Regulation of Paracellular Fluxes of Amino Acids by Claudin-8 in Normal Mouse Intestinal MCE301 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Cell Culture, Transfection, and Reporter Assay
2.4. Real-Time PCR
2.5. Western Blot Analysis
2.6. Immunocytochemistry
2.7. Paracellular Barrier to Electrolyte Ions and AAs
2.8. Statistic Analyses
3. Results
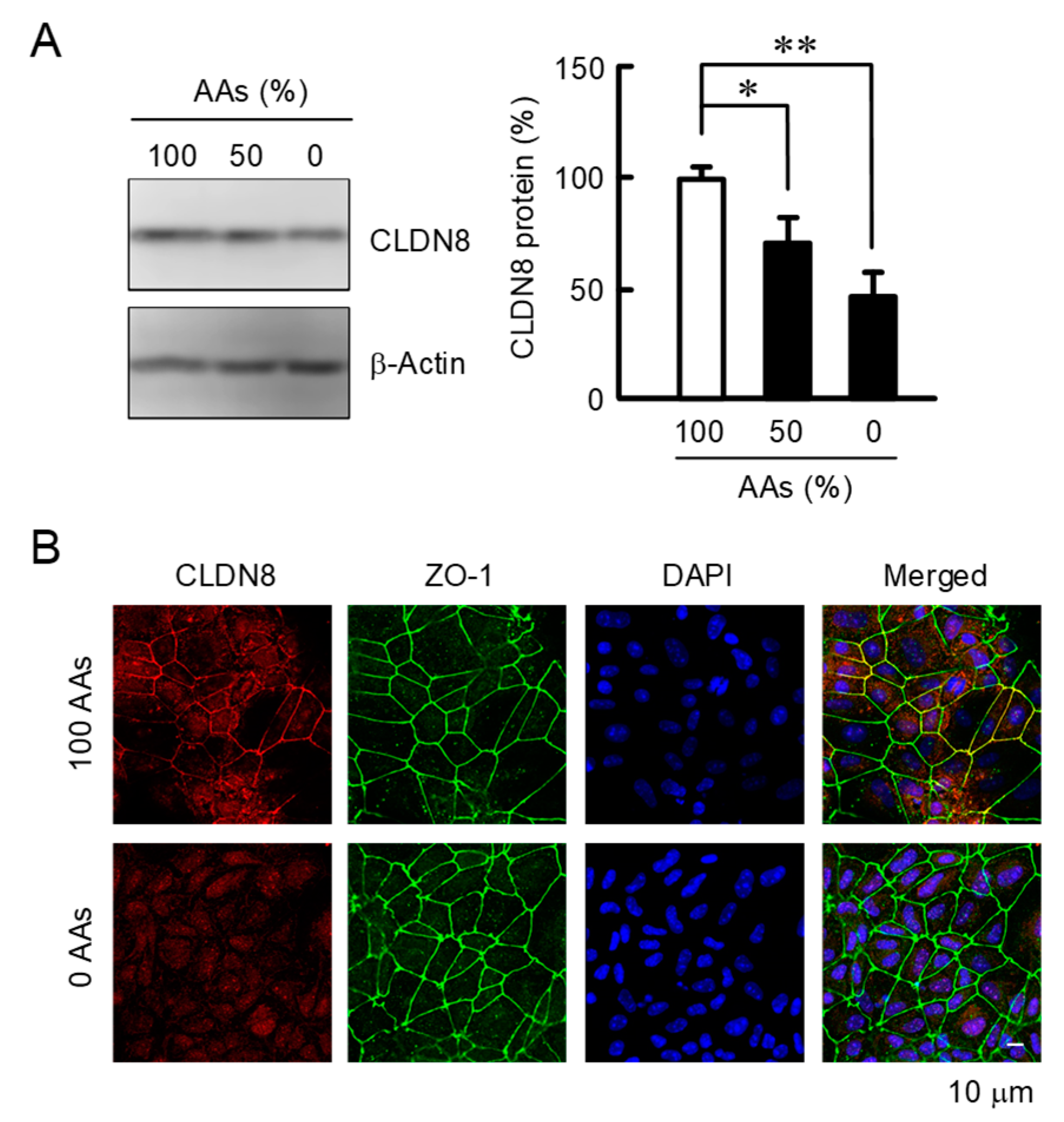
3.1. Effects of AAs Deprivation on the Expression of TJs Components
3.2. Effects of AAs Deprivation on the Expression and Cellular Localization of CLDN8 Protein
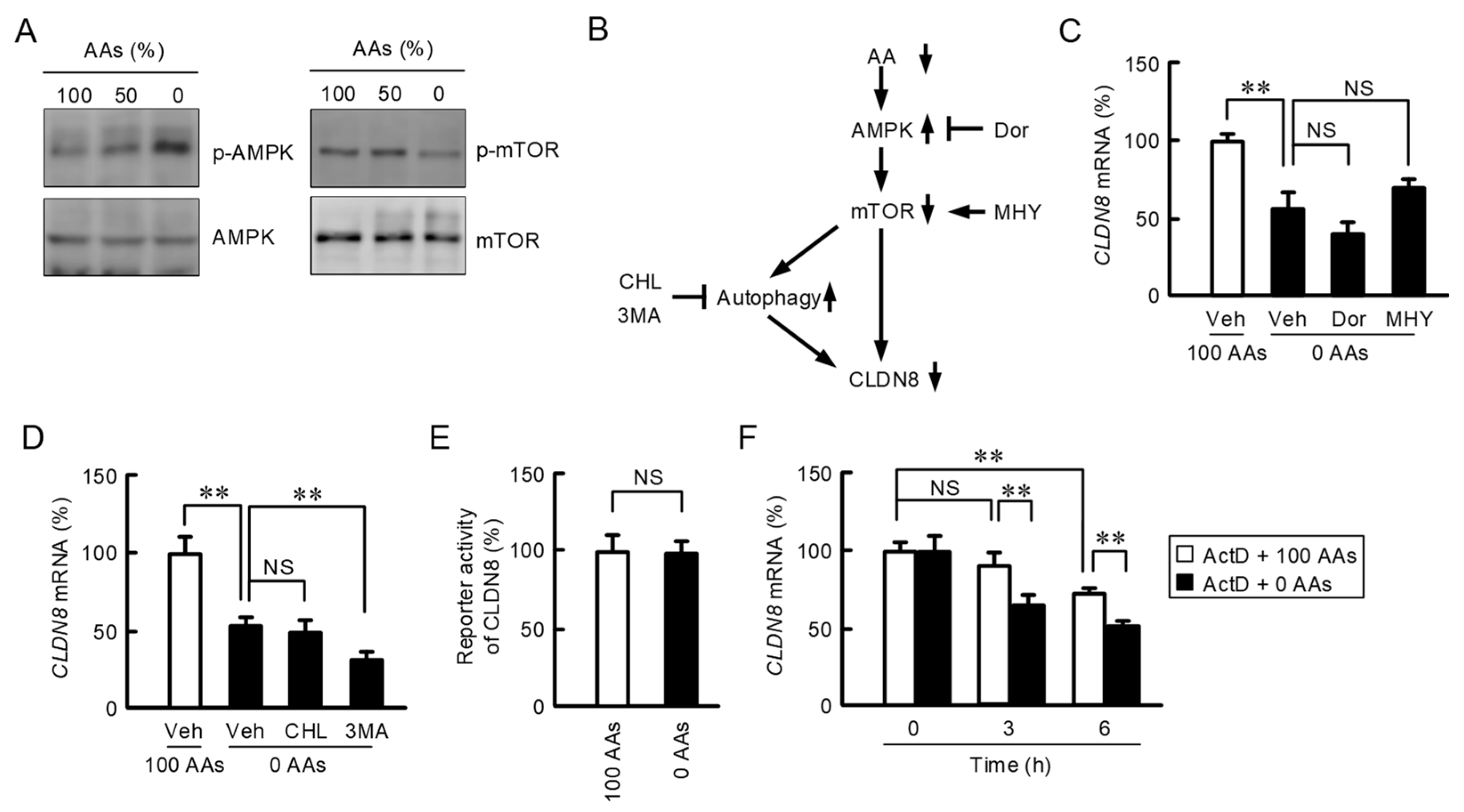
3.3. Regulatory Mechanism of AAs Deprivation-Induced Decline of CLDN8 Expression
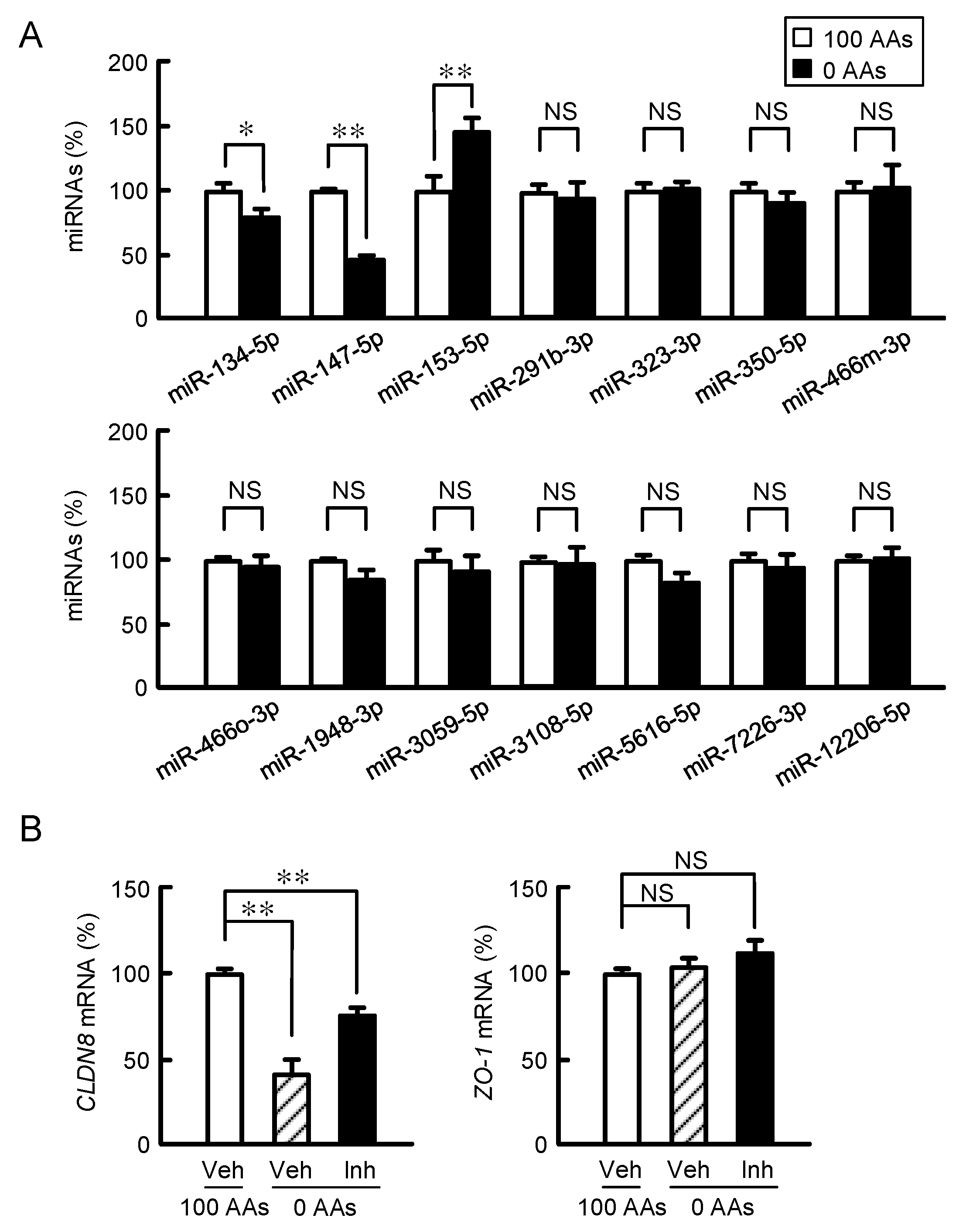
3.4. Involvement of miRNA-153-5p in the AAs Deprivation-Induced Decline of CLDN8 mRNA
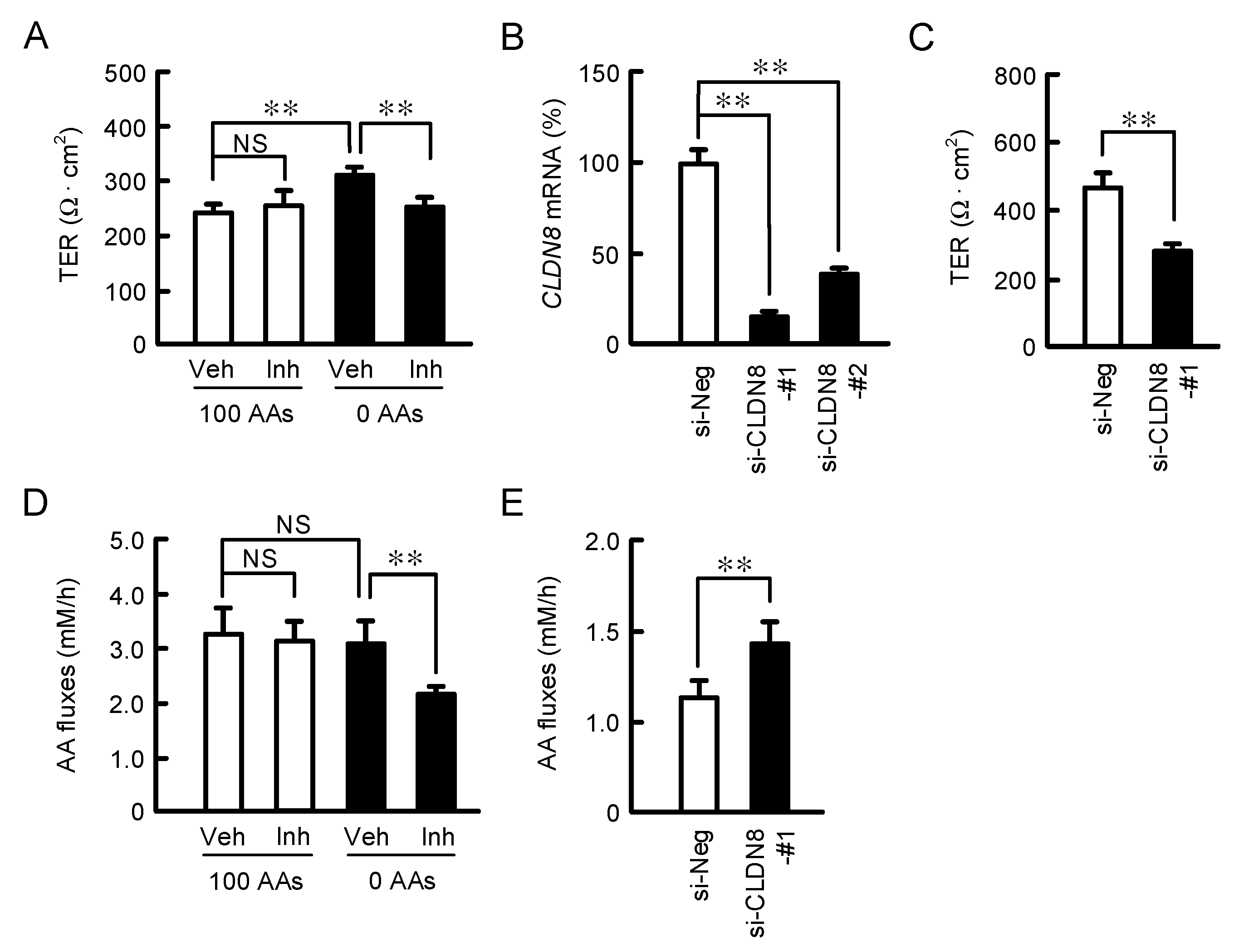
3.5. Effect of CLDN8 Expression on TER and Paracellular AA Fluxes
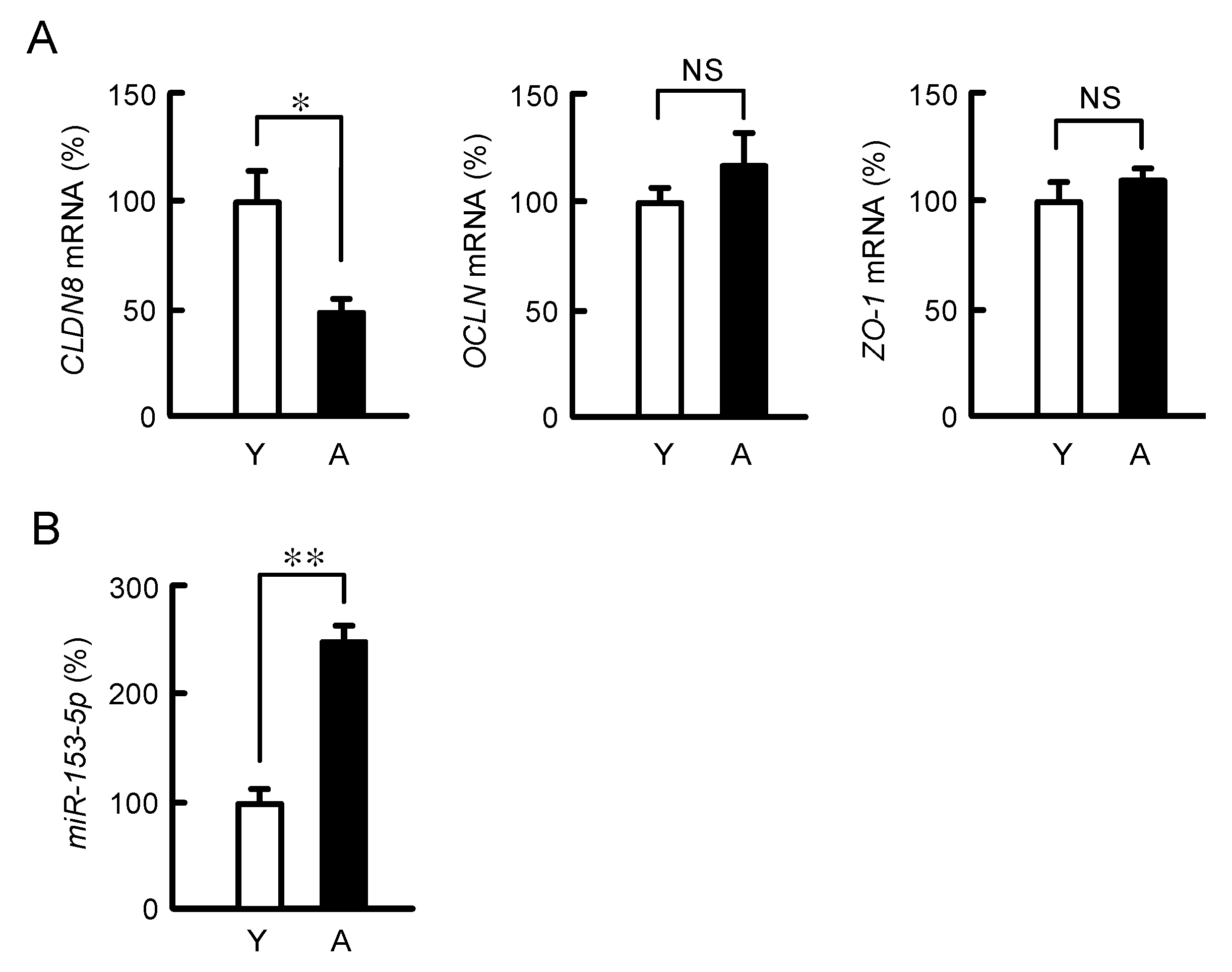
3.6. Effect of Aging on Colonic CLDN8 and miR-153-5p Expression
4. Discussion
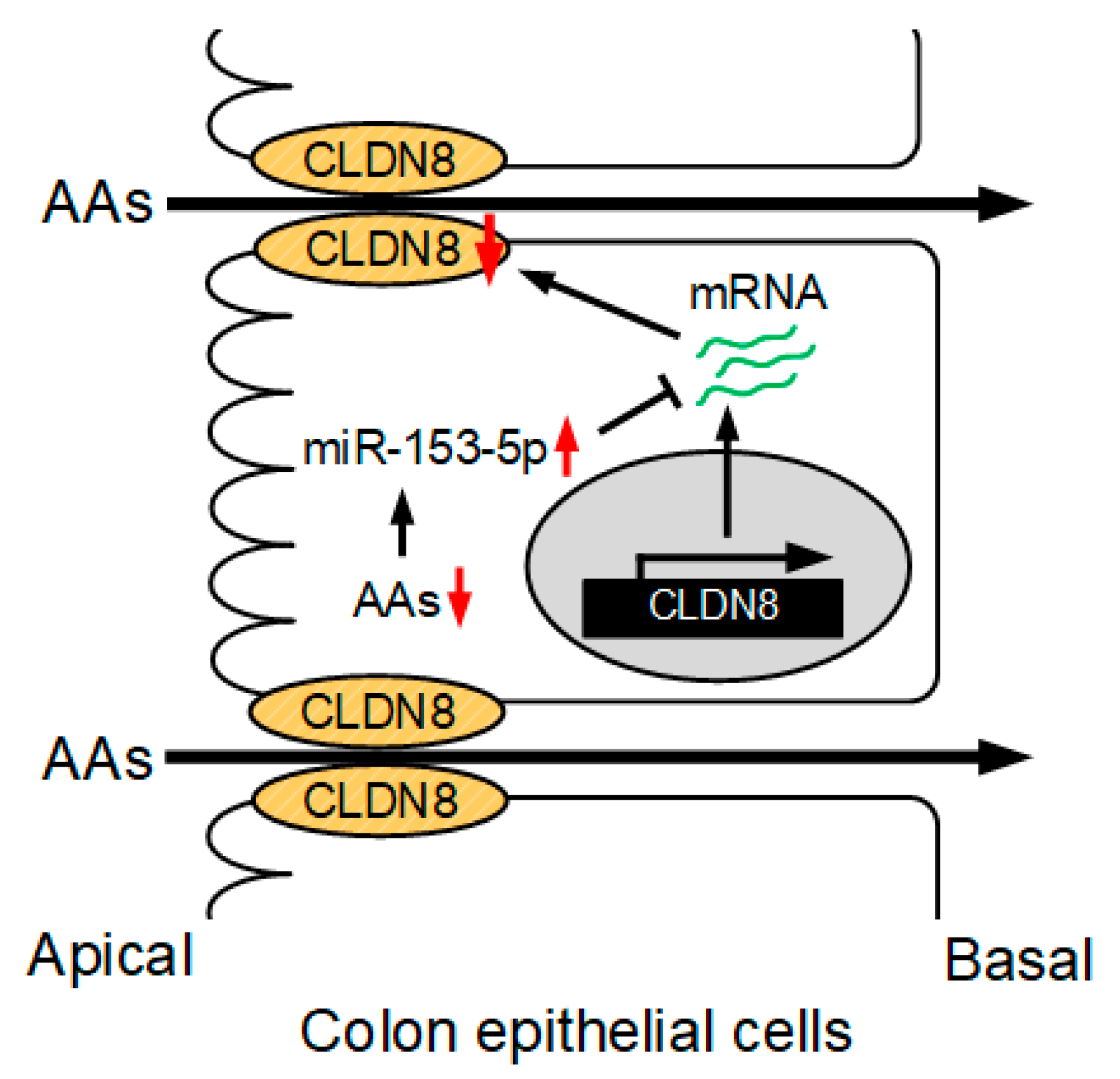
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | Amino acid |
| CLDN | Claudin |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DMEM | Dulbecco’s modified Eagle’s medium |
| 3MA | 3-methyladenine |
| miRNA | MicroRNA |
| OCLN | Occludin |
| p-mTOR | Phospho-mammalian target of rapamycin |
| PCR | Polymerase chain reaction |
| TER | Transepithelial electrical resistance |
| TJ | Tight junction |
| ZO-1 | Zonula occludens-1 |
References
- Chen, Y.; Dinges, M.M.; Green, A.; Cramer, S.E.; Larive, C.K.; Lytle, C. Absorptive transport of amino acids by the rat colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G189–G202. [Google Scholar] [CrossRef] [PubMed]
