Chrysanthemum morifolium and Its Bioactive Substance Enhanced the Sleep Quality in Rodent Models via Cl− Channel Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
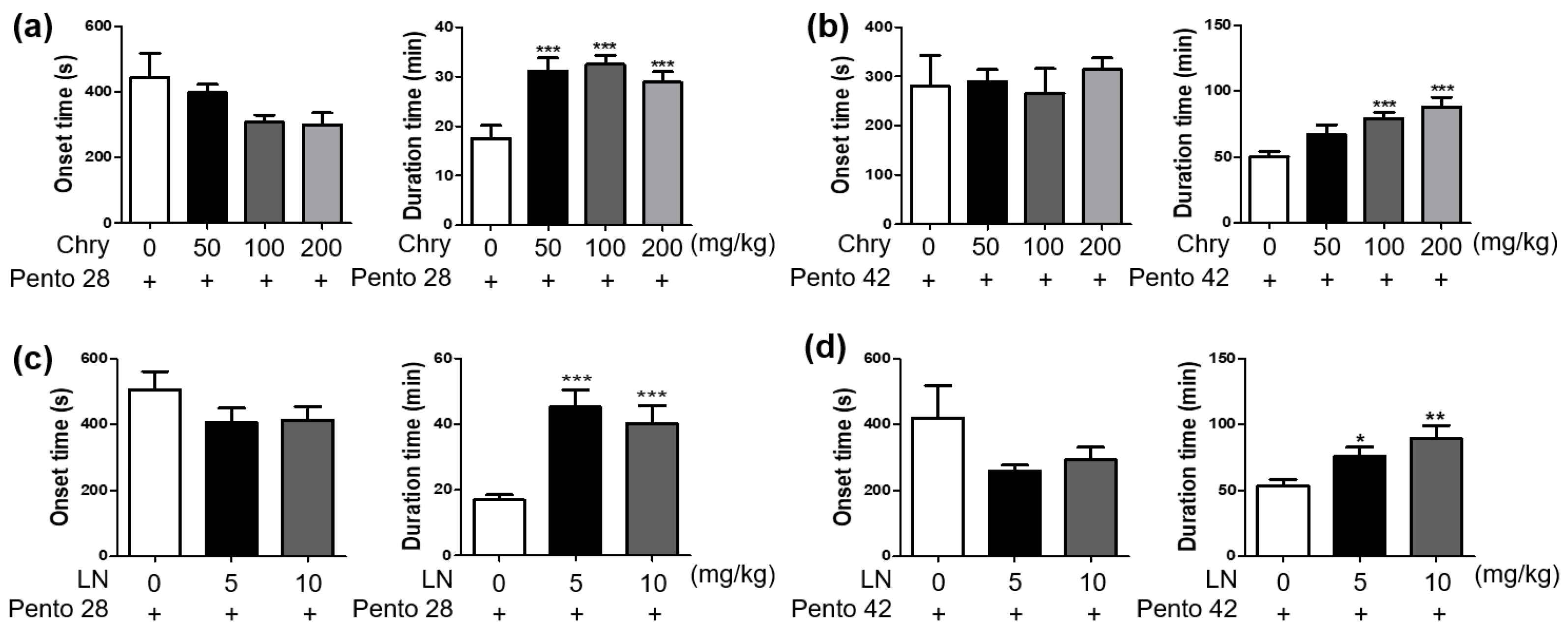
2.3. Pentobarbital-Induced Sleeping Test
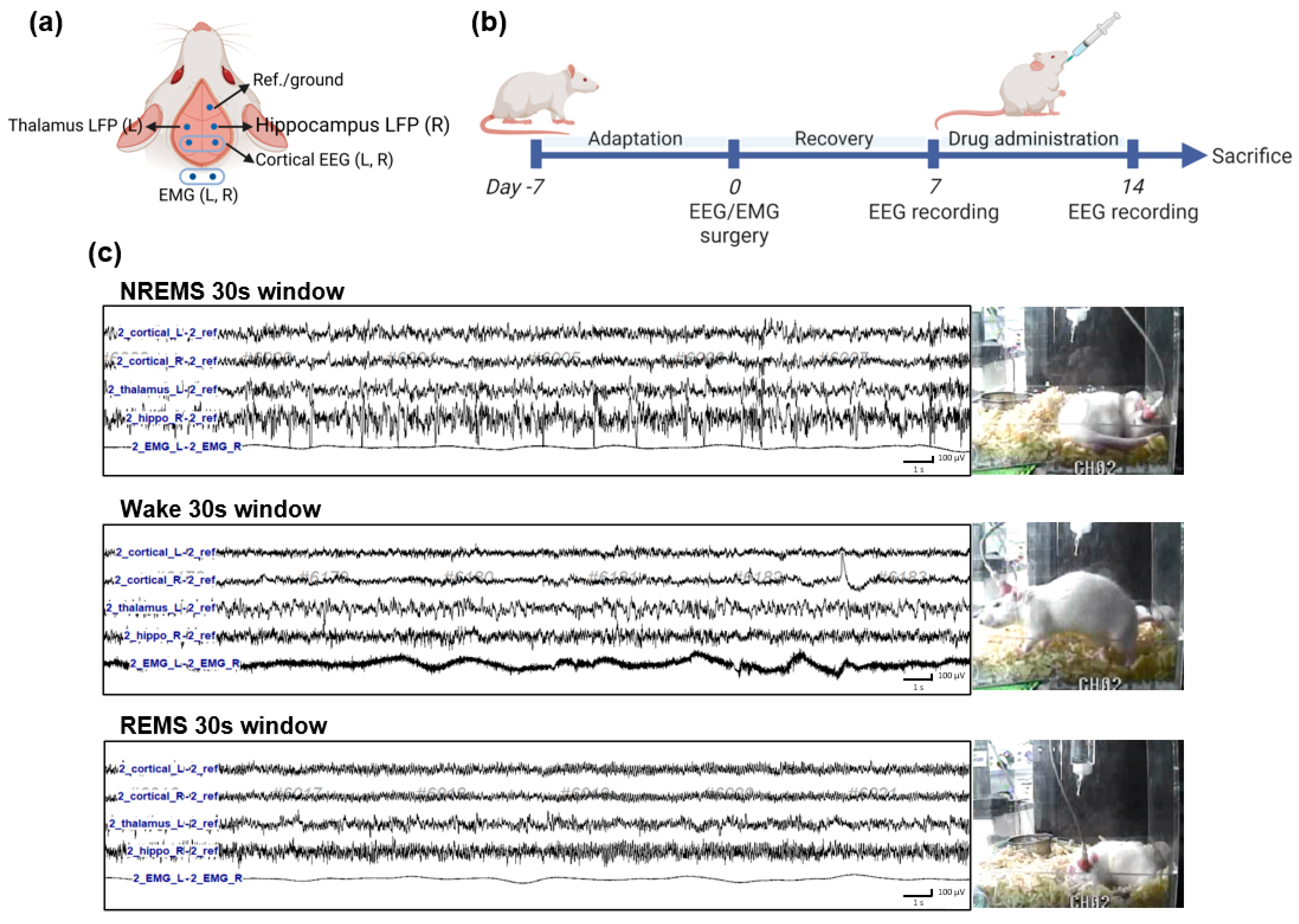
2.4. Electroencephalography (EEG) and Electromyogram (EMG) Implantation Surgery
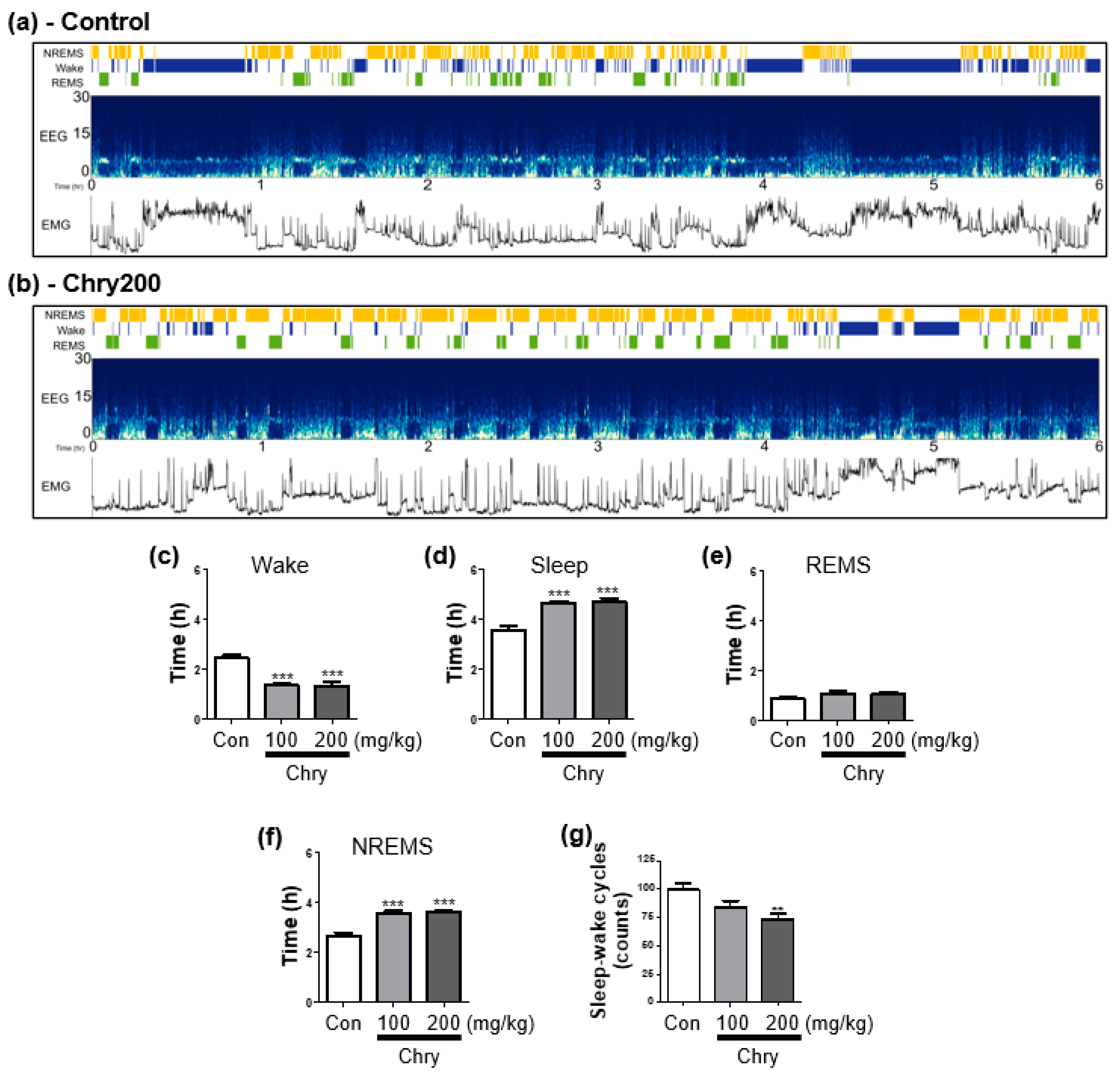
2.5. EEG Recording and Sleep–Wake State Analysis
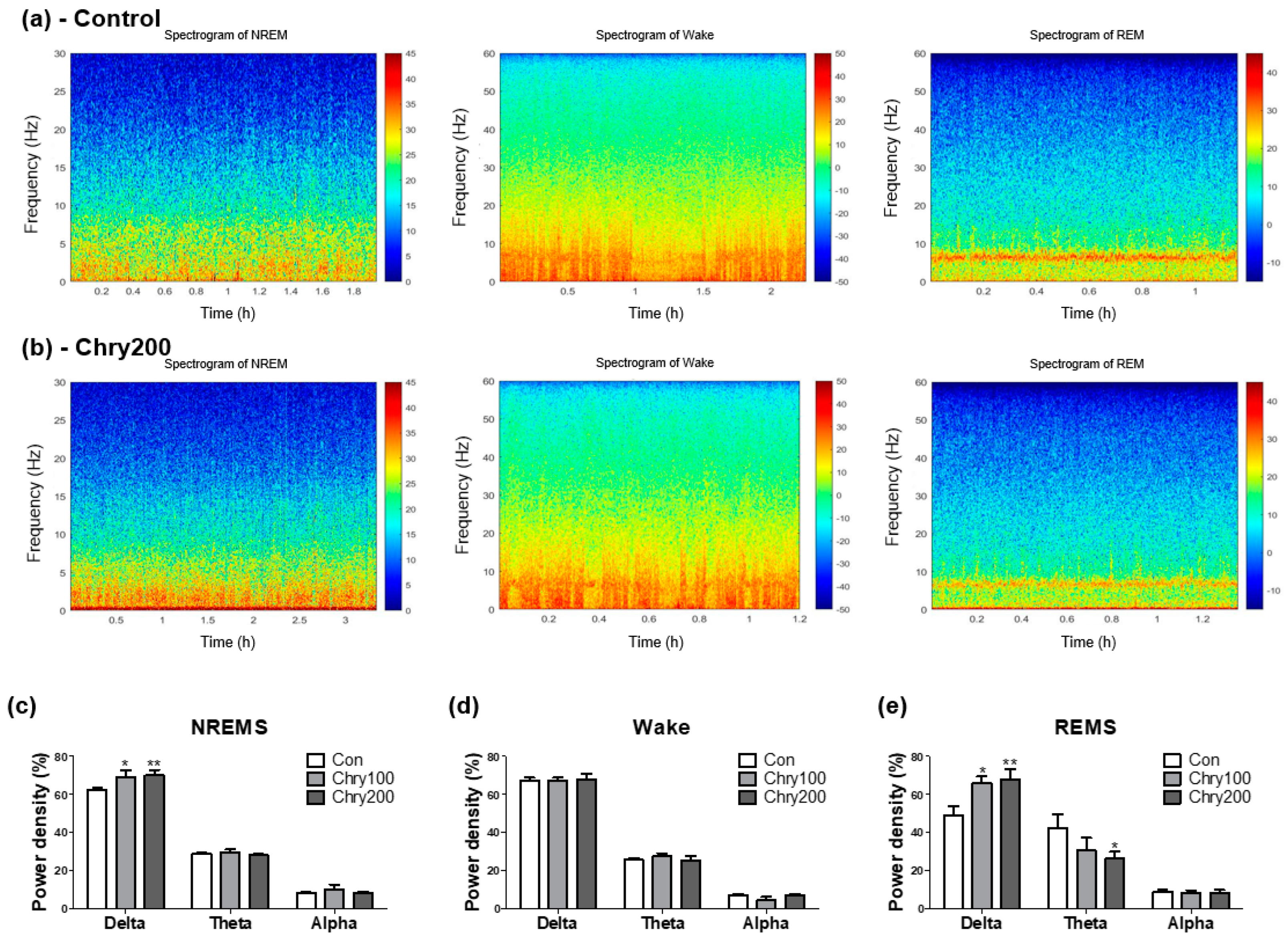
2.6. Time-Dependent Power Spectrum Heatmaps
2.7. Western Blotting
2.8. Intracellular Chloride Ion Measurement Assay
2.9. Statistical Analysis
3. Results
3.1. Extract of Chrysanthemum morifolium and Its Bioactive Substance Enhances Sleep Duration in Pentobarbital-Induced Sleep in Mice
3.2. Extract of Chrysanthemum morifolium Improves Sleep Quality in Rats
3.3. Extract of Chrysanthemum morifolium Improves Sleep Quality in Power Spectrogram Analysis
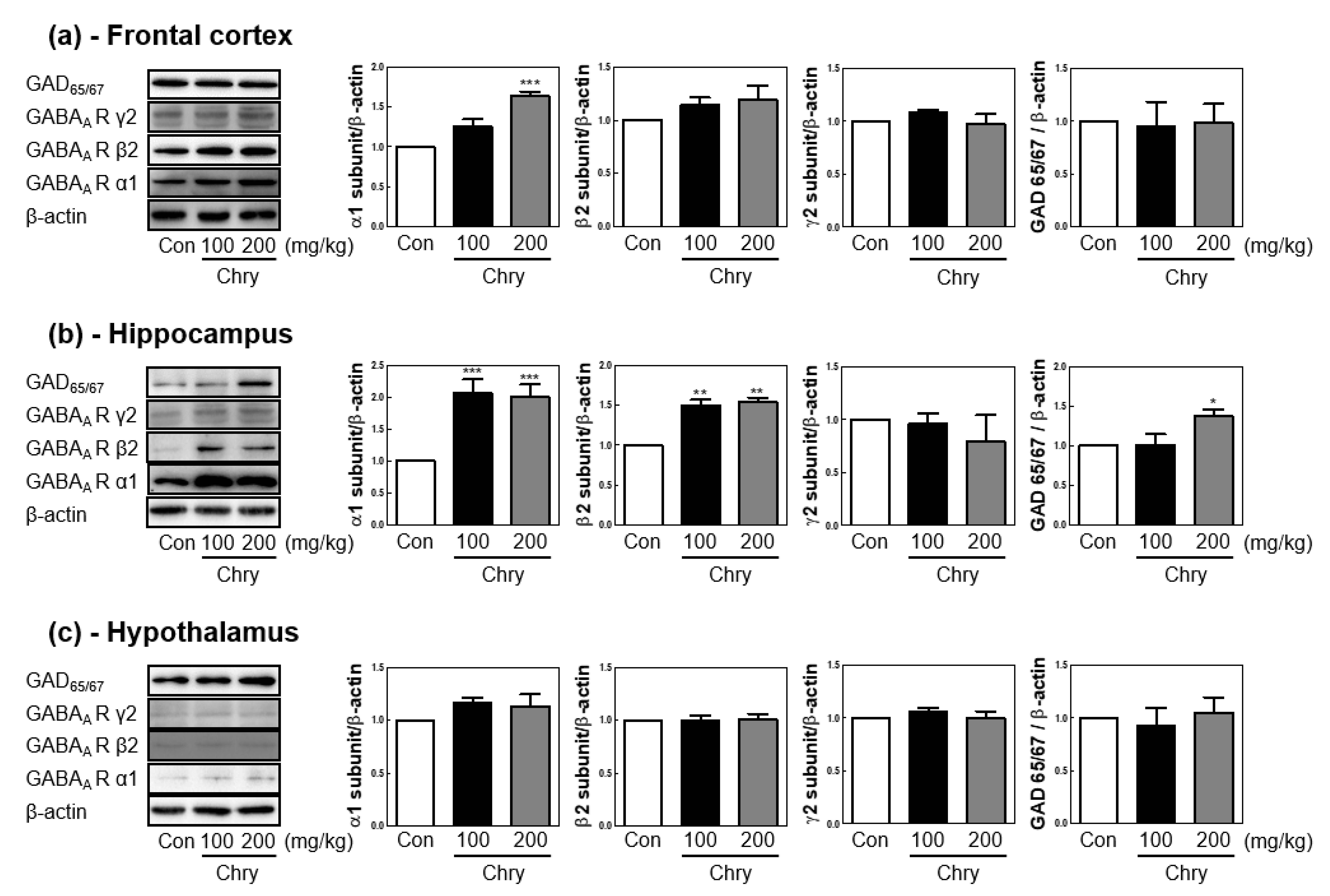
3.4. Extract of Chrysanthemum morifolium Modulates Expression of Subtypes of GABAA Receptors in the Rat Brain
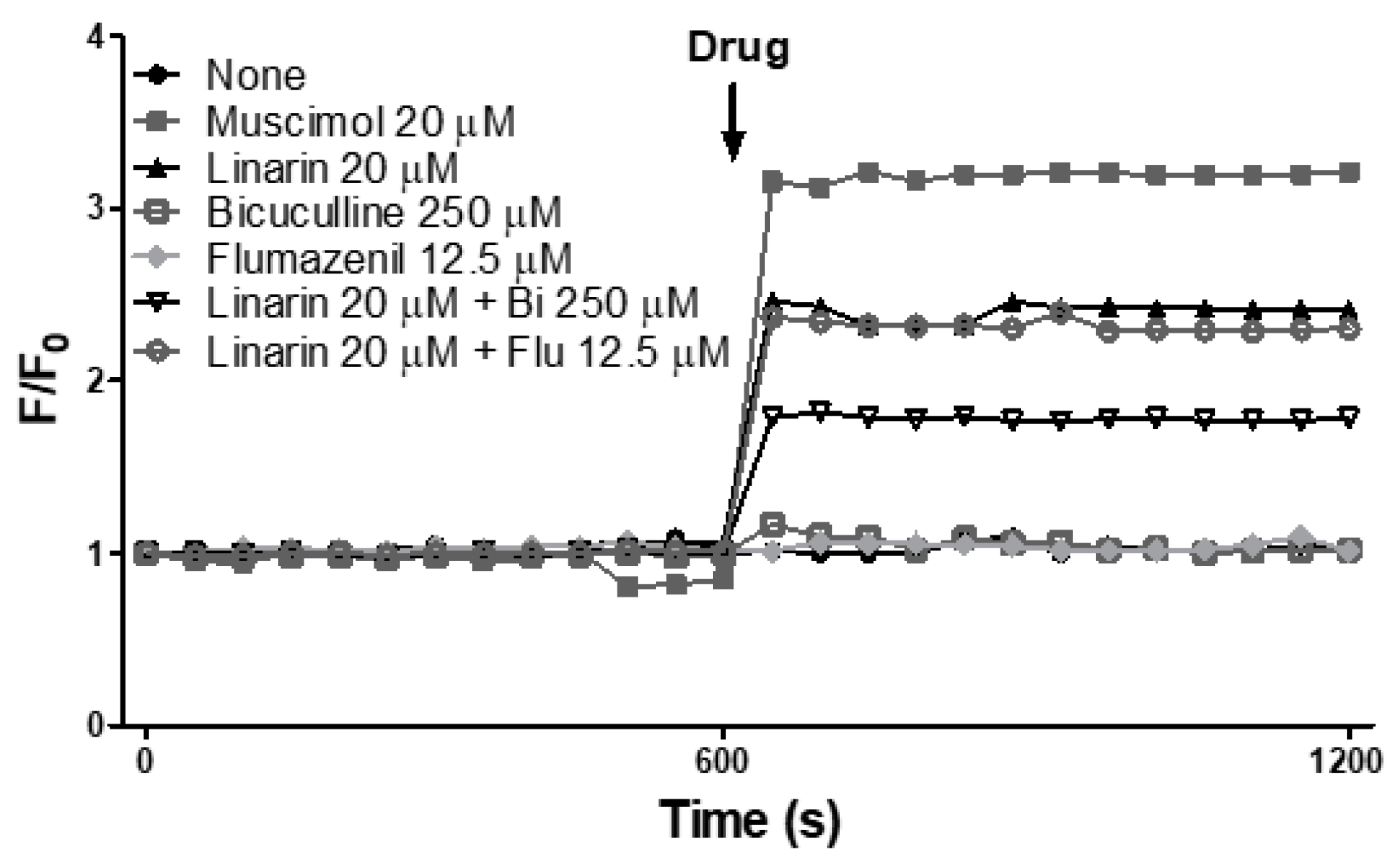
3.5. Linarin Activates the Chloride Ion Channels of GABAA Receptors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Chattu, V.K.; Manzar, M.D.; Kumary, S.; Burman, D.; Spence, D.W.; Pandi-Perumal, S.R. The Global Problem of Insufficient Sleep and Its Serious Public Health Implications. Healthcare 2019, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Patke, A.; Young, M.W.; Axelrod, S. Molecular mechanisms and physiological importance of circadian rhythms. Nat. Rev. Mol. Cell Biol. 2020, 21, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Tsujino, N.; Kuramoto, E.; Koyama, Y.; Susaki, E.A.; Chikahisa, S.; Funato, H. Sleep as