Association between Antibiotic Exposure and Type 2 Diabetes Mellitus in Middle-Aged and Older Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Urine Testing and Medical Information Collection
2.3. Antibiotic Health Risk Assessment
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castanon, J.I. History of the use of antibiotic as growth promoters in European poultry feeds. Poult. Sci. 2007, 86, 2466–2471. [Google Scholar] [CrossRef]
- Dibner, J.J.; Richards, J.D. Antibiotic growth promoters in agriculture: History and mode of action. Poult. Sci. 2005, 84, 634–643. [Google Scholar] [CrossRef]
- Roberts, P.J. Penicillin man: Alexander Fleming and the antibiotic revolution. J. Hosp. Infect. 2006, 62, 393. [Google Scholar] [CrossRef]
- Carvalho, I.T.; Santos, L. Antibiotics in the aquatic environments: A review of the European scenario. Environ. Int. 2016, 94, 736–757. [Google Scholar] [PubMed]
- Chu, L.; Wang, H.X.; Su, D.Q.; Zhang, H.W.; Yimingniyazi, B.H.; Aili, D.L.; Luo, T.; Zhang, Z.W.; Dai, J.H.; Jiang, Q.W. Urinary Antibiotics and Dietary Determinants in Adults in Xinjiang, West China. Nutrients 2022, 14, 4748. [Google Scholar] [CrossRef]
- Wang, H.; Yang, J.; Yu, X.; Zhao, G.; Zhao, Q.; Wang, N.; Jiang, Y.; Jiang, F.; He, G.; Chen, Y.; et al. Exposure of adults to antibiotics in a Shanghai Suburban Area and Health Risk Assessment: A biomonitoring-based study. Environ. Sci. Technol. 2018, 52, 13942–13950. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ren, L.; Yu, X.; Hu, J.; Chen, Y.; He, G.; Jiang, Q. Antibiotic residues in meat, milk and aquatic products in Shanghai and human exposure assessment. Food Control 2017, 80, 217–225. [Google Scholar] [CrossRef]
- Boursi, B.; Mamtani, R.; Haynes, K.; Yang, Y.X. The effect of past antibiotic exposure on diabetes risk. Eur. J. Endocrinol. 2015, 172, 639–648. [Google Scholar] [CrossRef]
- Mikkelsen, K.H.; Knop, F.K.; Frost, M.; Hallas, J.; Pottegard, A. Use of antibiotics and risk of type 2 diabetes: A population-based case-control study. J. Clin. Endocrinol. Metab. 2015, 100, 3633–3640. [Google Scholar] [CrossRef] [PubMed]
- Omori, M.; Kato-Kogoe, N.; Sakaguchi, S.; Kamiya, K.; Fukui, N.; Gu, Y.H.; Nakamura, S.; Nakano, T.; Hoshiga, M.; Imagawa, A.; et al. Characterization of salivary microbiota in elderly patients with type 2 diabetes mellitus: A matched case-control study. Clin. Oral Investig. 2022, 26, 493–504. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Chou, H.W.; Wang, J.L.; Chang, C.H.; Lee, J.J.; Shau, W.Y.; Lai, M.S. Risk of severe dysglycemia among diabetic patients receiving levofloxacin, ciprofloxacin, or moxifloxacin in Taiwan. Clin. Infect. Dis. 2013, 57, 971–980. [Google Scholar] [CrossRef]
- Park, S.J.; Park, Y.J.; Chang, J.; Choi, S.; Lee, G.; Son, J.S.; Kim, K.H.; Oh, Y.H.; Park, S.M. Association between antibiotics use and diabetes incidence in a nationally representative retrospective cohort among Koreans. Sci. Rep. 2021, 11, 21681. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Lin, Y.; Liu, Y.; Chen, K. Antibiotic exposure and risk of type 2 diabetes mellitus: A systematic review and meta-analysis. Environ. Sci. Pollut. Res. Int. 2021, 28, 65052–65061. [Google Scholar] [CrossRef]
