fibromyalgia OR headache OR hyperalgesia OR hypoalgesia OR hyperpathia OR maldynia OR migraine OR neuralgia OR nociceptive OR odontalgia OR opthalmodynia OR vulvodynia OR otalgia OR radiculopathy OR toothache OR orchidodynia OR coccygodynia OR CRPS OR nuchalgia OR lumbalgia OR lumboischialgia OR cervicobrachialgia |
| Study type | prospective OR randomised OR randomized OR controlled OR observational OR trial |
| Author Year | Country | Population | N | Females % | Drop Outs | Dose PEA | Micronization | Manufacturer | Evaluated Pain Scale | COI |
|---|---|---|---|---|---|---|---|---|---|---|
| Andresen 2016 [27] | Denmark | Spinal cord injury | 73 | 35.2 | 5/73 (6.8%) | 600 mg 2×/d | um | Epitech Group SpA | NRS | Medication provided by Epitech |
| Cobellis 2011 [29] | Italy | Chronic pelvic pain | 61 | 100 | 0/61 (0%) | 400 mg 2×/d | m | n.r. | VAS | n.r. |
| Cremon 2017 [25] | Italy | Irritable bowel syndrome | 54 | 50.0 | 0/54 (0%) | 200 mg 2×/d | co-m | Epitech Group SpA | Likert scale | Funded by Company |
| Faig-Marti 2017 [30] | Spain | Carpal tunnel syndrome | 68 | 60.7 | 7/68 (10.3%) | 300 mg 2×/d | n.a. | Valpharma SpA | VAS | no |
| Marini 2012 [31] | Italy | Temporomandibular joint arthritis | 24 | 33.3 | n.a. | (300 mg + 600 mg)/d (1–7.d), (2× 300 mg)/d (8–14.d) | m | Epitech Group SpA | VAS | n.r. |
| Murina 2013 [32] | Italy | Vestibulodynia | 20 | 100 | 0/20 (0%) | 400 mg 2×/d | n.r. | n.r. | VAS | n.r. |
| Orefice 2016 [28] | Italy | Multiple sclerosis | 29 | 51.7 | n.a. | 600 mg 1×/d | um | Epitech Group SpA | VAS | Medication provided by Epitech. |
| Ottaviani 2019 [33] | Italy | Burning mouth syndrome | 35 | 82.9 | 6/35 (17.1%) | 600 mg 2×/d | um | Epitech Group SpA | NRS | no |
| Pickering 2022 [34] | Australia | Diabetic neuropathic pain | 70 | 44.3 | 4/70 (5.7%) | 300 mg 2×/d | no | Gencor Pacific | NRS | no |
| Steels 2019 [26] | Australia | Knee osteoarthritis | 111 | 53.2 | 11/111 (12.2%) | 150 mg/300 mg 2×/d | no | Gencor Pacific | NRS | Funded by company |
| Tartaglia 2015 [35] | Italy | Dysmenorrhea | 220 | 100 | 0/220 (0%) | 400 mg 1×/d | n.r. | n.r. | VAS | no |
| Author Year | Intervention | Control | Application | Dose PEA | Duration | Primary Outcome | Secondary Outcome | AE |
|---|---|---|---|---|---|---|---|---|
| Andresen 2016 [27] | PEA | Plc. | s.l. | 600 mg 2×/d | 12 w | No sig. differences | Rescue medication intake in PEA group sig. reduced, no sig. improvement in QoL | no |
| Cobellis 2011 [29] | PEA + transpolydatin | Plc. or Celecoxib 200 mg 2×/d | p.o. | 400 mg 2×/d | 3 m | Sig. better pain reduction compared to placebo | Satisfaction with therapy in celecoxib and PEA group sig. higher than in placebo group | no |
| Cremon 2017 [25] | PEA + transpolydatin | Plc. | p.o. | 200 mg 2×/d | 12 w | Sig. better pain reduction compared to placebo | General wellbeing questionnaire in both groups without sig. difference Improved, rescue medication intake without sig. differences | no |
| Faig-Marti 2017 [30] | PEA | Plc. | p.o. | 300 mg 2×/d | 60 d | No sig. differences | no sig. differences in the two groups in function and seveity | n.r. |
| Marini 2012 [31] | PEA | Ibuprofen 600 mg 3×/d | p.o. | (300 mg + 600 mg)/d (1–7 d), (2× 300 mg)/d (8–14 d) | 14 d | Sig. pain reduction compared to ibuprofen | Change in maximum mouth opening after therapy in PEA group sig. higher than in ibuprofen group | no |
| Murina 2013 [32] | PEA + transpolydatin | Plc. | p.o. | 400 mg 2×/d | 60 d | Sig. pain reduction in both groups; no sig. benefit between placebo and PEA | Marinoff Dyspareuniae scale in both groups sig. improves but no sig. difference between placebo and PEA | 2 AE’s in PEA, 1 in Plc. Group (mild, transient gastrointestinal symptoms) |
| Orefice 2016 [28] | PEA | Plc. | p.o. | 600 mg 1×/d | 12 m | Sig. better pain reduction compared to placebo | QoL with sig. improvement at 12 months compared to placebo, no sig. changes in the EDSS score | no |
| Ottaviani 2019 [33] | PEA | Plc. | s.l. | 600 mg 2×/d | 60 d | Sig. better pain reduction compared to placebo | None | no |
| Pickering 2022 [34] | PEA | Plc. | p.o. | 300 mg 2×/d | 8 w | Sig. improvement in neuropathic pain scale | Improved sleep quality and mood | no |
| Steels 2019 [26] | PEA | Plc. | p.o. | 150 mg/300 mg 2×/d | 8 w | Sig. better pain reduction compared to placebo | WOMAC scores in PEA group sign. better, reduction of rescue medication, improvement in anxiety score, remaining scores unchanged | no |
| Tartaglia 2015 [35] | PEA + transpolydatin | Plc. | p.o. | 400 mg 1×/d | 10 d | Sig. better pain reduction compared to placebo | None | no |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lang-Illievich, K.; Klivinyi, C.; Lasser, C.; Brenna, C.T.A.; Szilagyi, I.S.; Bornemann-Cimenti, H. Palmitoylethanolamide in the Treatment of Chronic Pain: A Systematic Review and Meta-Analysis of Double-Blind Randomized Controlled Trials. Nutrients 2023, 15, 1350. https://doi.org/10.3390/nu15061350
Lang-Illievich K, Klivinyi C, Lasser C, Brenna CTA, Szilagyi IS, Bornemann-Cimenti H. Palmitoylethanolamide in the Treatment of Chronic Pain: A Systematic Review and Meta-Analysis of Double-Blind Randomized Controlled Trials. Nutrients. 2023; 15(6):1350. https://doi.org/10.3390/nu15061350
Chicago/Turabian StyleLang-Illievich, Kordula, Christoph Klivinyi, Christian Lasser, Connor T. A. Brenna, Istvan S. Szilagyi, and Helmar Bornemann-Cimenti. 2023. "Palmitoylethanolamide in the Treatment of Chronic Pain: A Systematic Review and Meta-Analysis of Double-Blind Randomized Controlled Trials" Nutrients 15, no. 6: 1350. https://doi.org/10.3390/nu15061350
APA StyleLang-Illievich, K., Klivinyi, C., Lasser, C., Brenna, C. T. A., Szilagyi, I. S., & Bornemann-Cimenti, H. (2023). Palmitoylethanolamide in the Treatment of Chronic Pain: A Systematic Review and Meta-Analysis of Double-Blind Randomized Controlled Trials. Nutrients, 15(6), 1350. https://doi.org/10.3390/nu15061350