a biological problem: An overview of frontiers in sleep research. J. Physiol. Sci. 2016, 66, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Besedovsky, L.; Lange, T.; Haack, M. The Sleep-Immune Crosstalk in Health and Disease. Physiol. Rev. 2019, 99, 1325–1380. [Google Scholar] [CrossRef]
- Riemann, D.; Krone, L.B.; Wulff, K.; Nissen, C. Sleep, insomnia, and depression. Neuropsychopharmacology 2020, 45, 74–89. [Google Scholar] [CrossRef]
- Vanda, D.; Zajdel, P.; Soural, M. Imidazopyridine-based selective and multifunctional ligands of biological targets associated with psychiatric and neurodegenerative diseases. Eur. J. Med. Chem. 2019, 181, 111569. [Google Scholar] [CrossRef]
- Monti, J.M.; Spence, D.W.; Buttoo, K.; Pandi-Perumal, S.R. Zolpidem’s use for insomnia. Asian J. Psychiatr. 2017, 25, 79–90. [Google Scholar] [CrossRef]
- Skibiski, J.; Abdijadid, S. Barbiturates. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Sigel, E.; Ernst, M. The Benzodiazepine Binding Sites of GABA(A) Receptors. Trends Pharmacol. Sci. 2018, 39, 659–671. [Google Scholar] [CrossRef]
- Engin, E.; Benham, R.S.; Rudolph, U. An Emerging Circuit Pharmacology of GABA(A) Receptors. Trends Pharmacol. Sci. 2018, 39, 710–732. [Google Scholar] [CrossRef]
- Jembrek, M.J.; Vlainic, J. GABA Receptors: Pharmacological Potential and Pitfalls. Curr. Pharm. Des. 2015, 21, 4943–4959. [Google Scholar] [CrossRef]
- Edinoff, A.N.; Wu, N.; Ghaffar, Y.T.; Prejean, R.; Gremillion, R.; Cogburn, M.; Chami, A.A.; Kaye, A.M.; Kaye, A.D. Zolpidem: Efficacy and Side Effects for Insomnia. Health Psychol. Res. 2021, 9, 24927. [Google Scholar] [CrossRef] [PubMed]
