The Hypolipidemic Characteristics of a Methanol Extract of Fermented Green Tea and Spore of Eurotium cristatum SXHBTBU1934 in Golden Hamsters
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Material, FBT, Spore of E. cristatum, and Extraction
2.2. HPLC-MS Analysis of Extracts
2.3. Compound Isolation
2.4. Structure Determination
2.5. Animal Experimental Design
2.6. Serum and Liver Collection/Biochemical Analyses
2.7. Cell Culture and Treatment
2.8. Cell Viability Assay
2.9. Bodipy Staining
2.10. Oil Red O Staining
2.11. Statistical Analysis
3. Results
3.1. Spores of E. cristatum Share Similiar Components as E. cristatum Fermented Green Tea
3.2. Methanol Extract of Fermented Green Tea, as Well as Spore Suspension Alleviated HFD-Induced Body Weight and Ratio of Liver Weight to Body Weight
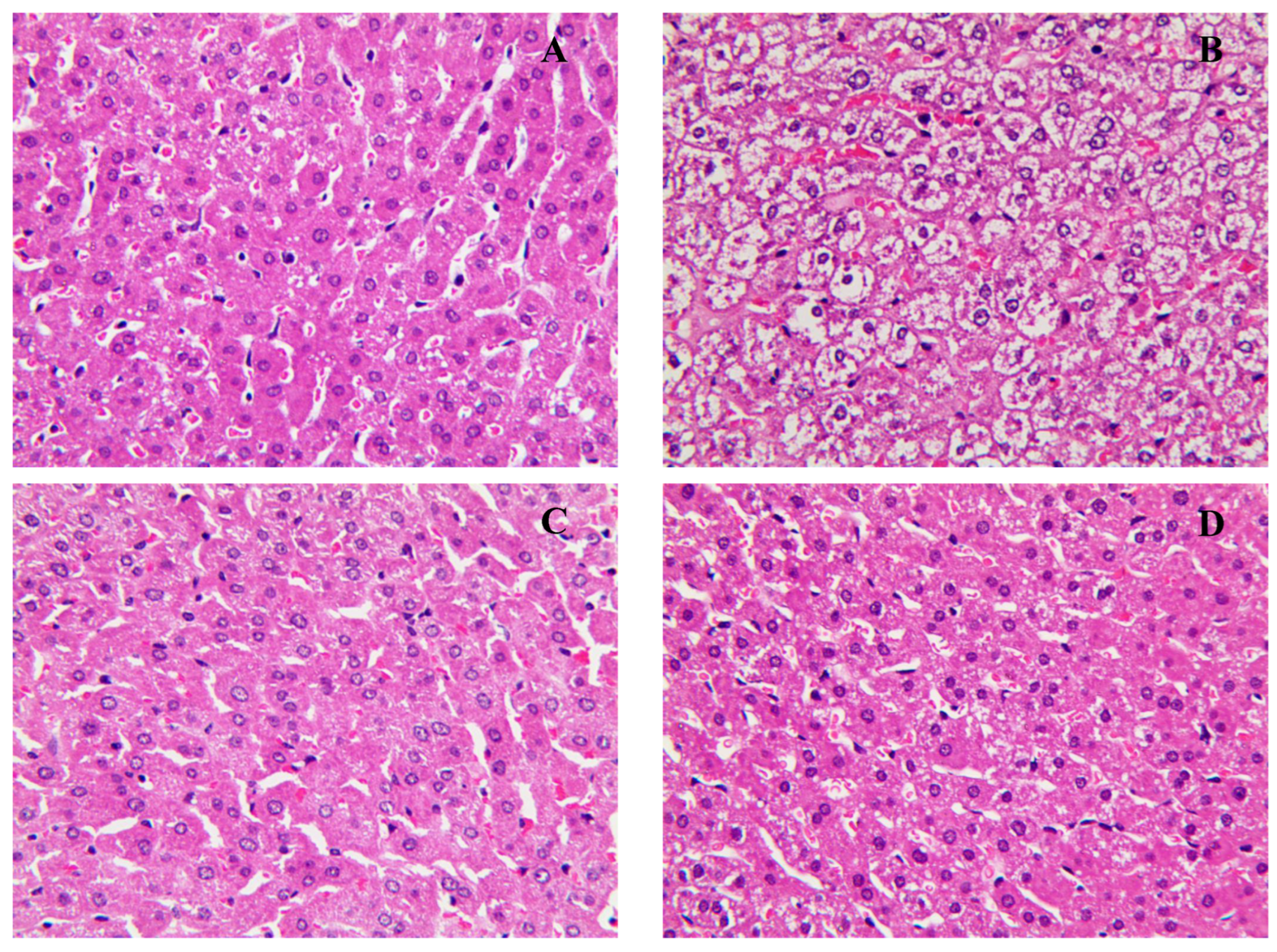
3.3. Effect of Methanol Extract of Fermented Green Tea and Spore Suspension of E. cristatum on Lipid Levels in Serum and Liver
3.4. Methanol Extract of Fermented Green Tea and Spore Suspension Improve Diabetes-Related Biomarkers in Serum
3.5. Isolation and Structure Elucidation of Compounds from Spore
3.6. Compound 1 Attenuated OA-Induced Lipid Accumulation in HepG2 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Makri, E.; Goulas, A.; Polyzos, S.A. Epidemiology, pathogenesis, diagnosis and emerging treatment of nonalcoholic fatty liver disease. Arch. Med. Res. 2021, 52, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Piche, M.-E.; Tchernof, A.; Despres, J.-P. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef]
- Klein, S.; Gastaldelli, A.; Yki-Jarvinen, H.; Scherer, P.E. Why does obesity cause diabetes? Cell Metab. 2022, 34, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Martel, J.; Ojcius, D.M.; Chang, C.-J.; Lin, C.-S.; Lu, C.-C.; Ko, Y.-F.; Tseng, S.-F.; Lai, H.-C.; Young, J.D. Anti-obesogenic and antidiabetic effects of plants and mushrooms. Nat. Rev. Endocrinol. 2017, 13, 149–160. [Google Scholar] [CrossRef]
- Lim, H.J.; Lim, T.J.; Lee, J.H.; Lee, J.H.; Kim, M.O.; Park, J.Y.; Kim, J.T.; Kim, M.J.; Jang, S.H.; Choi, S.H. Anti-obesity effects of dark tea extracts by down-regulation of C/EBPalpha and PPARgamma. In Vivo 2022, 36, 1753–1760. [Google Scholar] [CrossRef]
- Luo, Z.M.; Du, H.X.; Li, L.X.; An, M.Q.; Zhang, Z.Z.; Wan, X.C.; Bao, G.H.; Zhang, L.; Ling, T.J. Fuzhuanins A and B: The B-ring fission lactones of flavan-3-ols from Fuzhuan brick-tea. J. Agric. Food. Chem. 2013, 61, 6982–6990. [Google Scholar] [CrossRef]
- Luo, Z.M.; Ling, T.J.; Li, L.X.; Zhang, Z.Z.; Zhu, H.T.; Zhang, Y.J.; Wan, X.C. A new norisoprenoid and other compounds from Fuzhuan brick tea. Molecules 2012, 17, 3539–3546. [Google Scholar] [CrossRef]
