Vitamin D Deficiency in Childhood Cancer Survivors: Results from Southern Thailand
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Anthropometric Data Collection
2.2. Vitamin D Levels and Biochemistry Analyses
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Participants
3.2. Demographic Characteristics at the Follow-Up Visit
3.3. Vitamin D Status and Biochemistry Measurements
3.4. Risk Factors for Vitamin D Deficiency
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yeh, J.M.; Ward, Z.J.; Chaudhry, A.; Liu, Q.; Yasui, Y.; Armstrong, G.T.; Gibson, T.M.; Howell, R.; Hudson, M.M.; Krull, K.R.; et al. Life Expectancy of Adult Survivors of Childhood Cancer over 3 Decades. JAMA Oncol. 2020, 6, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.H.; Katsoulis, M.; Tan, Y.Y.; Mueller, S.H.; Green, K.; Lai, A.G. Late Effects of Cancer in Children, Teenagers and Young Adults: Population-Based Study on the Burden of 183 Conditions, in-Patient and Critical Care Admissions and Years of Life Lost. Lancet Reg. Health Eur. 2022, 12, 100248. [Google Scholar] [CrossRef] [PubMed]
- Poon, L.H.J.; Yu, C.P.; Peng, L.; Ewig, C.L.; Zhang, H.; Li, C.K.; Cheung, Y.T. Clinical Ascertainment of Health Outcomes in Asian Survivors of Childhood Cancer: A Systematic Review. J. Cancer Surviv. Res. Pract. 2019, 13, 374–396. [Google Scholar] [CrossRef] [PubMed]
- Al-Mahayri, Z.N.; AlAhmad, M.M.; Ali, B.R. Long-Term Effects of Pediatric Acute Lymphoblastic Leukemia Chemotherapy: Can Recent Findings Inform Old Strategies? Front. Oncol. 2021, 11, 710163. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Endocrine Society Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Munns, C.F.; Shaw, N.; Kiely, M.; Specker, B.L.; Thacher, T.D.; Ozono, K.; Michigami, T.; Tiosano, D.; Mughal, M.Z.; Mäkitie, O.; et al. Global Consensus Recommendations on Prevention and Management of Nutritional Rickets. J. Clin. Endocrinol. Metab. 2016, 101, 394–415. [Google Scholar] [CrossRef]
- Palacios, C.; Gonzalez, L. Is Vitamin D Deficiency a Major Global Public Health Problem? J. Steroid Biochem. Mol. Biol. 2014, 144, 138–145. [Google Scholar] [CrossRef]
- Reesukumal, K.; Manonukul, K.; Jirapongsananuruk, O.; Krobtrakulchai, W.; Hanyongyuth, S.; Chatsiricharoenkul, S.; Pratumvinit, B. Hypovitaminosis D in Healthy Children in Central Thailand: Prevalence and Risk Factors. BMC Public Health 2015, 15, 248. [Google Scholar] [CrossRef]
- Rojroongwasinkul, N.; Kijboonchoo, K.; Wimonpeerapattana, W.; Purttiponthanee, S.; Yamborisut, U.; Boonpraderm, A.; Kunapan, P.; Thasanasuwan, W.; Khouw, I. SEANUTS: The Nutritional Status and Dietary Intakes of 0.5–12-Year-Old Thai Children. Br. J. Nutr. 2013, 110, S36–S44. [Google Scholar] [CrossRef]
- Poh, B.K.; Rojroongwasinkul, N.; Nguyen, B.K.L.; Sandjaja; Ruzita, A.T.; Yamborisut, U.; Hong, T.N.; Ernawati, F.; Deurenberg, P.; Parikh, P. 25-Hydroxy-Vitamin D Demography and the Risk of Vitamin D Insufficiency in the South East Asian Nutrition Surveys (SEANUTS). Asia Pac. J. Clin. Nutr. 2016, 25, 538–548. [Google Scholar]