- Pandey, J.; Bastola, T.; Dhakal, B.; Poudel, A.; Devkota, H.P. Chrysanthemum morifolium Ramat.: A Medicinal Plant with Diverse Traditional Uses, Bioactive Constituents, and Pharmacological Activities. In Medicinal Plants of the Asteraceae Family: Traditional Uses, Phytochemistry and Pharmacological Activities; Devkota, H.P., Aftab, T., Eds.; Springer Nature: Singapore, 2022; pp. 125–143. [Google Scholar]
- Kim, J.-W.; Han, J.-Y.; Hong, J.T.; Li, R.; Eun, J.S.; Oh, K.-W. Ethanol Extract of the Flower Chrysanthemum morifolium Augments Pentobarbital-Induced Sleep Behaviors: Involvement of Cl− Channel Activation. Evid.-Based Complement. Altern. Med. 2011, 2011, 109164. [Google Scholar] [CrossRef] [PubMed]
- Nugroho, A.; Lim, S.-C.; Choi, J.; Park, H.-J. Identification and quantification of the sedative and anticonvulsant flavone glycoside from Chrysanthemum boreale. Arch. Pharmacal Res. 2013, 36, 51–60. [Google Scholar] [CrossRef]
- Zhan, G.; Long, M.; Shan, K.; Xie, C.; Yang, R. Antioxidant Effect of Chrysanthemum morifolium (Chuju) Extract on H2O2-Treated L-O2 Cells as Revealed by LC/MS-Based Metabolic Profiling. Antioxidants 2022, 11, 1068. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, P.; Luo, Y.; Gao, B.; Sun, J.; Lu, W.; Liu, J.; Chen, P.; Zhang, Y.; Yu, L.L. Chemical compositions of chrysanthemum teas and their anti-inflammatory and antioxidant properties. Food Chem. 2019, 286, 8–16. [Google Scholar] [CrossRef]
- Wu, J.Y.; Chen, Y.J.; Fu, X.Q.; Li, J.K.; Chou, J.Y.; Yin, C.L.; Bai, J.X.; Wu, Y.; Wang, X.Q.; Li, A.S.; et al. Chrysoeriol suppresses hyperproliferation of rheumatoid arthritis fibroblast-like synoviocytes and inhibits JAK2/STAT3 signaling. BMC Complement. Med. Ther. 2022, 22, 73. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.J.; Su, S.L.; Yan, H.; Guo, S.; Qian, D.W.; Duan, J.A. Evaluation of Anti-Inflammatory and Antioxidant Effectsof Chrysanthemum Stem and Leaf Extract on Zebrafish Inflammatory Bowel Disease Model. Molecules 2022, 27, 2114. [Google Scholar] [CrossRef]