- Tao, L.; Tian, T.; Liu, L.; Zhang, Z.; Sun, Q.; Sun, G.; Dai, J.; Yan, H. Cohort profile: The Xinjiang Multiethnic Cohort (XMC) study. BMJ Open 2022, 12, e048242. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, J.; Collins, R.; Guo, Y.; Peto, R.; Wu, F.; Li, L. China Kadoorie Biobank collaborative, g. China Kadoorie Biobank of 0.5 million people: Survey methods, baseline characteristics and long-term follow-up. Int. J. Epidemiol. 2011, 40, 1652–1666. [Google Scholar] [CrossRef] [PubMed]
- Saravia, L.; Miguel-Berges, M.L.; Iglesia, I.; Nascimento-Ferreira, M.V.; Perdomo, G.; Bove, I.; Slater, B.; Moreno, L.A. Relative validity of FFQ to assess food items, energy, macronutrient and micronutrient intake in children and adolescents: A systematic review with meta-analysis. Br. J. Nutr. 2021, 125, 792–818. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhu, B.B.; Tao, X.Y.; Zhu, Y.D.; Tao, X.G.; Tao, F.B. Temporal variability of cumulative risk assessment on phthalates in Chinese pregnant women: Repeated measurement analysis. Environ. Sci. Technol. 2018, 52, 6585–6591. [Google Scholar] [CrossRef]
- Guo, Y.; Alomirah, H.; Cho, H.S.; Minh, T.B.; Mohd, M.A.; Nakata, H.; Kannan, K. Occurrence of phthalate metabolites in human urine from several Asian countries. Environ. Sci. Technol. 2011, 45, 3138–3144. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, N.; Qian, J.; Hu, L.; Huang, P.; Su, M.; Yu, X.; Fu, C.; Jiang, F.; Zhao, Q.; et al. Urinary antibiotics of pregnant women in Eastern China and cumulative health risk assessment. Environ. Sci. Technol. 2017, 51, 3518–3525. [Google Scholar] [CrossRef]
- Soeborg, T.; Frederiksen, H.; Andersson, A.M. Cumulative risk assessment of phthalate exposure of Danish children and adolescents using the hazard index approach. Int. J. Androl. 2012, 35, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Wang, J.; Chen, K.; Wang, J.; Chen, K.; Yan, W.; Wang, A.; Wang, W.; Gao, Z.; Tang, X.; et al. A high triglyceride glucose index is more closely associated with hypertension than lipid or glycemic parameters in elderly individuals: A cross-sectional survey from the Reaction Study. Cardiovasc. Diabetol. 2020, 19, 112. [Google Scholar] [CrossRef]
- Wang, H.; Wang, B.; Zhao, Q.; Zhao, Y.; Fu, C.; Feng, X.; Wang, N.; Su, M.; Tang, C.; Jiang, F.; et al. Antibiotic body burden of Chinese school children: A multisite biomonitoring-based study. Environ. Sci. Technol. 2015, 49, 5070–5079. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Kho, Y.; Park, C.; Paek, D.; Ryu, P.; Paek, D.; Kim, M.; Kim, P.; Choi, K. Influence of water and food consumption on inadvertent antibiotics intake among general population. Environ. Res. 2010, 110, 641–649. [Google Scholar] [CrossRef]