- Kasemsripitak, S.; Jaruratanasirikul, S.; Boonrusmee, S.; Saengkaew, T.; Sriplung, H. Prevalence and Risk Factors for Vitamin D Insufficiency in 6–12-Month-Old Infants: A Cross-Sectional Study in Southern Thailand. BMC Pediatr. 2022, 22, 729. [Google Scholar] [CrossRef]
- Skversky, A.L.; Kumar, J.; Abramowitz, M.K.; Kaskel, F.J.; Melamed, M.L. Association of Glucocorticoid Use and Low 25-Hydroxyvitamin D Levels: Results from the National Health and Nutrition Examination Survey (NHANES): 2001–2006. J. Clin. Endocrinol. Metab. 2011, 96, 3838–3845. [Google Scholar] [CrossRef]
- Vierucci, F.; Del Pistoia, M.; Fanos, M.; Gori, M.; Carlone, G.; Erba, P.; Massimetti, G.; Federico, G.; Saggese, G. Vitamin D Status and Predictors of Hypovitaminosis D in Italian Children and Adolescents: A Cross-Sectional Study. Eur. J. Pediatr. 2013, 172, 1607–1617. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Jin, S.; Bi, X.; Chen, D.; Zhang, D.; Liu, L.; Jing, H.; Na, L. Association of Serum 25-Hydroxyvitamin D with Vitamin D Intervention and Outdoor Activity among Children in North China: An Observational Study. BMC Pediatr. 2020, 20, 542. [Google Scholar] [CrossRef]
- Pulungan, A.; Soesanti, F.; Tridjaja, B.; Batubara, J. Vitamin D Insufficiency and Its Contributing Factors in Primary School-Aged Children in Indonesia, a Sun-Rich Country. Ann. Pediatr. Endocrinol. Metab. 2021, 26, 92–98. [Google Scholar] [CrossRef]
- Revuelta Iniesta, R.; Rush, R.; Paciarotti, I.; Rhatigan, E.B.; Brougham, F.H.M.; McKenzie, J.M.; Wilson, D.C. Systematic Review and Meta-Analysis: Prevalence and Possible Causes of Vitamin D Deficiency and Insufficiency in Pediatric Cancer Patients. Clin. Nutr. Edinb. Scotl. 2016, 35, 95–108. [Google Scholar] [CrossRef]
- Rosen, G.P.; Beebe, K.L.; Shaibi, G.Q. Vitamin D Levels Differ by Cancer Diagnosis and Decline over Time in Survivors of Childhood Cancer. Pediatr. Blood Cancer 2013, 60, 949–952. [Google Scholar] [CrossRef]
- Esbenshade, A.J.; Sopfe, J.; Zhao, Z.; Li, Z.; Campbell, K.; Simmons, J.H.; Friedman, D.L. Screening for Vitamin D Insufficiency in Pediatric Cancer Survivors. Pediatr. Blood Cancer 2014, 61, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, R.; Teh, J.B.; Herrera, C.; Echevarria, M.; Lindenfeld, L.; Wong, F.L.; Wilson, K.; Armenian, S.H. Prevalence and Risk Factors for Vitamin D Deficiency in Long-Term Childhood Cancer Survivors. Pediatr. Blood Cancer 2021, 68, e29048. [Google Scholar] [CrossRef]
- Choudhary, A.; Chou, J.; Heller, G.; Sklar, C. Prevalence of Vitamin D Insufficiency in Survivors of Childhood Cancer. Pediatr. Blood Cancer 2013, 60, 1237–1239. [Google Scholar] [CrossRef]
- Modan-Moses, D.; Pinhas-Hamiel, O.; Munitz-Shenkar, D.; Temam, V.; Kanety, H.; Toren, A. Vitamin D Status in Pediatric Patients with a History of Malignancy. Pediatr. Res. 2012, 72, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Janjai, S.; Kirdsiri, K.; Masiri, I.; Nunez, M. An Investigation of Solar Erythemal Ultraviolet Radiation in the Tropics: A Case Study at Four Stations in Thailand. Int. J. Climatol. 2010, 30, 1893–1903. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. BMI Calculator for Child and Teen. Available online: https://www.cdc.gov/healthyweight/bmi/calculator.html (accessed on 12 May 2022).