- Mottaghipisheh, J.; Taghrir, H.; Boveiri Dehsheikh, A.; Zomorodian, K.; Irajie, C.; Mahmoodi Sourestani, M.; Iraji, A. Linarin, a Glycosylated Flavonoid, with Potential Therapeutic Attributes: A Comprehensive Review. Pharmaceuticals 2021, 14, 1104. [Google Scholar] [CrossRef]
- Qi, W.; Chen, Y.; Sun, S.; Xu, X.; Zhan, J.; Yan, Z.; Shang, P.; Pan, X.; Liu, H. Inhibiting TLR4 signaling by linarin for preventing inflammatory response in osteoarthritis. Aging 2021, 13, 5369–5382. [Google Scholar] [CrossRef]
- Yayeh, T.; Leem, Y.H.; Kim, K.M.; Jung, J.C.; Schwarz, J.; Oh, K.W.; Oh, S. Administration of Alpha(s1)-Casein Hydrolysate Increases Sleep and Modulates GABA(A) Receptor Subunit Expression. Biomol. Ther. 2018, 26, 268–273. [Google Scholar] [CrossRef]
- Yoon, M.; Jung, J.; Kim, M.; Lee, C.; Cho, S.; Um, M. Effect of Black Pepper (Piper nigrum) Extract on Caffeine-Induced Sleep Disruption and Excitation in Mice. Nutrients 2022, 14, 2249. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, J.; Kim, S.; Yoon, M.; Um, M.; Kim, D.; Kwon, S.; Cho, S. Arousal-Inducing Effect of Garcinia cambogia Peel Extract in Pentobarbital-Induced Sleep Test and Electroencephalographic Analysis. Nutrients 2021, 13, 2845. [Google Scholar] [CrossRef] [PubMed]
- Rensing, N.; Moy, B.; Friedman, J.L.; Galindo, R.; Wong, M. Longitudinal analysis of developmental changes in electroencephalography patterns and sleep-wake states of the neonatal mouse. PLoS ONE 2018, 13, e0207031. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Kao, H.-Y.; Yang, T.; Wang, Y. Early and Bi-hemispheric seizure onset in a rat glioblastoma Multiforme model. Neurosci. Lett. 2022, 766, 136351. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.A.; Gallego, R.; Ramos, M.; Lopez, J.M.; de Arcas, G.; Gonzalez-Nieto, D. Sleep-Wake Cycle and EEG-Based Biomarkers during Late Neonate to Adult Transition. Brain Sci. 2021, 11, 298. [Google Scholar] [CrossRef]