- Ling, T.J.; Wan, X.C.; Ling, W.W.; Zhang, Z.Z.; Xia, T.; Li, D.X.; Hou, R.Y. New triterpenoids and other constituents from a special microbial-fermented tea-Fuzhuan brick tea. J. Agric. Food. Chem. 2010, 58, 4945–4950. [Google Scholar] [CrossRef]
- Liu, G.; Duan, Z.; Wang, P.; Fan, D.; Zhu, C. Purification, characterization, and hypoglycemic properties of eurocristatine from Eurotium cristatum spores in Fuzhuan brick tea. RSC Adv. 2020, 10, 22234–22241. [Google Scholar] [CrossRef]
- Fu, D.; Ryan, E.P.; Huang, J.; Liu, Z.; Weir, T.L.; Snook, R.L.; Ryan, T.P. Fermented Camellia sinensis, Fu Zhuan Tea, regulates hyperlipidemia and transcription factors involved in lipid catabolism. Food Res. Int. 2011, 44, 2999–3005. [Google Scholar] [CrossRef]
- Kang, D.; Su, M.; Duan, Y.; Huang, Y. Eurotium cristatum, a potential probiotic fungus from Fuzhuan brick tea, alleviated obesity in mice by modulating gut microbiota. Food Funct. 2019, 10, 5032–5045. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.J.; Xie, M.H.; Dai, Z.Q.; Wan, P.; Ye, H.; Zeng, X.X.; Sun, Y. Kudingcha and Fuzhuan brick tea prevent obesity and modulate gut microbiota in high-fat diet fed mice. Mol. Nutr. Food Res. 2018, 62, e1700485. [Google Scholar] [CrossRef]
- Liu, D.M.; Huang, J.A.; Luo, Y.; Wen, B.B.; Wu, W.L.; Zeng, H.L.; Liu, Z.H. Fuzhuan brick tea attenuates high-fat diet-induced obesity and associated metabolic disorders by shaping gut microbiota. J. Agric. Food Chem. 2019, 67, 13589–13604. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, Z.; Huang, J.; Luo, G.; Liang, Q.; Wang, D.; Ye, X.; Wu, C.; Wang, L.; Hu, J. Anti-obesity and hypolipidemic effects of Fuzhuan brick tea water extract in high-fat diet-induced obese rats. J. Sci. Food Agric. 2013, 93, 1310–1316. [Google Scholar] [CrossRef]
- Xiang, X.; Xiang, Y.; Jin, S.; Wang, Z.; Xu, Y.; Su, C.; Shi, Q.; Chen, C.; Yu, Q.; Song, C. The hypoglycemic effect of extract/fractions from Fuzhuan Brick-Tea in streptozotocin-induced diabetic mice and their active components characterized by LC-QTOF-MS/MS. J. Food Sci. 2020, 85, 2933–2942. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhou, Y.; Lin, L.; Yuan, D.; Peng, Y.; Li, L.; Xiao, W.; Gong, Z. Hypoglycemic effects of black brick tea with fungal growth in hyperglycemic mice model. Food Sci. Hum. Wellness 2022, 11, 711–718. [Google Scholar] [CrossRef]
- Liu, B.; Yang, T.; Zeng, L.N.; Shi, L.M.; Li, Y.; Xia, Z.G.; Xia, X.P.; Lin, Q.L.; Luo, F.J. Crude extract of Fuzhuan brick tea ameliorates DSS-induced colitis in mice. Int. J. Food Sci. Technol. 2016, 51, 2574–2582. [Google Scholar] [CrossRef]
- Keller, A.C.; Weir, T.L.; Broeckling, C.D.; Ryan, E.P. Antibacterial activity and phytochemical profile of fermented Camellia sinensis (fuzhuan tea). Food Res. Int. 2013, 53, 945–949. [Google Scholar] [CrossRef]