- Marshall, W.A.; Tanner, J.M. Variations in Pattern of Pubertal Changes in Girls. Arch. Dis. Child. 1969, 44, 291. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.J.; Brion, L.P.; Spitzer, A. The Use of Plasma Creatinine Concentration for Estimating Glomerular Filtration Rate in Infants, Children, and Adolescents. Pediatr. Clin. N. Am. 1987, 34, 571–590. [Google Scholar] [CrossRef]
- Schwartz, G.J.; Work, D.F. Measurement and Estimation of GFR in Children and Adolescents. Clin. J. Am. Soc. Nephrol. 2009, 4, 1832–1843. [Google Scholar] [CrossRef]
- Sinha, A.; Avery, P.; Turner, S.; Bailey, S.; Cheetham, T. Vitamin D Status in Paediatric Patients with Cancer. Pediatr. Blood Cancer 2011, 57, 594–598. [Google Scholar] [CrossRef]
- Gunes, A.M.; Can, E.; Saglam, H.; Ilçöl, Y.O.; Baytan, B. Assessment of Bone Mineral Density and Risk Factors in Children Completing Treatment for Acute Lymphoblastic Leukemia. J. Pediatr. Hematol. Oncol. 2010, 32, e102–e107. [Google Scholar] [CrossRef]
- Simmons, J.H.; Chow, E.J.; Koehler, E.; Esbenshade, A.; Smith, L.-A.; Sanders, J.; Friedman, D. Significant 25-Hydroxyvitamin D Deficiency in Child and Adolescent Survivors of Acute Lymphoblastic Leukemia: Treatment with Chemotherapy Compared with Allogeneic Stem Cell Transplant. Pediatr. Blood Cancer 2011, 56, 1114–1119. [Google Scholar] [CrossRef]
- Delvin, E.; Alos, N.; Rauch, F.; Marcil, V.; Morel, S.; Boisvert, M.; Lecours, M.-A.; Laverdière, C.; Sinnett, D.; Krajinovic, M.; et al. Vitamin D Nutritional Status and Bone Turnover Markers in Childhood Acute Lymphoblastic Leukemia Survivors: A PETALE Study. Clin. Nutr. 2019, 38, 912–919. [Google Scholar] [CrossRef]
- Schündeln, M.M.; Hauffa, P.K.; Munteanu, M.; Kiewert, C.; Unger, N.; Bauer, J.J.; Hauffa, B.P.; Grasemann, C. Prevalence of Osteopathologies in Children and Adolescents after Diagnosis of Acute Lymphoblastic Leukemia. Front. Pediatr. 2020, 8, 509. [Google Scholar] [CrossRef]
- Bilariki, K.; Anagnostou, E.; Masse, V.; Elie, C.; Grill, J.; Valteau-Couanet, D.; Kalifa, C.; Doz, F.; Sainte-Rose, C.; Zerah, M.; et al. Low Bone Mineral Density and High Incidences of Fractures and Vitamin D Deficiency in 52 Pediatric Cancer Survivors. Horm. Res. Paediatr. 2010, 74, 319–327. [Google Scholar] [CrossRef]