- Topchiy, I.; Fink, A.M.; Maki, K.A.; Calik, M.W. Validation of PiezoSleep Scoring Against EEG/EMG Sleep Scoring in Rats. Nat. Sci. Sleep 2022, 14, 1877–1886. [Google Scholar] [CrossRef]
- Barger, Z.; Frye, C.G.; Liu, D.; Dan, Y.; Bouchard, K.E. Robust, automated sleep scoring by a compact neural network with distributional shift correction. PLoS ONE 2019, 14, e0224642. [Google Scholar] [CrossRef]
- Park, S.H.; Hyun, J.Y.; Shin, I. A lysosomal chloride ion-selective fluorescent probe for biological applications. Chem. Sci. 2019, 10, 56–66. [Google Scholar] [CrossRef]
- Stephen, G.B.; Mody, I. Extrasynaptic GABAA Receptors: Their Function in the CNS and Implications for Disease. Neuron 2012, 73, 23–34. [Google Scholar] [CrossRef]
- Palagini, L.; Bianchini, C. Pharmacotherapeutic management of insomnia and effects on sleep processes, neural plasticity, and brain systems modulating stress: A narrative review. Front. Neurosci. 2022, 16, 893015. [Google Scholar] [CrossRef]
- Woo, J.; Yang, H.; Yoon, M.; Gadhe, C.G.; Pae, A.N.; Cho, S.; Lee, C.J. 3-Carene, a Phytoncide from Pine Tree Has a Sleep-enhancing Effect by Targeting the GABA(A)-benzodiazepine Receptors. Exp. Neurobiol. 2019, 28, 593–601. [Google Scholar] [CrossRef]
- Lin, A.; Shih, C.-T.; Huang, C.-L.; Wu, C.-C.; Lin, C.-T.; Tsai, Y.-C. Hypnotic Effects of Lactobacillus fermentum PS150TM on Pentobarbital-Induced Sleep in Mice. Nutrients 2019, 11, 2409. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Rogawski, M.A. How theories evolved concerning the mechanism of action of barbiturates. Epilepsia 2012, 53, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jo, K.; Hong, K.B.; Han, S.H.; Suh, H.J. GABA and l-theanine mixture decreases sleep latency and improves NREM sleep. Pharm. Biol. 2019, 57, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.R.; Lee, S.Y.; Lee, S.M.; Shim, I.; Lee, M.Y. Effect of Hibiscus syriacus Linnaeus extract and its active constituent, saponarin, in animal models of stress-induced sleep disturbances and pentobarbital-induced sleep. Biomed. Pharmacother. 2022, 146, 112301. [Google Scholar] [CrossRef] [PubMed]
- Le Bon, O. Relationships between REM and NREM in the NREM-REM sleep cycle: A review on competing concepts. Sleep Med. 2020, 70, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Hossain, M.A.; Jany, R.; Bari, M.A.; Uddin, M.; Kamal, A.R.M.; Ku, Y.; Kim, J.-S. Quantitative Evaluation of EEG-Biomarkers for Prediction of Sleep Stages. Sensors 2022, 22, 3079. [Google Scholar] [CrossRef]
- Lucey, B.P.; Mcleland, J.S.; Toedebusch, C.D.; Boyd, J.; Morris, J.C.; Landsness, E.C.; Yamada, K.; Holtzman, D.M. Comparison of a single-channel EEG sleep study to polysomnography. J. Sleep Res. 2016, 25, 625–635. [Google Scholar] [CrossRef]
- Park, I.; Díaz, J.; Matsumoto, S.; Iwayama, K.; Nabekura, Y.; Ogata, H.; Kayaba, M.; Aoyagi, A.; Yajima, K.; Satoh, M.; et al. Exercise improves the quality of slow-wave sleep by increasing slow-wave stability. Sci. Rep. 2021, 11, 4410. [Google Scholar] [CrossRef]
- Xiong, X.; Ren, Y.; Gao, S.; Luo, J.; Liao, J.; Wang, C.; Yi, S.; Liu, R.; Xiang, Y.; He, J. EEG microstate in obstructive sleep apnea patients. Sci. Rep. 2021, 11, 17178. [Google Scholar] [CrossRef]