- Starck, C.S.; Wolfe, R.R.; Moughan, P.J. Endogenous Amino Acid Losses from the Gastrointestinal Tract of the Adult Human—A Quantitative Model. J. Nutr. 2018, 148, 1871–1881. [Google Scholar] [CrossRef] [PubMed]
- van der Wielen, N.; Moughan, P.J.; Mensink, M. Amino Acid Absorption in the Large Intestine of Humans and Porcine Models. J. Nutr. 2017, 147, 1493–1498. [Google Scholar] [CrossRef]
- Fuller, M. Determination of protein and amino acid digestibility in foods including implications of gut microbial amino acid synthesis. Br. J. Nutr. 2012, 108 (Suppl. S2), S238–S246. [Google Scholar] [CrossRef]
- Gilani, G.S.; Sepehr, E. Protein digestibility and quality in products containing antinutritional factors are adversely affected by old age in rats. J. Nutr. 2003, 133, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.S.; Currier, G.J.; Wabner, C.L. Intestinal transport during the life span of the mouse. J. Gerontol. 1990, 45, B129–B133. [Google Scholar] [CrossRef] [PubMed]
- Pitkanen, H.T.; Oja, S.S.; Kemppainen, K.; Seppa, J.M.; Mero, A.A. Serum amino acid concentrations in aging men and women. Amino Acids 2003, 24, 413–421. [Google Scholar] [CrossRef]
- Wu, G. Functional amino acids in nutrition and health. Amino Acids 2013, 45, 407–411. [Google Scholar] [CrossRef]
- Calvani, R.; Picca, A.; Marini, F.; Biancolillo, A.; Gervasoni, J.; Persichilli, S.; Primiano, A.; Coelho-Junior, H.J.; Bossola, M.; Urbani, A.; et al. A Distinct Pattern of Circulating Amino Acids Characterizes Older Persons with Physical Frailty and Sarcopenia: Results from the BIOSPHERE Study. Nutrients 2018, 10, 1691. [Google Scholar] [CrossRef]