- Zhang, Q.-A.; Zhang, X.-L.; Yan, Y.-Y.; Fan, X.-H. Antioxidant evaluation and composition analysis of extracts from uzhuan brick tea and its comparison with two instant tea products. J. AOAC Int. 2017, 100, 653–660. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Q.; Zhao, Y.; Aimy, A.; Yang, X. Consumption of post-fermented Jing-Wei Fuzhuan brick tea alleviates liver dysfunction and intestinal microbiota dysbiosis in high fructose diet-fed mice. RSC Adv. 2019, 9, 17501–17513. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, M.; Wu, Y.; Zhong, K.; Gao, H. Structural characteristics and hypolipidemic activity of theabrownins from dark tea fermented by single species Eurotium cristatum PW-1. Biomolecules 2020, 10, 204. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.F.; Lin, P.; Kook, M.; Yi, T.H.; Li, C.T. Immune activation effects of Eurotium cristatum on T cells through NF-B signaling pathways in humans. Food Agric. Immunol. 2017, 28, 388–402. [Google Scholar] [CrossRef]
- Mangiapane, E.H.; McAteer, M.A.; Benson, G.M.; White, D.A.; Salter, A.M. Modulation of the regression of atherosclerosis in the hamster by dietary lipids: Comparison of coconut oil and olive oil. Br. J. Nutr. 1999, 82, 401–409. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, C.; Zhang, X.; Li, N.; Dong, Z.; Sun, G.; Sun, X. Atorvastatin promotes AMPK signaling to protect against high fat diet-induced non-alcoholic fatty liver in golden hamsters. Exp. Ther. Med. 2020, 19, 2133–2142. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xiao, C.; Peng, C.; Liang, L.; He, X.; Zhao, S.; Zhang, G. Probiotic strains improve high-fat diet-induced hypercholesterolemia through modulating gut microbiota in ways different from atorvastatin. Food Funct. 2019, 10, 6098–6109. [Google Scholar] [CrossRef]
- Li, N.; Sun, Y.R.; He, L.B.; Huang, L.; Li, T.T.; Wang, T.Y.; Tang, L. Amelioration by Idesia polycarpa Maxim. var. vestita Diels. of oleic acid-induced nonalcoholic fatty liver in HepG2 cells through antioxidant and modulation of lipid metabolism. Oxid. Med. Cell Longev. 2020, 2020, 1208726. [Google Scholar] [CrossRef]
- Xia, H.; Zhu, X.; Zhang, X.; Jiang, H.; Li, B.; Wang, Z.; Li, D.; Jin, Y. Alpha-naphthoflavone attenuates non-alcoholic fatty liver disease in oleic acid-treated HepG2 hepatocytes and in high fat diet-fed mice. Biomed. Pharmacother. 2019, 118, 109287. [Google Scholar] [CrossRef]
- Im, A.R.; Kim, Y.H.; Lee, H.W.; Song, K.H. Water extract of Dolichos lablab attenuates hepatic lipid accumulation in a cellular nonalcoholic fatty liver disease model. J. Med. Food 2016, 19, 495–503. [Google Scholar] [CrossRef]
- Li, J.; Xie, S.; Teng, W. Sulforaphane attenuates nonalcoholic fatty liver disease by inhibiting hepatic steatosis and apoptosis. Nutrients 2021, 14, 76. [Google Scholar] [CrossRef]