- Um, M.Y.; Yoon, M.; Lee, J.; Jung, J.; Cho, S. A Novel Potent Sleep-Promoting Effect of Turmeric: Turmeric Increases Non-Rapid Eye Movement Sleep in Mice Via Histamine H1Receptor Blockade. Mol. Nutr. Food Res. 2021, 65, 2100100. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Lee, C.J. Sleep-enhancing Effects of Phytoncide Via Behavioral, Electrophysiological, and Molecular Modeling Approaches. Exp. Neurobiol. 2020, 29, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Petersen, C.; Walsh, C.M.; Bittencourt, J.C.; Neylan, T.C.; Grinberg, L.T. The role of co-neurotransmitters in sleep and wake regulation. Mol. Psychiatry 2019, 24, 1284–1295. [Google Scholar] [CrossRef] [PubMed]
- Holst, S.C.; Valomon, A.; Landolt, H.P. Sleep Pharmacogenetics: Personalized Sleep-Wake Therapy. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 577–603. [Google Scholar] [CrossRef]
- Holst, S.C.; Landolt, H.P. Sleep-Wake Neurochemistry. Sleep Med. Clin. 2022, 17, 151–160. [Google Scholar] [CrossRef]
- Kim, J.J.; Gharpure, A.; Teng, J.; Zhuang, Y.; Howard, R.J.; Zhu, S.; Noviello, C.M.; Walsh, R.M.; Lindahl, E.; Hibbs, R.E. Shared structural mechanisms of general anaesthetics and benzodiazepines. Nature 2020, 585, 303–308. [Google Scholar] [CrossRef]
- Greenfield Jr, L.J. Molecular mechanisms of antiseizure drug activity at GABAA receptors. Seizure 2013, 22, 589–600. [Google Scholar] [CrossRef]
- Saper, C.B.; Lowell, B.B. The hypothalamus. Curr. Biol. 2014, 24, R1111–R1116. [Google Scholar] [CrossRef]
- Zhu, S.; Noviello, C.M.; Teng, J.; Walsh, R.M., Jr.; Kim, J.J.; Hibbs, R.E. Structure of a human synaptic GABA(A) receptor. Nature 2018, 559, 67–72. [Google Scholar] [CrossRef]
- Krone, L.B.; Yamagata, T.; Blanco-Duque, C.; Guillaumin, M.C.C.; Kahn, M.C.; van der Vinne, V.; McKillop, L.E.; Tam, S.K.E.; Peirson, S.N.; Akerman, C.J.; et al. A role for the cortex in sleep–wake regulation. Nat. Neurosci. 2021, 24, 1210–1215. [Google Scholar] [CrossRef]