- Li, N.; Ho, K.W.K.; Ying, G.G.; Deng, W.J. Veterinary antibiotics in food, drinking water, and the urine of preschool children in Hong Kong. Environ. Int. 2017, 108, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, N.; Wang, B.; Fang, H.; Fu, C.; Tang, C.; Jiang, F.; Zhou, Y.; He, G.; Zhao, Q.; et al. Antibiotics detected in urines and adipogenesis in school children. Environ. Int. 2016, 89–90, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.J.; Zhu, F.; Zheng, D.Y.; Gao, M.M.; Guo, B.F.; Zhang, N.; Meng, Y.; Wu, G.L.; Zhou, Y.L.; Huo, X. Detection of antibiotics in the urine of children and pregnant women in Jiangsu, China. Environ. Res. 2021, 196, 110945. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, L.; Chen, Q.; Yu, K.; Zhao, S.; Zhang, L.; Zhang, J.; Zhang, W.; Huang, L. Maternal antibiotic concentrations in pregnant women in Shanghai and their determinants: A biomonitoring-based prospective study. Environ. Int. 2020, 2138, 105638. [Google Scholar] [CrossRef]
- Geng, M.; Liu, K.; Huang, K.; Zhu, Y.; Ding, P.; Zhang, J.; Wang, B.; Liu, W.; Han, Y.; Gao, H.; et al. Urinary antibiotic exposure across pregnancy from Chinese pregnant women and health risk assessment: Repeated measures analysis. Environ. Int. 2020, 145, 106164. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, W.; Wang, Y.; Nian, M.; Jiang, F.; Zhang, J.; Chen, Q. Environmental antibiotics exposure in school-age children in Shanghai and health risk assessment: A population-based representative investigation. Sci. Total Environ. 2022, 824, 153859. [Google Scholar] [CrossRef]
- Wang, H.; Tang, C.; Yang, J.; Wang, N.; Jiang, F.; Xia, Q.; He, G.; Chen, Y.; Jiang, Q. Predictors of urinary antibiotics in children of Shanghai and health risk assessment. Environ. Int. 2018, 121, 507–514. [Google Scholar] [CrossRef]
- Feng, L.; Cheng, Y.; Zhang, Y.; Li, Z.; Yu, Y.; Feng, L.; Zhang, S.; Xu, L. Distribution and human health risk assessment of antibiotic residues in large-scale drinking water sources in Chongqing area of the Yangtze River. Environ. Res. 2020, 185, 109386. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.Y.; Hao, L.J.; Qiu, P.Z.; Chen, R.; Xu, J.; Kong, X.J.; Shan, Z.J.; Wang, N. Pollution characteristics of 23 veterinary antibiotics in livestock manure and manure-amended soils in Jiangsu province, China. J. Environ. Sci. Health B 2016, 51, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Wu, H.; Wang, J.; Zhang, H.; Zhang, Z.; Zhang, Y.; Lin, H.; Ma, J. Occurrence of trace elements and antibiotics in manure-based fertilizers from the Zhejiang Province of China. Sci. Total Environ. 2016, 559, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Cunha, B.A. Antibiotic Essentials; Jones & Bartlett: Burlington, MA, USA, 2013. [Google Scholar]
- Alvarez-Silva, C.; Kashani, A.; Hansen, T.H.; Pinna, N.K.; Anjana, R.M.; Dutta, A.; Saxena, S.; Støy, J.; Kampmann, U.; Nielsen, T.; et al. Trans-ethnic gut microbiota signatures of type 2 diabetes in Denmark and India. Genome Med. 2021, 13, 37. [Google Scholar] [CrossRef]
- Doumatey, A.P.; Adeyemo, A.; Zhou, J.; Lei, L.; Adebamowo, S.N.; Adebamowo, C.; Rotimi, C.N. Gut microbiome profiles are associated with type 2 diabetes in Urban Africans. Front. Cell Infect. Microbiol. 2020, 10, 63. [Google Scholar] [CrossRef]