- Wei, X.; Feng, C.; Wang, S.Y.; Zhang, D.M.; Li, X.H.; Zhang, C.X. New indole diketopiperazine alkaloids from soft coral-associated epiphytic fungus Aspergillus sp. EGF 15-0-3. Chem. Biodivers. 2020, 17, e2000106. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Kamisuki, S.; Kimoto, M.; Ohnishi, K.; Takakusagi, Y.; Kuramochi, K.; Takeda, Y.; Nakazaki, A.; Kuroiwa, K.; Ohuchi, T.; et al. Total synthesis of (-)-neoechinulin A. Synlett 2006, 2006, 677–680. [Google Scholar] [CrossRef]
- Bovio, E.; Garzoli, L.; Poli, A.; Luganini, A.; Villa, P.; Musumeci, R.; McCormack, G.P.; Cocuzza, C.E.; Gribaudo, G.; Mehiri, M.; et al. Marine fungi from the sponge Grantia compressa: Biodiversity, chemodiversity, and biotechnological potential. Mar. Drugs 2019, 17, 220. [Google Scholar] [CrossRef]
- Wang, W.-L.; Lu, Z.-Y.; Tao, H.-W.; Zhu, T.-J.; Fang, Y.-C.; Gu, Q.-Q.; Zhu, W.-M. Isoechinulin-type alkaloids, variecolorins A-L, from halotolerant Aspergillus variecolor. J. Nat. Prod. 2007, 70, 1558–1564. [Google Scholar] [CrossRef]
- Miyake, Y.; Ito, C.; Itoigawa, M.; Osawa, T. Antioxidants produced by Eurotium herbariorum of filamentous fungi used for the manufacture of karebushi, dried bonito (Katsuobushi). Biosci. Biotechnol. Biochem. 2009, 73, 1323–1327. [Google Scholar] [CrossRef]
- Huang, P.L. A comprehensive definition for metabolic syndrome. Dis. Model. Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.S.Z.H.; Imani, S.; Hosseinifard, H.; Wen, Q.-L.; Shahzad, M.N.; Ijaz, I.; Deng, Y.; Guo, M.; Xu, Y. Associations of serum low-density lipoprotein and systolic blood pressure levels with type 2 diabetic patients with and without peripheral neuropathy: Systemic review, meta-analysis and meta-regression analysis of observational studies. BMC Endocr. Disord. 2019, 19, 125. [Google Scholar] [CrossRef]
- Chen, G.; Peng, Y.; Xie, M.; Xu, W.; Chen, C.; Zeng, X.; Liu, Z. A critical review of Fuzhuan brick tea: Processing, chemical constituents, health benefits and potential risk. Crit. Rev. Food Sci. Nutr. 2021. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Chen, J.J.; Ji, X.M.; Hu, X.; Ling, T.J.; Zhang, Z.Z.; Bao, G.H.; Wan, X.C. Changes of major tea polyphenols and production of four new B-ring fission metabolites of catechins from post-fermented Jing-Wei Fu brick tea. Food Chem. 2015, 170, 110–117. [Google Scholar] [CrossRef]
- Jia, W.; Shi, Q.; Shi, L.; Qin, J.; Chang, J.; Chu, X. A strategy of untargeted foodomics profiling for dynamic changes during Fu brick tea fermentation using ultrahigh-performance liquid chromatography-high resolution mass spectrometry. J. Chromatogr. A 2020, 1618, 460900. [Google Scholar] [CrossRef]
