Exposure to Obesogenic Environments during Perinatal Development Modulates Offspring Energy Balance Pathways in Adipose Tissue and Liver of Rodent Models
Abstract
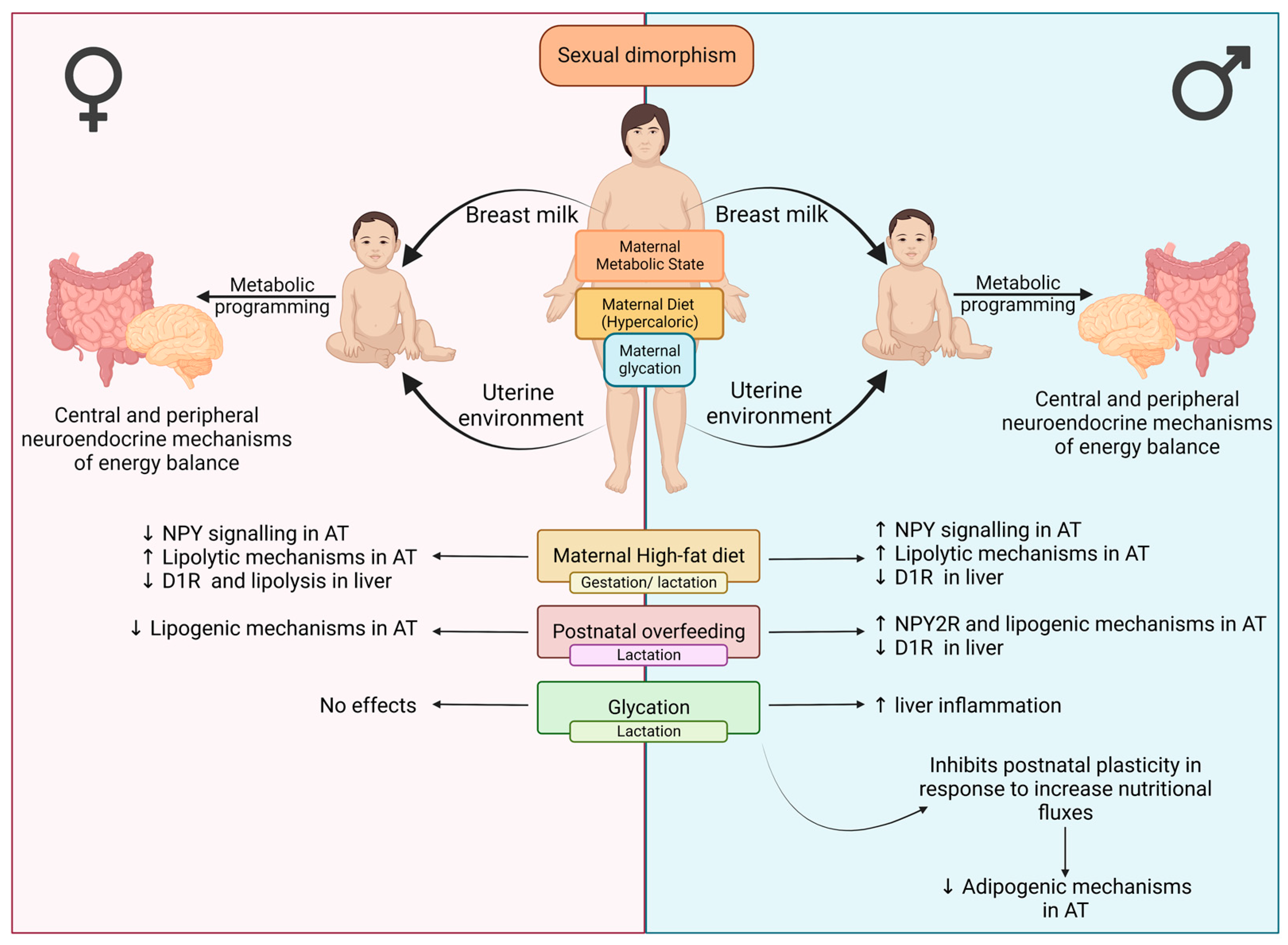
1. Introduction
2. Materials and Methods
2.1. In Vivo Models of Obesogenic Metabolic Programming