- Shi, A.; Li, T.; Zheng, Y.; Song, Y.; Wang, H.; Wang, N.; Dong, L.; Shi, H. Chlorogenic acid improves NAFLD by regulating gut microbiota and GLP-1. Front. Pharmacol. 2021, 12, 693048. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, A.; Sandhu, A.K.; Edirisinghe, I.; Burton-Freeman, B.M. Functional Deficits in gut microbiome of young and middle-aged adults with prediabetes apparent in metabolizing bioactive (poly)phenols. Nutrients 2020, 12, 3595. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ni, Y.; Qian, L.; Fang, Q.; Zheng, T.; Zhang, M.; Gao, Q.; Zhang, Y.; Ni, J.; Hou, X.; et al. Decreased abundance of Akkermansia muciniphila leads to the impairment of insulin secretion and glucose homeostasis in lean type 2 diabetes. Adv. Sci. 2021, 8, e2100536. [Google Scholar] [CrossRef]
- Herrera, C.R.; Vidal, G.X. Cardiovascular events and mortality among type 2 diabetes mellitus patients newly prescribed first-line blood glucose-lowering drugs monotherapies: A population-based cohort study in the Catalan electronic medical record database, SIDIAP, 2010–2015. Prim. Care Diabetes 2021, 15, 323–331. [Google Scholar] [CrossRef]
- Blaser, M.J. Antibiotic use and its consequences for the normal microbiome. Science 2016, 352, 544–545. [Google Scholar] [CrossRef]
- Yu, F.; Han, W.; Zhan, G.; Li, S.; Jiang, X.; Wang, L.; Xiang, S.; Zhu, B.; Yang, L.; Luo, A.; et al. Abnormal gut microbiota composition contributes to the development of type 2 diabetes mellitus in db/db mice. Aging 2019, 11, 10454–10467. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The microbiota-gut-brain axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Grasset, E.; Burcelin, R. The gut microbiota to the brain axis in the metabolic control. Rev. Endocr. Metab. Disord. 2019, 20, 427–438. [Google Scholar] [CrossRef]
- Muller, P.A.; Matheis, F.; Schneeberger, M.; Kerner, Z.; Jové, V.; Mucida, D. Microbiota-modulated CART+ enteric neurons autonomously regulate blood glucose. Science 2020, 370, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B. Globalization of diabetes: The role of diet, lifestyle, and genes. Diabetes Care 2011, 34, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Lalrohlui, F.; Ghatak, S.; Zohmingthanga, J.; Hruaii, V.; Kumar, N.S. Fermented pork fat (Sa-um) and lifestyle risk factors as potential indicators for type 2 diabetes among the Mizo population, Northeast India. J. Health Popul. Nutr. 2021, 40, 32. [Google Scholar] [CrossRef] [PubMed]
- Patyra, E.; Kwiatek, K. HPLC-DAD analysis of florfenicol and thiamphenicol in medicated feedingstuffs. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess 2019, 36, 1184–1190. [Google Scholar] [CrossRef]
- Ahmed, A.; Lager, A.; Fredlund, P.; Elinder, L.S. Consumption of fruit and vegetables and the risk of type 2 diabetes: A 4-year longitudinal study among Swedish adults. J. Nutr. Sci. 2020, 9, e14. [Google Scholar] [CrossRef]
- Tadic, D.; Bleda Hernandez, M.J.; Cerqueira, F.; Matamoros, V.; Pina, B.; Bayona, J.M. Occurrence and human health risk assessment of antibiotics and their metabolites in vegetables grown in field-scale agricultural systems. J. Hazard Mater. 2021, 401, 123424. [Google Scholar] [CrossRef]
- Lam, B.Q.; Srivastava, R.; Morvant, J.; Shankar, S.; Srivastava, R.K. Association of diabetes mellitus and alcohol abuse with cancer: Molecular mechanisms and clinical significance. Cells 2021, 10, 3077. [Google Scholar] [CrossRef]