| Variable † | Total (%) |
|---|---|
| Cancer Diagnosis | |
| Leukemia/lymphoma | 101 (49.0) |
| Solid tumor | 83 (40.3) |
| Brain tumor | 22 (10.7) |
| Leukemia/lymphoma | (N = 101) |
| Acute lymphoblastic leukemia | 62 (61.4) |
| Acute myeloid leukemia | 12 (11.9) |
| Non-Hodgkin lymphoma | 17 (16.8) |
| Hodgkin lymphoma | 10 (9.9) |
| Solid tumor | (N = 83) |
| Ewing sarcoma | 7 (8.4) |
| Rhabdomyosarcoma | 6 (7.2) |
| Osteosarcoma | 10 (12.0) |
| Neuroblastoma | 6 (7.2) |
| Hepatoblastoma | 5 (6.0) |
| Wilm tumor | 8 (9.6) |
| Retinoblastoma | 13 (15.7) |
| Germ cell tumor | 14 (16.9) |
| Langerhans cell histiocytosis | 12 (14.5) |
| Others | 2 (2.4) |
| Brain tumor | (N = 22) |
| Medulloblastoma | 9 (40.9) |
| Astrocytoma | 5 (22.7) |
| Primitive neuro-ectodermal tumor | 4 (18.2) |
| Germ cell tumor | 4 (18.2) |
| Treatment | |
| Intrathecal chemotherapy | |
| Yes | 87 (42.2) |
| No | 119 (57.8) |
| Steroids | |
| Yes | 101 (49.0) |
| No | 105 (51.0) |
| Cumulative steroids (mg/m2) | 6400 (4600–8400) |
| Surgery | |
| Yes | 94 (45.6) |
| No | 112 (54.4) |
| Radiation | |
| Yes | 55 (26.7) |
| No | 151 (73.3) |
| Cumulative radiation dosage (Gray) | 46 (30–54) |
| Variable † | Total (N = 206) | Vitamin D Deficiency (N = 74) | No Vitamin D Deficiency (N = 132) | p-Value |
|---|---|---|---|---|
| Demographic and Clinical Characteristics | ||||
| Age at follow-up visit (years) | 10.8 ± 4.7 | 12.5 ± 4.0 | 9.9 ± 4.8 | <0.001 |
| Sex | 0.014 | |||
| Male | 122 (59.2) | 35 (47.3) | 87 (65.9) | |
| Female | 84 (40.8) | 39 (52.7) | 45 (34.1) | |
| Diagnosis | 0.519 | |||
| Leukemia/lymphoma | 101 (49.0) | 39 (52.7) | 62 (47.0) | |
| Solid tumor/brain tumor | 105 (51.0) | 35 (47.3) | 70 (53.0) | |
| Weight (kg) | 38.6 (21.2–52.1) | 45.0 (36.2–56.7) | 28.2 (18.6–48.4) | <0.001 |
| Height (cm) | 140.0 (119.0–157.0) | 150.5 (138.5–160.8) | 129.8 (111.0–152.0) | <0.001 |
| BMI (kg/m2) | 17.9 (15.6–22.3) | 19.9 (17.3–24.6) | 17.0 (15.1–20.5) | <0.001 |