- Lu, Y.; He, Y.J.; Zhu, S.H.; Zhong, X.H.; Chen, D.; Liu, Z.H. New acylglycosides flavones from Fuzhuan brick tea and simulation analysis of their bioactive effects. Int. J. Mol. Sci. 2019, 20, 494. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ge, B.; Zhang, X.; Wang, K.; Zhou, C.; Fu, D. Metabolomics analysis reveals the effects of compound Fuzhuan brick tea (CFBT) on regulating dyslipidemia and metabolic disorders in mice induced by high-fat diet. Nutrients 2022, 14, 1128. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Dai, W.; Li, H.; Zhang, X.; Xu, X.; Ma, L.; Wang, L. Characterization of hypolipidemic phenol analogues from fermented tea by Eurotium cristatum. Foods 2022, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Ali, K.M.; Wonnerth, A.; Huber, K.; Wojta, J. Cardiovascular disease risk reduction by raising HDL cholesterol—Current therapies and future opportunities. Br. J. Pharmacol. 2012, 167, 1177–1194. [Google Scholar] [CrossRef]
- Wadhera, R.K.; Steen, D.L.; Khan, I.; Giugliano, R.P.; Foody, J.M. A review of low-density lipoprotein cholesterol, treatment strategies, and its impact on cardiovascular disease morbidity and mortality. J. Clin. Lipidol. 2016, 10, 472–489. [Google Scholar] [CrossRef]
- Powell, E.E.; Wong, V.W.-S.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef]
- Cobbina, E.; Akhlaghi, F. Non-alcoholic fatty liver disease (NAFLD)—Pathogenesis, classification, and effect on drug metabolizing enzymes and transporters. Drug Metab. Rev. 2017, 49, 197–211. [Google Scholar] [CrossRef]
- Stefan, N.; Haering, H.-U.; Cusi, K. Non-alcoholic fatty liver disease: Causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. 2019, 7, 313–324. [Google Scholar] [CrossRef]
- Li, J.; Wu, H.; Liu, Y.; Yang, L. High fat diet induced obesity model using four strains of mice: Kunming, C57BL/6, BALB/c and ICR. Exp. Anim. 2020, 69, 326–335. [Google Scholar] [CrossRef]
- Frayn, K.N. Non-esterified fatty acid metabolism and postprandial lipaemia. Atherosclerosis 1998, 141 (Suppl. S1), S41–S46. [Google Scholar] [CrossRef]
- Hara, T.; Kashihara, D.; Ichimura, A.; Kimura, I.; Tsujimoto, G.; Hirasawa, A. Role of free fatty acid receptors in the regulation of energy metabolism. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2014, 1841, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, L.; Gurley, E.; Studer, E.; Shang, J.; Wang, T.; Wang, C.; Yan, M.; Jiang, Z.; Hylemon, P.B.; et al. Prevention of free fatty acid-induced hepatic lipotoxicity by 18 beta-glycyrrhetinic acid through lysosomal and mitochondrial pathways. Hepatology 2008, 47, 1905–1915. [Google Scholar] [CrossRef] [PubMed]
- Feldstein, A.E.; Werneburg, N.W.; Canbay, A.; Guicciardi, M.E.; Bronk, S.F.; Rydzewski, R.; Burgart, L.J.; Gores, G.J. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology 2004, 40, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Fiorenza, C.G.; Chou, S.H.; Mantzoros, C.S. Lipodystrophy: Pathophysiology and advances in treatment. Nat. Rev. Endocrinol. 2011, 7, 137–150. [Google Scholar] [CrossRef]