- Mallet, V.; Parlati, L.; Martinino, A.; Scarano Pereira, J.P.; Jimenez, C.N.; Sakka, M.; Bouam, S.; Retbi, A.; Krasteva, D.; Meritet, J.F.; et al. Demosthenes research, g. Burden of liver disease progression in hospitalized patients with type 2 diabetes mellitus. J. Hepatol. 2022, 76, 265–274. [Google Scholar] [CrossRef]
- Moschona, A.; Liakopoulou-Kyriakides, M. Encapsulation of biological active phenolic compounds extracted from wine wastes in alginate-chitosan microbeads. J. Microencapsul. 2018, 35, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.J.; Liu, M.; Alemi, F.; Jensen, A.; Avramovic, S.; Levy, E.; Hayes, R.B.; Schwartz, M.D. Prior antibiotic exposure and risk of type 2 diabetes among Veterans. Prim. Care Diabetes 2019, 13, 49–56. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, Y.; Liu, Y.; Chen, K. Response to Antibiotic exposure and risk of type 2 diabetes mellitus: A systematic review and meta-analysis by Maryam et al. (2021) by J Zhou and K Chen (2021). Environ. Sci. Pollut. Res. Int. 2022, 29, 18298–18299. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Robson, P.J.; Eurich, D.T.; Vena, J.E.; Xu, J.-Y.; Johnson, J.A. Systemic use of antibiotics and risk of diabetes in adults: A nested case-control study of Alberta’s Tomorrow Project. Diabetes Obes Metab. 2018, 20, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Baik, S.H.; McDonald, C.J. Independent effects of 15 commonly prescribed drugs on all-cause mortality among US elderly patients with type 2 diabetes mellitus. BMJ Open Diabetes Res. Care 2020, 8, e000940. [Google Scholar] [CrossRef] [PubMed]
- Ida, S.; Kaneko, R.; Imataka, K.; Okubo, K.; Shirakura, Y.; Azuma, K.; Fujiwara, R.; Takahashi, H.; Murata, K. Effects of oral antidiabetic drugs and glucagon-like peptide-1 receptor agonists on left ventricular diastolic function in patients with type 2 diabetes mellitus: A systematic review and network meta-analysis. Heart Fail. Rev. 2021, 26, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
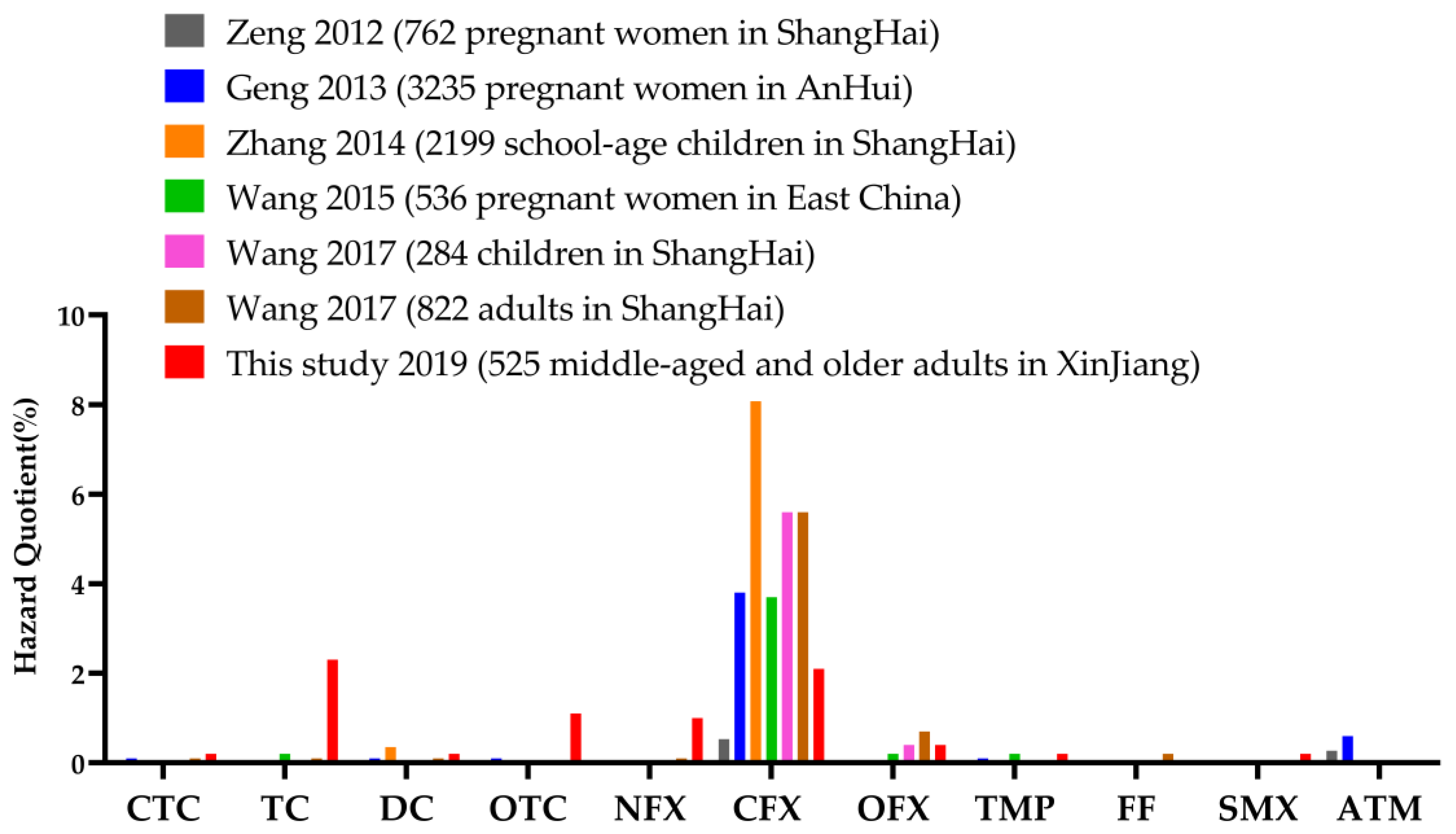
| Antibiotic | Usage | All (n = 525) n (%) a | Non-T2DM (n = 461) | T2DM (n = 64) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n (%) a | Percentiles | Maximum | n (%) a | Percentiles | Maximum | |||||
| 95th | 99th | 95th | 99th | |||||||
| All antibiotics b | 268 (51.0) | 228 (49.5) | 78.09 | 20,991.06 | 44,588.26 | 40 (62.5) | 2363.48 | 24,282.26 | 24,282.26 | |
| HAs | 54 (10.3) | 43 (9.3) | 0.87 | 77.06 | 4615.54 | 11 (17.2) | 41.30 | 2989.35 | 2989.35 | |
| VAs | 60 (11.4) | 48 (10.4) | 2.02 | 148.17 | 36,112.85 | 12 (18.8) | 4.48 | 2090.43 | 2090.43 | |
| PVAs | 224 (42.7) | 190 (41.2) | 44.97 | 7343.72 | 44,418.14 | 34 (53.1) | 272.99 | 24,282.26 | 24,282.26 | |
| Tetracyclines c | 79 (15.0) | 64 (13.9) | 13.35 | 20,991.06 | 44,565.07 | 15 (23.4) | 42.71 | 2362.84 | 2362.84 | |
| Chlortetracycline | VA | 4 (0.8) | 4 (0.9) | — | — | 119.02 | 0 (0) | — | — | — |
| Tetracycline | PVA | 60 (11.4) | 50 (10.8) | 7.41 | 626.39 | 44,416.90 | 10 (15.6) | 12.48 | 272.42 | 272.42 |
| Doxycycline | PVA | 3 (0.6) | 3 (0.7) | — | — | 11,315.50 | 0 (0) | — | — | — |
| Oxytetracycline | VA | 38 (7.2) | 30 (6.5) | 1.43 | 75.75 | 36,112.85 | 8 (12.5) | 4.48 | 2090.43 | 2090.43 |