- Havekes, R.; Abel, T. The tired hippocampus: The molecular impact of sleep deprivation on hippocampal function. Curr. Opin. Neurobiol. 2017, 44, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Hibbs, R.E. Direct Structural Insights into GABA(A) Receptor Pharmacology. Trends Biochem. Sci. 2021, 46, 502–517. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.; Aricescu, A.R. A structural perspective on GABAA receptor pharmacology. Curr. Opin. Struct. Biol. 2019, 54, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Johnston, G.A. Muscimol as an ionotropic GABA receptor agonist. Neurochem. Res. 2014, 39, 1942–1947. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Kim, Y.; Lee, H.W.; Jung, J.-C.; Oh, S. Chrysanthemum morifolium and Its Bioactive Substance Enhanced the Sleep Quality in Rodent Models via Cl− Channel Activation. Nutrients 2023, 15, 1309. https://doi.org/10.3390/nu15061309
Kim M, Kim Y, Lee HW, Jung J-C, Oh S. Chrysanthemum morifolium and Its Bioactive Substance Enhanced the Sleep Quality in Rodent Models via Cl− Channel Activation. Nutrients. 2023; 15(6):1309. https://doi.org/10.3390/nu15061309
Chicago/Turabian StyleKim, Mijin, YuJaung Kim, Hyang Woon Lee, Jae-Chul Jung, and Seikwan Oh. 2023. "Chrysanthemum morifolium and Its Bioactive Substance Enhanced the Sleep Quality in Rodent Models via Cl− Channel Activation" Nutrients 15, no. 6: 1309. https://doi.org/10.3390/nu15061309
APA StyleKim, M., Kim, Y., Lee, H. W., Jung, J.-C., & Oh, S. (2023). Chrysanthemum morifolium and Its Bioactive Substance Enhanced the Sleep Quality in Rodent Models via Cl− Channel Activation. Nutrients, 15(6), 1309. https://doi.org/10.3390/nu15061309