| Obese | 0.025 | |||
| Yes | 65 (31.6) | 31 (41.9) | 34 (25.8) | |
| No | 141 (68.4) | 43 (58.1) | 98 (74.2) | |
| Pubertal status | <0.001 | |||
| Prepuberty | 105 (51.0) | 24 (32.4) | 81 (61.4) | |
| Puberty | 101 (49.0) | 50 (67.6) | 51 (38.6) | |
| Outdoor activities | <0.001 | |||
| Yes | 140 (68.0) | 32 (43.2) | 108 (81.8) | |
| No | 66 (32.0) | 42 (56.8) | 24 (18.2) | |
| Duration of outdoor activities (hours/week) | 3.0 (0–5.0) | 0 (0–2.0) | 4.0 (2.0–5.0) | <0.001 |
| Dietary dairy intake (mL/week) | 1250.0 (750.0–2400.0) | 1000.0 (500.0–1237.5) | 1500.0 (1000.0–3000.0) | <0.001 |
| Steroids | 0.949 | |||
| Yes | 101 (49.0) | 37 (50.0) | 64 (48.5) | |
| No | 105 (51.0) | 37 (50.0) | 68 (51.5) | |
| Cumulative steroids (mg/m2) | 6400 (4600–8400) | 6400 (4600–8400) | 6850 (4175–8400) | 0.466 |
| Surgery | 0.341 | |||
| Yes | 94 (45.6) | 30 (40.5) | 64 (48.5) | |
| No | 112 (54.4) | 44 (59.5) | 68 (51.5) | |
| Radiation | 0.567 | |||
| Yes | 55 (26.7) | 22 (29.7) | 33 (25.0) | |
| No | 151 (73.3) | 52 (70.3) | 99 (75.0) | |
| Intrathecal chemotherapy | 0.212 | |||
| Yes | 87 (42.2) | 36 (48.6) | 51 (38.6) | |
| No | 119 (57.8) | 38 (51.4) | 81 (61.4) | |
| Follow-up time (years) | 2.3 (1.0–3.9) | 2.3 (0.7–3.8) | 2.4 (1.1–3.9) | 0.744 |
| Laboratory Parameters | ||||
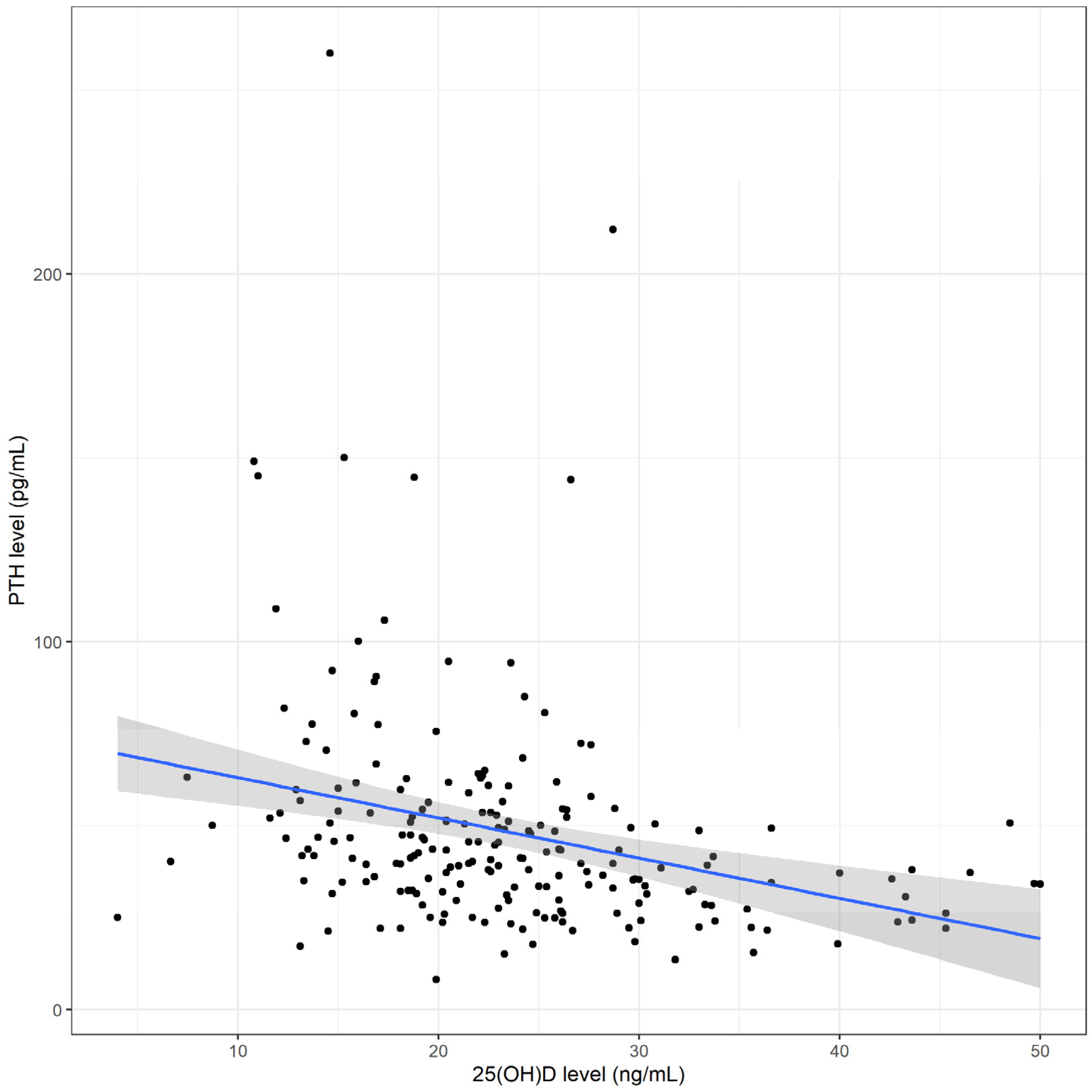
| PTH (pg/mL) | 41.2 (32.0–53.8) | 47.4 (39.5–65.9) | 38.0 (28.5–50.2) | <0.001 |
| Calcium (mg/dL) | 9.8 ± 0.4 | 9.7 ± 0.4 | 9.8 ± 0.4 | 0.009 |
| Phosphorus (mg/dL) | 4.5 ± 0.7 | 4.4 ± 0.8 | 4.5 ± 0.7 | 0.443 |
| Alkaline phosphatase (U/L) | 243.5 (171.5–304.0) | 229.5 (127.2–341.2) | 249.5 (193.8–301.2) | 0.402 |
| LDH (U/L) | 224.0 (192.0–263.5) | 211.0 (168.5–257.5) | 234.0 (195.8–265.0) | 0.045 |
| ALT (U/L) | 17.0 (13.0–24.0) | 17.0 (12.0–25.0) | 17.0 (13.0–23.0) | 0.868 |
| Albumin (g/dL) | 4.6 (4.4–4.7) | 4.5 (4.4–4.7) | 4.6 (4.4–4.8) | 0.156 |
| Hb (g/dL) | 13.0 (12.2–13.9) | 13.3 (12.1–14.1) | 12.9 (12.2–13.8) | 0.406 |
| Serum iron (μmol/L) | 13.0 (9.1–16.3) | 13.1 (9.7–17.2) | 12.6 (8.9–16.1) | 0.288 |
| TIBC (μmol/L) | 54.7 (49.0–60.1) | 54.0 (47.9–60.9) | 54.8 (49.2–59.8) | 0.82 |