| Fluoroquinolones c | 170 (32.4) | 142 (30.8) | 7.09 | 726.97 | 2876.96 | 28 (43.8) | 67.69 | 24,282.26 | 24,282.26 | |
| Enrofloxacin | VA | 11 (2.1) | 7 (1.5) | — | 0.21 | 9.40 | 4 (6.3) | 0.20 | 0.96 | 0.96 |
| Norfloxacin | PVA | 66 (12.6) | 55 (11.9) | 1.28 | 18.14 | 1012.23 | 11 (17.2) | 1.11 | 24,282.26 | 24,282.26 |
| Ciprofloxacin | PVA | 35 (6.7) | 29 (6.3) | 0.57 | 6.24 | 928.11 | 6 (9.4) | 3.86 | 8.66 | 8.66 |
| Ofloxacin | PVA | 105 (20.0) | 92 (20.0) | 1.63 | 39.86 | 2876.96 | 13 (20.3) | 1.08 | 66.89 | 66.89 |
| Macrolides c | 29 (5.5) | 24 (5.2) | 0.87 | 77.06 | 4615.54 | 5 (7.8) | 41.30 | 2989.35 | 2989.35 | |
| Azithromycin | HA | 15 (2.9) | 13 (2.8) | — | 5.11 | 479.12 | 2 (3.1) | — | 248.70 | 248.70 |
| Clarithromycin | HA | 3 (0.6) | 3 (0.7) | — | — | 4615.54 | 0 (0) | — | — | — |
| Roxithromycin | HA | 15 (2.9) | 12 (2.6) | — | 23.10 | 132.94 | 3 (4.7) | — | 2989.35 | 2989.35 |
| Sulfonamides c | 47 (9.0) | 40 (8.7) | 0.25 | 1.81 | 7232.59 | 7 (10.9) | 0.21 | 2.74 | 2.74 | |
| Sulfamethazine | PVA | 16 (3.0) | 13 (2.8) | — | 0.28 | 11.66 | 3 (4.7) | — | 0.28 | 0.28 |
| Sulfadiazine | PVA | 1 (0.2) | 1 (0.2) | — | — | 10.51 | 0 (0) | — | — | — |
| Sulfamethoxazole | PVA | 9 (1.7) | 9 (2.0) | — | 0.69 | 5546.27 | 0 (0) | — | — | — |
| Trimethoprim | PVA | 33 (6.3) | 29 (6.3) | 0.12 | 1.12 | 1686.32 | 4 (6.3) | 0.06 | 2.74 | 2.74 |
| Phenicols c | 46 (8.8) | 37 (8.0) | 0.05 | 0.63 | 30.93 | 9 (14.1) | 0.24 | 1.04 | 1.04 | |
| Chloramphenicol | HA | 30 (5.7) | 23 (5.0) | — | 0.12 | 9.98 | 7 (10.9) | 0.05 | 0.78 | 0.78 |
| Florfenicol | VA | 18 (3.4) | 15 (3.3) | — | 0.43 | 9.86 | 3 (4.7) | — | 0.43 | 0.43 |
| Thiamphenicol | PVA | 3 (0.6) | 2 (0.4) | — | — | 11.10 | 1 (1.6) | — | 0.08 | 0.08 |
| Non-T2DM (n = 461) | T2DM (n = 64) | |||||
|---|---|---|---|---|---|---|
| Percentiles | Maximum | Percentiles | Maximum | |||
| Antibiotic | 95th | 99th | 95th | 99th | ||
| Tetracyclines a | 0.44 | 569.61 | 1619.25 | 1.27 | 91.25 | 91.25 |
| Chlortetracycline | 0.00 | 0.00 | 5.44 | 0.00 | 0.00 | 0.00 |
| Tetracycline | 0.32 | 25.64 | 1612.89 | 0.45 | 11.91 | 11.91 |
| Doxycycline | 0.00 | 0.00 | 336.85 | 0.00 | 0.00 | 0.00 |
| Oxytetracycline | 0.06 | 3.11 | 1443.79 | 0.16 | 79.34 | 79.34 |
| Fluoroquinolones a | 0.22 | 37.14 | 57.11 | 2.13 | 873.98 | 873.98 |
| Enrofloxacin | 0.00 | 0.03 | 0.89 | 0.03 | 0.11 | 0.11 |
| Norfloxacin | 0.05 | 0.76 | 45.57 | 0.04 | 873.98 | 873.98 |
| Ciprofloxacin | 0.03 | 0.24 | 51.21 | 0.17 | 0.37 | 0.37 |
| Ofloxacin | 0.04 | 0.96 | 57.11 | 0.02 | 2.09 | 2.09 |
| Macrolides a | 0.04 | 4.59 | 383.10 | 1.01 | 108.08 | 108.08 |
| Azithromycin | 0.00 | 1.57 | 167.41 | 0.00 | 108.08 | 108.08 |
| Clarithromycin | 0.00 | 0.00 | 383.10 | 0.00 | 0.00 | 0.00 |
| Roxithromycin | 0.00 | 0.83 | 3.85 | 0.00 | 94.88 | 94.88 |
| Sulfonamides a | 0.01 | 0.12 | 610.71 | 0.01 | 0.10 | 0.10 |
| Sulfamethazine | 0.00 | 0.01 | 0.35 | 0.00 | 0.01 | 0.01 |
| Sulfadiazine | 0.00 | 0.00 | 0.40 | 0.00 | 0.00 | 0.00 |
| Sulfamethoxazole | 0.00 | 0.07 | 536.56 | 0.00 | 0.00 | 0.00 |
| Trimethoprim | 0.00 | 0.05 | 74.15 | 0.00 | 0.10 | 0.10 |
| Phenicols a | 0.00 | 0.03 | 1.02 | 0.01 | 0.03 | 0.03 |