- Broer, S.; Fairweather, S.J. Amino Acid Transport Across the Mammalian Intestine. Compr. Physiol. 2018, 9, 343–373. [Google Scholar]
- Kiela, P.R.; Ghishan, F.K. Physiology of Intestinal Absorption and Secretion. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Qiao, S.; Ren, M.; Zeng, X.; Ma, X.; Wu, Z.; Thacker, P.; Wu, G. Supplementation with branched-chain amino acids to a low-protein diet regulates intestinal expression of amino acid and peptide transporters in weanling pigs. Amino Acids 2013, 45, 1191–1205. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, H.; Ning, L.; Li, F. Rabbit SLC15A1, SLC7A1 and SLC1A1 genes are affected by site of digestion, stage of development and dietary protein content. Animal 2019, 13, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Tsukita, S.; Furuse, M.; Itoh, M. Structural and signalling molecules come together at tight junctions. Curr. Opin. Cell Biol. 1999, 11, 628–633. [Google Scholar] [CrossRef]
- Furuse, M.; Fujita, K.; Hiiragi, T.; Fujimoto, K.; Tsukita, S. Claudin-1 and -2: Novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J. Cell Biol. 1998, 141, 1539–1550. [Google Scholar] [CrossRef]
- Mineta, K.; Yamamoto, Y.; Yamazaki, Y.; Tanaka, H.; Tada, Y.; Saito, K.; Tamura, A.; Igarashi, M.; Endo, T.; Takeuchi, K.; et al. Predicted expansion of the claudin multigene family. FEBS Lett. 2011, 585, 606–612. [Google Scholar] [CrossRef]
- Turksen, K.; Troy, T.C. Barriers built on claudins. J. Cell Sci. 2004, 117, 2435–2447. [Google Scholar] [CrossRef]
- Hou, J.; Gomes, A.S.; Paul, D.L.; Goodenough, D.A. Study of claudin function by RNA interference. J. Biol. Chem. 2006, 281, 36117–36123. [Google Scholar] [CrossRef]