- Seoane-Collazo, P.; Martinez-Sanchez, N.; Milbank, E.; Contreras, C. Incendiary leptin. Nutrients 2020, 12, 472. [Google Scholar] [CrossRef]
- Lee, S.; Kweon, O.-K.; Kim, W.H. Associations between serum leptin levels, hyperlipidemia, and cholelithiasis in dogs. PLoS ONE 2017, 12, e0187315. [Google Scholar] [CrossRef]







| Position | 1 | ||
|---|---|---|---|
| δC | δH (J in Hz) | HMBC | |
| 1 | 9.64 s | 2, 3, 3a, 7a, | |
| 2 | 144.6 | ||
| 3 | 103.2 | ||
| 3a | 126.3 | ||
| 4 | 117.8 | 7.19 d (8.0) | 3, 6, 7a |
| 5 | 121.1 | 7.08 dd (8.0, 8.0) | 3a, 7 |
| 6 | 122.7 | 6.99 d (8.0) | 4, 7a, 21 |
| 7 | 122.2 | ||
| 7a | 134.2 | ||
| 8 | 112.2 | 7.22, s | 2, 3a, 10 |
| 9 | 124.0 | ||
| 10 | 160.1 | ||
| 12 | 51.8 | 4.29, qd (7.0, 1.5) | 10, 13, 21 |
| 13 | 165.8 | ||
| 14 | 7.47, s | 8, 9, 10, 12, 13 | |
| 15 | 39.3 | ||
| 16 | 144.3 | 6.07 dd (17.5, 10.5) | 2, 15, 18,19 |
| 17a | 112.9 | 5.15 d (17.5) | |
| 17b | 5.17 d (10.5) | ||
| 18 | 27.4 | 1.55 s | 2, 15, 16 |
| 19 | 27.5 | 1.55 s | 2, 15, 16 |
| 20 | 21.0 | 1.60 d (7.0) | 12, 13 |
| 21a | 33.7 | 2.92 d (15.0, 9.5) | 6, 7, 7a, 22, 23 |
| 21b | 3.24 dd (15.0) | 6, 7, 7a, 22, 23 | |
| 22 | 64.4 | 3.01 d (9.5) | 21 |
| 23 | 60.4 | ||
| 24 | 19.2 | 1.51 s | |
| 25 | 25.0 | 1.40 s | |
| Compound | Concentration (μg/mL) | Inhibitory Rate (%) | Cell Viability (%) |
|---|---|---|---|
| 1 | 100 | 37.30 | 110.29 |
| 2 | 100 | 6.19 | 93.97 |
| 3 | 100 | −67.35 | 11.15 |
| 4 | 100 | −15.27 | 78.06 |
| 5 | 25 | 5.46 | 94.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, F.; Zhang, K.; Yang, J.; Wilson, A.S.; Chen, C.; Xu, X. The Hypolipidemic Characteristics of a Methanol Extract of Fermented Green Tea and Spore of Eurotium cristatum SXHBTBU1934 in Golden Hamsters. Nutrients 2023, 15, 1329. https://doi.org/10.3390/nu15061329
Song F, Zhang K, Yang J, Wilson AS, Chen C, Xu X. The Hypolipidemic Characteristics of a Methanol Extract of Fermented Green Tea and Spore of Eurotium cristatum SXHBTBU1934 in Golden Hamsters. Nutrients. 2023; 15(6):1329. https://doi.org/10.3390/nu15061329
Chicago/Turabian StyleSong, Fuhang, Kai Zhang, Jinpeng Yang, Annette S. Wilson, Caixia Chen, and Xiuli Xu. 2023. "The Hypolipidemic Characteristics of a Methanol Extract of Fermented Green Tea and Spore of Eurotium cristatum SXHBTBU1934 in Golden Hamsters" Nutrients 15, no. 6: 1329. https://doi.org/10.3390/nu15061329
APA StyleSong, F., Zhang, K., Yang, J., Wilson, A. S., Chen, C., & Xu, X. (2023). The Hypolipidemic Characteristics of a Methanol Extract of Fermented Green Tea and Spore of Eurotium cristatum SXHBTBU1934 in Golden Hamsters. Nutrients, 15(6), 1329. https://doi.org/10.3390/nu15061329