| Chloramphenicol | 0.00 | 0.00 | 0.21 | 0.00 | 0.02 | 0.02 |
| Florfenicol | 0.00 | 0.03 | 0.41 | 0.00 | 0.02 | 0.02 |
| Thiamphenicol | 0.00 | 0.00 | 0.40 | 0.00 | 0.00 | 0.00 |
| All antibiotics b | 3.82 | 615.82 | 1619.98 | 95.29 | 873.98 | 873.98 |
| Antibiotics | Effect End Point | ADI (μg/kg/day) | Overall N (%) | Non-T2DM (n = 461) | T2DM (n = 64) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Percentiles | Maximum | Percentiles | Maximum | ||||||
| 95th | 99th | 95th | 99th | ||||||
| Hazard index a | |||||||||
| Chlortetracycline | Microbiological | 3 | 1 (0.2) | 0.000 | 0.000 | 1.813 | 0.000 | 0.000 | 0.000 |
| Tetracycline | Microbiological | 3 | 12 (2.3) | 0.107 | 8.547 | 537.630 | 0.150 | 3.970 | 3.970 |
| Doxycycline | Microbiological | 3 | 1 (0.2) | 0.000 | 0.000 | 112.284 | 0.000 | 0.000 | 0.000 |
| Oxytetracycline | Microbiological | 3 | 6 (1.1) | 0.019 | 1.038 | 481.264 | 0.052 | 26.448 | 26.448 |
| Enrofloxacin | Microbiological | 6.2 | 0 (0) | 0.000 | 0.005 | 0.143 | 0.004 | 0.017 | 0.017 |
| Norfloxacin | Microbiological | 14 | 5 (1.0) | 0.004 | 0.054 | 3.255 | 0.003 | 62.427 | 62.427 |
| Ciprofloxacin | Microbiological | 0.15 | 11 (2.1) | 0.202 | 1.589 | 341.393 | 1.129 | 2.464 | 2.464 |
| Ofloxacin | Microbiological | 3.2 | 2 (0.4) | 0.014 | 0.299 | 17.847 | 0.008 | 0.653 | 0.653 |
| Trimethoprim | Microbiological | 4.2 | 1 (0.2) | 0.001 | 0.012 | 17.656 | 0.001 | 0.025 | 0.025 |
| Florfenicol | Microbiological | 3 | 0 (0) | 0.000 | 0.010 | 0.137 | 0.000 | 0.007 | 0.007 |
| Thiamphenicol | Microbiological | 2.5 | 0 (0) | 0.000 | 0.000 | 0.159 | 0.000 | 0.002 | 0.002 |
| Hazard index b | |||||||||
| Sulfamethazine | Toxicological | 1.6 | 0 (0) | 0.000 | 0.006 | 0.220 | 0.000 | 0.008 | 0.008 |
| Sulfadiazine | Toxicological | 20 | 0 (0) | 0.000 | 0.000 | 0.020 | 0.000 | 0.000 | 0.000 |
| Sulfamethoxazole | Toxicological | 130 | 1 (0.2) | 0.000 | 0.001 | 4.127 | 0.000 | 0.000 | 0.000 |
| Variable | Blood Glucose (mmol/L) a | p-Value d | All Antibiotics (ng/mL) c | p-Value b |
|---|---|---|---|---|
| Gender | 0.698 | 0.090 | ||
| Male (n = 264) | 5.90 ± 1.68 | 0 (1.78) | ||
| Female (n = 261) | 5.84 ± 1.79 | 0.11 (2.45) | ||
| Age | 0.373 | 0.041 | ||
| 45–59 (n = 432) | 5.73 ± 1.43 | 0 (1.65) | ||
| 60–74 (n = 227) | 6.15 ± 2.18 | 0.17 (2.27) | ||
| Education | 0.457 | 0.101 | ||
| ≤Primary (n = 383) | 5.89 ± 1.86 | 0.08 (1.78) | ||
| Secondary (n = 112) | 5.92 ± 1.46 | 0 (1.82) | ||
| ≥High school (n = 30) | 5.49 ± 0.48 | 0 (0.36) | ||
| Income level | 0.022 | 0.821 | ||
| <10,000 RMB/year (n = 76) | 6.27 ± 2.40 | 0.16 (1.63) | ||
| 10,000–35,000 RMB/year (n = 332) | 5.89 ± 1.71 | 0.03 (1.69) | ||
| ≥35,000 RMB/year (n = 116) | 5.57 ± 1.14 | 0 (2.37) | ||
| Physical activity | 0.824 | 0.267 | ||
| Never (n = 427) | 5.87 ± 1.78 | 0.04 (2.09) | ||
| Occasionally (n = 98) | 5.90 ± 1.52 | 0 (0.82) | ||
| Pork | 0.101 | 0.094 | ||
| Do not eat (n = 444) | 5.82 ± 1.73 | 0.05 (2.03) | ||
| Occasionally eat (n = 78) | 6.17 ± 1.77 | 0 (0.77) | ||
| Beef | 0.974 | 0.478 | ||
| Do not eat (n = 155) | 5.88 ± 1.63 | 0.07 (1.69) | ||
| Occasionally eat (n = 281) | 5.86 ± 1.88 | 0 (1.45) | ||
| Eat every day (n = 87) | 5.91 ± 1.41 | 0.17 (2.27) | ||