- Krug, S.M.; Schulzke, J.D.; Fromm, M. Tight junction, selective permeability, and related diseases. Semin. Cell Dev. Biol. 2014, 36, 166–176. [Google Scholar] [CrossRef]
- Tabuchi, Y.; Ohta, S.; Arai, Y.; Kawahara, M.; Ishibashi, K.; Sugiyama, N.; Horiuchi, T.; Furusawa, M.; Obinata, M.; Fuse, H.; et al. Establishment and characterization of a colonic epithelial cell line MCE301 from transgenic mice harboring temperature-sensitive simian virus 40 large T-antigen gene. Cell Struct. Funct. 2000, 25, 297–307. [Google Scholar] [CrossRef]
- Furukawa, C.; Ishizuka, N.; Hayashi, H.; Fujii, N.; Manabe, A.; Tabuchi, Y.; Matsunaga, T.; Endo, S.; Ikari, A. Up-regulation of claudin-2 expression by aldosterone in colonic epithelial cells of mice fed with NaCl-depleted diets. Sci. Rep. 2017, 7, 12223. [Google Scholar] [CrossRef]
- Ma, X.M.; Blenis, J. Molecular mechanisms of mTOR-mediated translational control. Nat. Rev. Mol. Cell Biol. 2009, 10, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Verschooten, L.; Barrette, K.; Van Kelst, S.; Rubio Romero, N.; Proby, C.; De Vos, R.; Agostinis, P.; Garmyn, M. Autophagy inhibitor chloroquine enhanced the cell death inducing effect of the flavonoid luteolin in metastatic squamous cell carcinoma cells. PLoS ONE 2012, 7, e48264. [Google Scholar] [CrossRef] [PubMed]
- Sassi, A.; Wang, Y.; Chassot, A.; Roth, I.; Ramakrishnan, S.; Olivier, V.; Staub, O.; Udwan, K.; Feraille, E. Expression of claudin-8 is induced by aldosterone in renal collecting duct principal cells. Am. J. Physiol. Renal Physiol. 2021, 321, F645–F655. [Google Scholar] [CrossRef] [PubMed]