| Transferrin saturation (%) | 24.1 (16.2–30.1) | 24.1 (16.4–33.2) | 23.9 (15.9–29.4) | 0.241 |
| Ferritin (ng/mL) | 79.5 (48.7–183.8) | 89.6 (44.0–338.8) | 77.0 (50.8–114.8) | 0.156 |
| Zinc (mg/L) | 0.8 (0.7–0.9) | 0.8 (0.7–0.9) | 0.8 (0.7–0.9) | 0.428 |
| eGFR (mL/min/1.73 m2) | 116.6 ± 28.0 | 117.8 ± 30.3 | 115.9 ± 26.7 | 0.632 |
| eGFR | 1 | |||
| Decreased | 36 (17.5) | 13 (17.6) | 23 (17.4) | |
| Normal | 170 (82.5) | 61 (82.4) | 109 (82.6) | |
| Risk Factor | Crude OR (95% CI) | Adjusted OR (95% CI) | p-Value |
|---|---|---|---|
| Female sex | 2.15 (1.20–3.85) | 2.11 (1.08–4.13) | 0.029 |
| Obesity | 2.08 (1.14–3.80) | 2.01 (1.00–4.04) | 0.05 |
| Lack of outdoor activities | 5.91 (3.12–11.2) | 4.14 (2.08–8.21) | <0.001 |
| Dietary dairy intake (mL/week) | 0.51 (0.38–0.68) | 0.59 (0.44–0.80) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kittivisuit, S.; Sripornsawan, P.; Songthawee, N.; Chavananon, S.; Yam-ubon, U.; McNeil, E.B.; Jaruratanasirikul, S.; Chotsampancharoen, T. Vitamin D Deficiency in Childhood Cancer Survivors: Results from Southern Thailand. Nutrients 2023, 15, 1328. https://doi.org/10.3390/nu15061328
Kittivisuit S, Sripornsawan P, Songthawee N, Chavananon S, Yam-ubon U, McNeil EB, Jaruratanasirikul S, Chotsampancharoen T. Vitamin D Deficiency in Childhood Cancer Survivors: Results from Southern Thailand. Nutrients. 2023; 15(6):1328. https://doi.org/10.3390/nu15061328
Chicago/Turabian StyleKittivisuit, Sirinthip, Pornpun Sripornsawan, Natsaruth Songthawee, Shevachut Chavananon, Umaporn Yam-ubon, Edward B. McNeil, Somchit Jaruratanasirikul, and Thirachit Chotsampancharoen. 2023. "Vitamin D Deficiency in Childhood Cancer Survivors: Results from Southern Thailand" Nutrients 15, no. 6: 1328. https://doi.org/10.3390/nu15061328
APA StyleKittivisuit, S., Sripornsawan, P., Songthawee, N., Chavananon, S., Yam-ubon, U., McNeil, E. B., Jaruratanasirikul, S., & Chotsampancharoen, T. (2023). Vitamin D Deficiency in Childhood Cancer Survivors: Results from Southern Thailand. Nutrients, 15(6), 1328. https://doi.org/10.3390/nu15061328