| Mutton | 0.057 | 0.138 | ||
| Do not eat (n = 128) | 6.19 ± 2.30 | 0.03 (1.72) | ||
| Occasionally eat (n = 205) | 5.82 ± 1.58 | 0 (0.94) | ||
| Eat every day (n = 189) | 5.73 ± 1.41 | 0.08 (3.07) | ||
| Fried food | 0.457 | 0.130 | ||
| Do not eat (n = 328) | 5.83 ± 1.64 | 0.07 (2.03) | ||
| Occasionally eat (n = 197) | 5.95 ± 1.88 | 0 (1.41) | ||
| Vegetables | 0.274 | 0.166 | ||
| Not every day (n = 28) | 5.52 ± 1.34 | 0 (0.68) | ||
| Every day (n = 497) | 5.89 ± 1.75 | 0.04 (1.78) | ||
| Fruits | 0.562 | 0.997 | ||
| Not every day (n = 384) | 5.85 ± 1.68 | 0.04 (1.69) | ||
| Every day (n = 141) | 5.95 ± 1.88 | 0.02 (2.70) | ||
| Smoking | 0.539 | 0.059 | ||
| Never (n = 397) | 5.92 ± 1.84 | 0.10 (2.05) | ||
| Occasionally (n = 16) | 5.88 ± 1.26 | 0.14 (4.36) | ||
| Every day (n = 112) | 5.71 ± 1.39 | 0 (0.96) | ||
| Drinking | 0.408 | 0.453 | ||
| Never (n = 440) | 5.85 ± 1.76 | 0.04 (1.75) | ||
| Occasionally (n = 85) | 6.02 ± 1.58 | 0 (1.39) |
| Model A | Model B | Model C | ||||
|---|---|---|---|---|---|---|
| Variables | Tertile 1 | Tertile 2 | Tertile 1 | Tertile 2 | Tertile 1 | Tertile 2 |
| Group based on hazard index | ||||||
| HI for microbiological effects | Ref. | 2.948 (1.287–6.756) * | Ref. | 3.391 (1.417–8.120) * | Ref. | 3.442 (1.423–8.327) * |
| HI for toxicological effects | Ref. | — | Ref. | — | Ref. | — |
| HI for veterinary antibiotics | Ref. | 0.962 (0.109–8.472) | Ref. | 1.004 (0.105–9.607) | Ref. | 0.952 (0.098–9.243) |
| HI for preferred veterinary antibiotics | Ref. | 2.928 (1.279–6.706) * | Ref. | 3.271 (1.371–7.802) * | Ref. | 3.348 (1.386–8.083) * |
| Group based on hazard quotient | ||||||
| HQ for tetracycline | Ref. | 1.164 (0.245–5.534) | Ref. | 1.195 (0.241–5.926) | Ref. | 1.090 (0.218–5.456) |
| HQ for oxytetracycline | Ref. | 1.195 (0.129–11.052) | Ref. | 1.398 (0.136–14.372) | Ref. | 1.238 (0.118–12.994) |
| HQ for norfloxacin | Ref. | 10.075 (1.612–62.952) * | Ref. | 11.243 (1.728–73.142) * | Ref. | 10.511 (1.571–70.344) * |
| HQ for ciprofloxacin | Ref. | 4.789 (1.336–17.175) * | Ref. | 5.241 (1.377–19.946) * | Ref. | 6.565 (1.676–25.715) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, L.; Su, D.; Wang, H.; Aili, D.; Yimingniyazi, B.; Jiang, Q.; Dai, J. Association between Antibiotic Exposure and Type 2 Diabetes Mellitus in Middle-Aged and Older Adults. Nutrients 2023, 15, 1290. https://doi.org/10.3390/nu15051290
Chu L, Su D, Wang H, Aili D, Yimingniyazi B, Jiang Q, Dai J. Association between Antibiotic Exposure and Type 2 Diabetes Mellitus in Middle-Aged and Older Adults. Nutrients. 2023; 15(5):1290. https://doi.org/10.3390/nu15051290
Chicago/Turabian StyleChu, Lei, Deqi Su, Hexing Wang, Dilihumaer Aili, Bahegu Yimingniyazi, Qingwu Jiang, and Jianghong Dai. 2023. "Association between Antibiotic Exposure and Type 2 Diabetes Mellitus in Middle-Aged and Older Adults" Nutrients 15, no. 5: 1290. https://doi.org/10.3390/nu15051290
APA StyleChu, L., Su, D., Wang, H., Aili, D., Yimingniyazi, B., Jiang, Q., & Dai, J. (2023). Association between Antibiotic Exposure and Type 2 Diabetes Mellitus in Middle-Aged and Older Adults. Nutrients, 15(5), 1290. https://doi.org/10.3390/nu15051290