- Kielgast, F.; Schmidt, H.; Braubach, P.; Winkelmann, V.E.; Thompson, K.E.; Frick, M.; Dietl, P.; Wittekindt, O.H. Glucocorticoids Regulate Tight Junction Permeability of Lung Epithelia by Modulating Claudin 8. Am. J. Respir. Cell Mol. Biol. 2016, 54, 707–717. [Google Scholar] [CrossRef]
- Li, M.; Zhao, J.; Cao, M.; Liu, R.; Chen, G.; Li, S.; Xie, Y.; Xie, J.; Cheng, Y.; Huang, L.; et al. Mast cells-derived MiR-223 destroys intestinal barrier function by inhibition of CLDN8 expression in intestinal epithelial cells. Biol. Res. 2020, 53, 12. [Google Scholar] [CrossRef]
- Liu, B.; Lu, B.; Wang, X.; Jiang, H.; Kuang, W. MiR-361-5p inhibits cell proliferation and induces cell apoptosis in retinoblastoma by negatively regulating CLDN8. Childs Nerv. Syst. 2019, 35, 1303–1311. [Google Scholar] [CrossRef]
- Cheng, B.; Rong, A.; Zhou, Q.; Li, W. LncRNA LINC00662 promotes colon cancer tumor growth and metastasis by competitively binding with miR-340-5p to regulate CLDN8/IL22 co-expression and activating ERK signaling pathway. J. Exp. Clin. Cancer Res. 2020, 39, 5. [Google Scholar] [CrossRef]
- Hu, X.; Guo, F. Amino Acid Sensing in Metabolic Homeostasis and Health. Endocr. Rev. 2021, 42, 56–76. [Google Scholar] [CrossRef]
- Ye, J.; Kumanova, M.; Hart, L.S.; Sloane, K.; Zhang, H.; De Panis, D.N.; Bobrovnikova-Marjon, E.; Diehl, J.A.; Ron, D.; Koumenis, C. The GCN2-ATF4 pathway is critical for tumour cell survival and proliferation in response to nutrient deprivation. EMBO J. 2010, 29, 2082–2096. [Google Scholar] [CrossRef]
- Yu, A.S.; Enck, A.H.; Lencer, W.I.; Schneeberger, E.E. Claudin-8 expression in Madin-Darby canine kidney cells augments the paracellular barrier to cation permeation. J. Biol. Chem. 2003, 278, 17350–17359. [Google Scholar] [CrossRef] [PubMed]
- Bucker, R.; Troeger, H.; Kleer, J.; Fromm, M.; Schulzke, J.D. Arcobacter butzleri induces barrier dysfunction in intestinal HT-29/B6 cells. J. Infect. Dis. 2009, 200, 756–764. [Google Scholar] [CrossRef]
- Nattramilarasu, P.K.; Bucker, R.; Lobo de Sa, F.D.; Fromm, A.; Nagel, O.; Lee, I.M.; Butkevych, E.; Mousavi, S.; Genger, C.; Klove, S.; et al. Campylobacter concisus Impairs Sodium Absorption in Colonic Epithelium via ENaC Dysfunction and Claudin-8 Disruption. Int. J. Mol. Sci. 2020, 21, 373. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.M.; Kim, J.C.; Hansen, C.F.; Mullan, B.P.; Hampson, D.J.; Pluske, J.R. Effects of feeding low protein diets to piglets on plasma urea nitrogen, faecal ammonia nitrogen, the incidence of diarrhoea and performance after weaning. Arch. Anim. Nutr. 2008, 62, 343–358. [Google Scholar] [CrossRef]
- Wada, M.; Tamura, A.; Takahashi, N.; Tsukita, S. Loss of claudins 2 and 15 from mice causes defects in paracellular Na+ flow and nutrient transport in gut and leads to death from malnutrition. Gastroenterology 2013, 144, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Renigunta, A.; Yang, J.; Waldegger, S. Claudin-4 forms paracellular chloride channel in the kidney and requires claudin-8 for tight junction localization. Proc. Natl. Acad. Sci. USA 2010, 107, 18010–18015. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yin, Y.L.; Li, D.; Kim, S.W.; Wu, G. Amino acids and immune function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef]
- Mirzaei, H.; Suarez, J.A.; Longo, V.D. Protein and amino acid restriction, aging and disease: From yeast to humans. Trends Endocrinol. Metab. 2014, 25, 558–566. [Google Scholar] [CrossRef]


| Primary Antibodies | Sources |
|---|---|
| Rabbit anti-CLDN8 antibody | Thermo Fisher Scientific (Rockford, IL, USA) |
| Mouse anti-ZO-1 antibody | Thermo Fisher Scientific (Rockford, IL, USA) |
| Goat anti-β-actin antibody | Santa Cruz Biotechnology (Santa Cruz, CA, USA) |
| Rabbit anti-phospho-mammalian targets of rapamycin (Ser2448) (p-mTOR) | Cell Signaling Technology (Danvers, MA, USA) |
| Rabbit anti-mTOR antibody | Cell Signaling Technology (Danvers, MA, USA) |
| Rabbit anti-AMPK antibody | ProteinTech (Rosemont, IL, USA) |
| Rabbit anti-phospho-AMPK (T183/T172) (p-AMPK) antibody | R&D Systems (Minneapolis, MN, USA) |
| Genes | Direction | Sequence (5′→3′) |
|---|---|---|
| CLDN1 | Sense | GTCTTCGATTCCTTGCTGAA |
| Antisense | CCTGGCCAAATTCATACCTG | |
| CLDN2 | Sense | TGCGACACACAGCACAGGCATCAC |
| Antisense | TCAGGAACCAGCGGCGAGTAGAA | |
| CLDN3 | Sense | CATCCTGCTGGCCGCCTTCG |
| Antisense | CCTGATGATGGTGTTGGCCGAC | |
| CLDN8 | Sense | CATGCCAACATCAGAATGCAGT |
| Antisense | CTGTGGTCCAGCCTATGTAGAG | |
| OCLN | Sense | TGGATCTATGTACGGCTCACAG |
| Antisense | AAAGCCACGATAATCATGAACC | |
| ZO-1 | Sense | CAGAGCCTCAGAAACCTCAAGT |
| Antisense | TCTTCGGTCAAAGTAGGAGAGC | |
| β-Actin | Sense | CCAACCGTGAAAAGATGACC |
| Antisense | CCAGAGGCATACAGGGACAG |
| Genes | Sequence (5′→3′) |
|---|---|
| miR-134-5p | TGTGACTGGTTGACCAGAGG |
| miR-147-5p | TGGAAACATTTCTGCACAAAC |
| miR-153-5p | GTCATTTTTGTGACGTTGCAG |
| miR-291b-3p | AAAGTGCATCCATTTTGTTTG |
| miR-323-3p | CACATTACACGGTCGACCTC |
| miR-350-5p | AAAGTGCATGCGCTTTGGG |
| miR-466m-3p | TACATACACACATACACACG |
| miR-466o-3p | TACATACATGCACACATAAG |
| miR-1948-3p | TTTAGGCAGAGCACTCGTAC |
| miR-3059-5p | TTTCCTCTCTGCCCCATAGG |
| miR-3108-5p | GTCTCTAAAGCTAGACGTTC |
| miR-5616-5p | TTTCCTCTCATCACAAGTTG |
| miR-7226-3p | TGACACAGCCATTCTCTGAG |
| miR-12206-5p | TACTATGCCTGGAAGGCACC |
| AAs | [M + H]+ |
|---|---|
| Phe | 166.0918 |
| Trp | 205.0967 |
| Leu_Ile | 132.1026 |
| Met | 150.06 |
| Tyr | 182.0836 |
| Pro | 116.0715 |
| Val | 118.0862 |
| Ala | 90.0551 |
| Thr | 120.0662 |
| Glu | 148.0616 |
| Asp | 134.0455 |
| Ser | 106.0506 |
| Gln | 147.077 |
| Asn | 133.0617 |
| (Cys)2 | 241.0299 |
| His | 156.0783 |
| Lys | 147.1142 |
| Arg | 175.1255 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okamoto, E.; Matsuda, S.; Yoshino, Y.; Morikawa, Y.; Suenami, K.; Tabuchi, Y.; Matsunaga, T.; Ikari, A. Regulation of Paracellular Fluxes of Amino Acids by Claudin-8 in Normal Mouse Intestinal MCE301 Cells. Nutrients 2023, 15, 1346. https://doi.org/10.3390/nu15061346
Okamoto E, Matsuda S, Yoshino Y, Morikawa Y, Suenami K, Tabuchi Y, Matsunaga T, Ikari A. Regulation of Paracellular Fluxes of Amino Acids by Claudin-8 in Normal Mouse Intestinal MCE301 Cells. Nutrients. 2023; 15(6):1346. https://doi.org/10.3390/nu15061346
Chicago/Turabian StyleOkamoto, Ema, Shunsuke Matsuda, Yuta Yoshino, Yoshifumi Morikawa, Koichi Suenami, Yoshiaki Tabuchi, Toshiyuki Matsunaga, and Akira Ikari. 2023. "Regulation of Paracellular Fluxes of Amino Acids by Claudin-8 in Normal Mouse Intestinal MCE301 Cells" Nutrients 15, no. 6: 1346. https://doi.org/10.3390/nu15061346
APA StyleOkamoto, E., Matsuda, S., Yoshino, Y., Morikawa, Y., Suenami, K., Tabuchi, Y., Matsunaga, T., & Ikari, A. (2023). Regulation of Paracellular Fluxes of Amino Acids by Claudin-8 in Normal Mouse Intestinal MCE301 Cells. Nutrients, 15(6), 1346. https://doi.org/10.3390/nu15061346