2.1.1. Maternal Diet-Induced Obesity (DIO) during Gestation and Lactation
2.1.2. Postnatal Overfeeding and Glycation Models
Postnatal Overfeeding Model
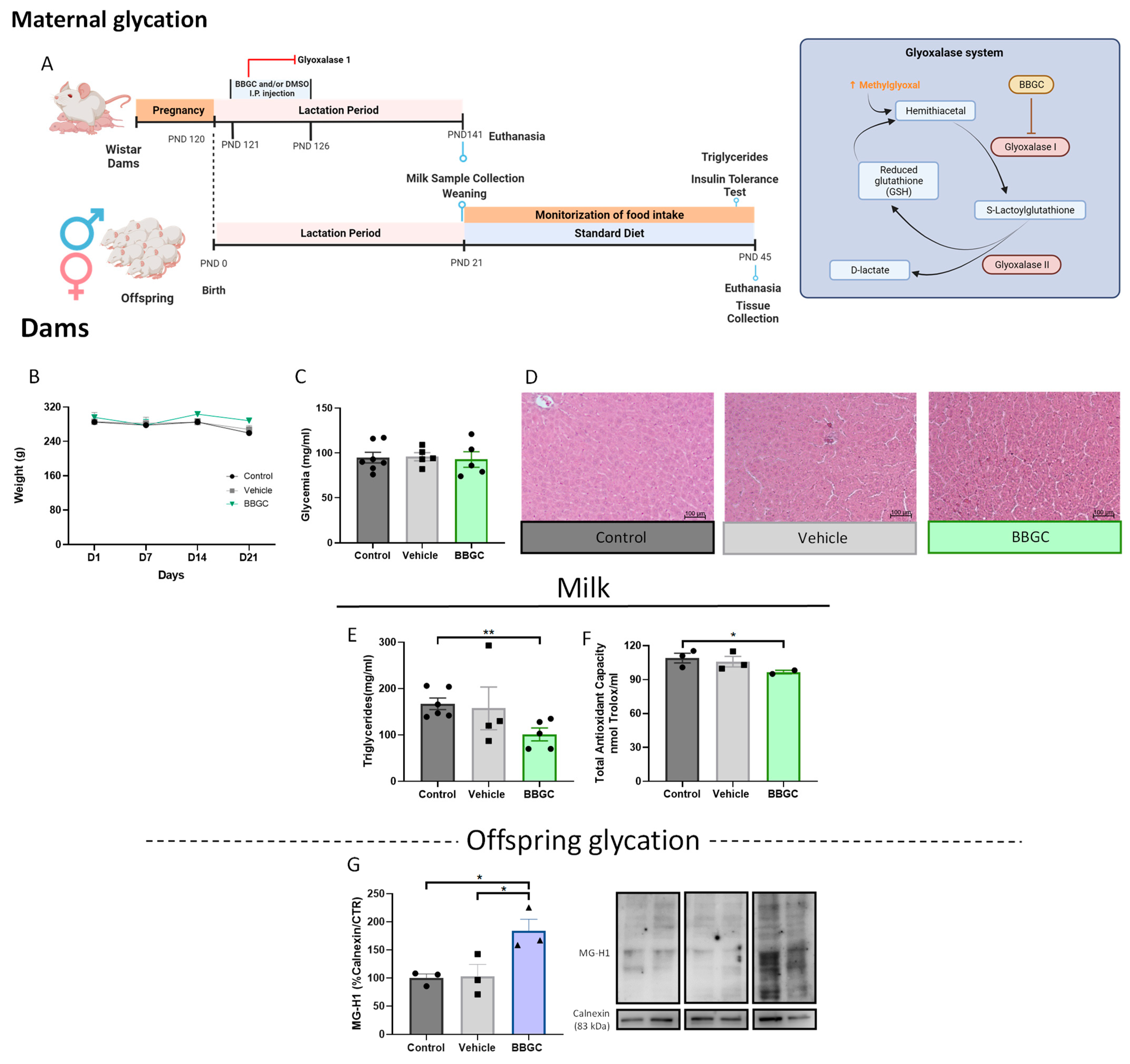
Maternal Glycation Model
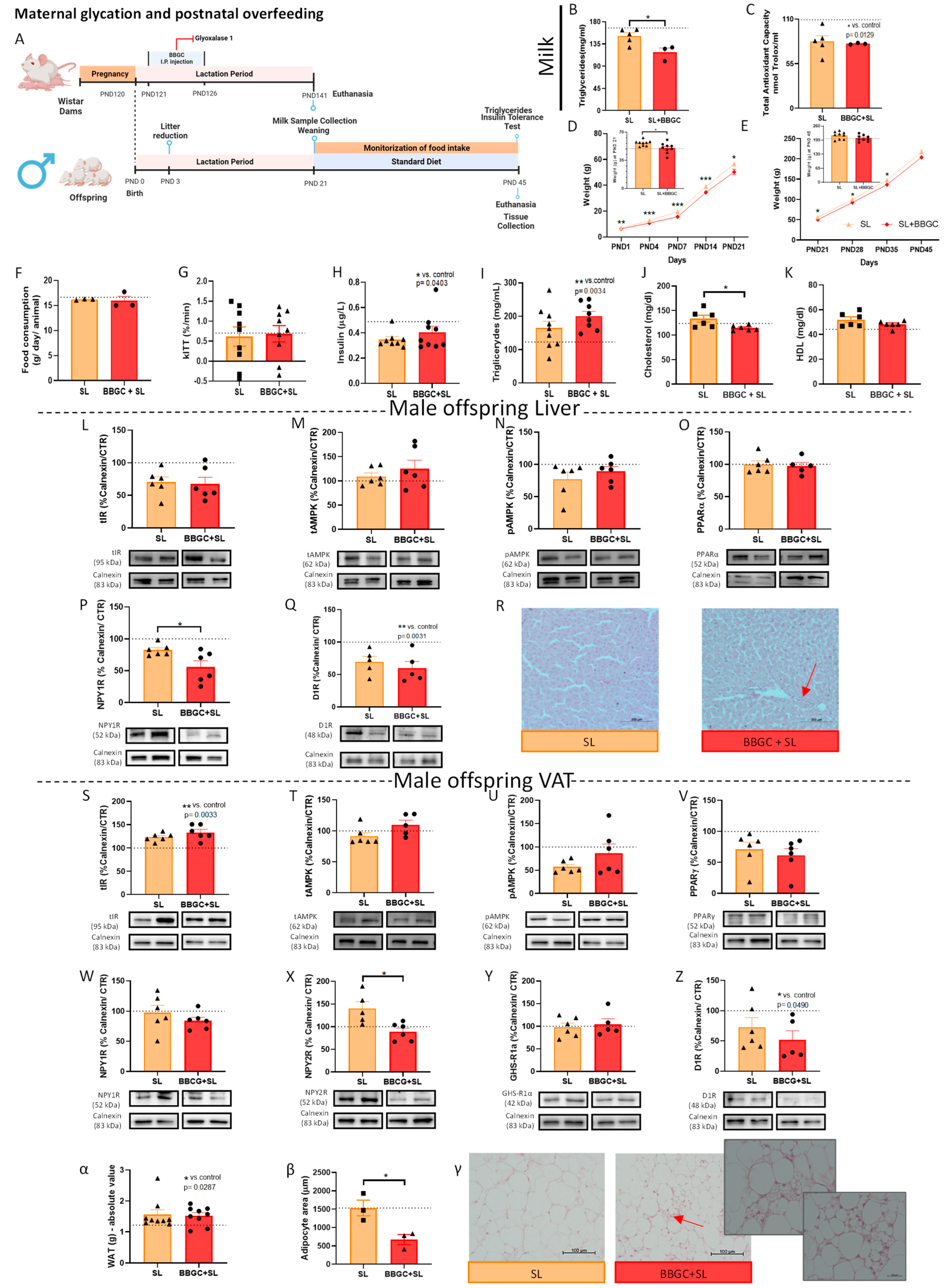
Postnatal Overfed Rats Exposed to Maternal Glycotoxins Model
2.2. Milk Sample Collection and Determination of Total Antioxidant Capacity and Triglycerides
2.3. Plasma Determinations
2.4. Histology—Haematoxylin-Eosin
2.5. Western Blot
2.6. Statistical Analyses
3. Results
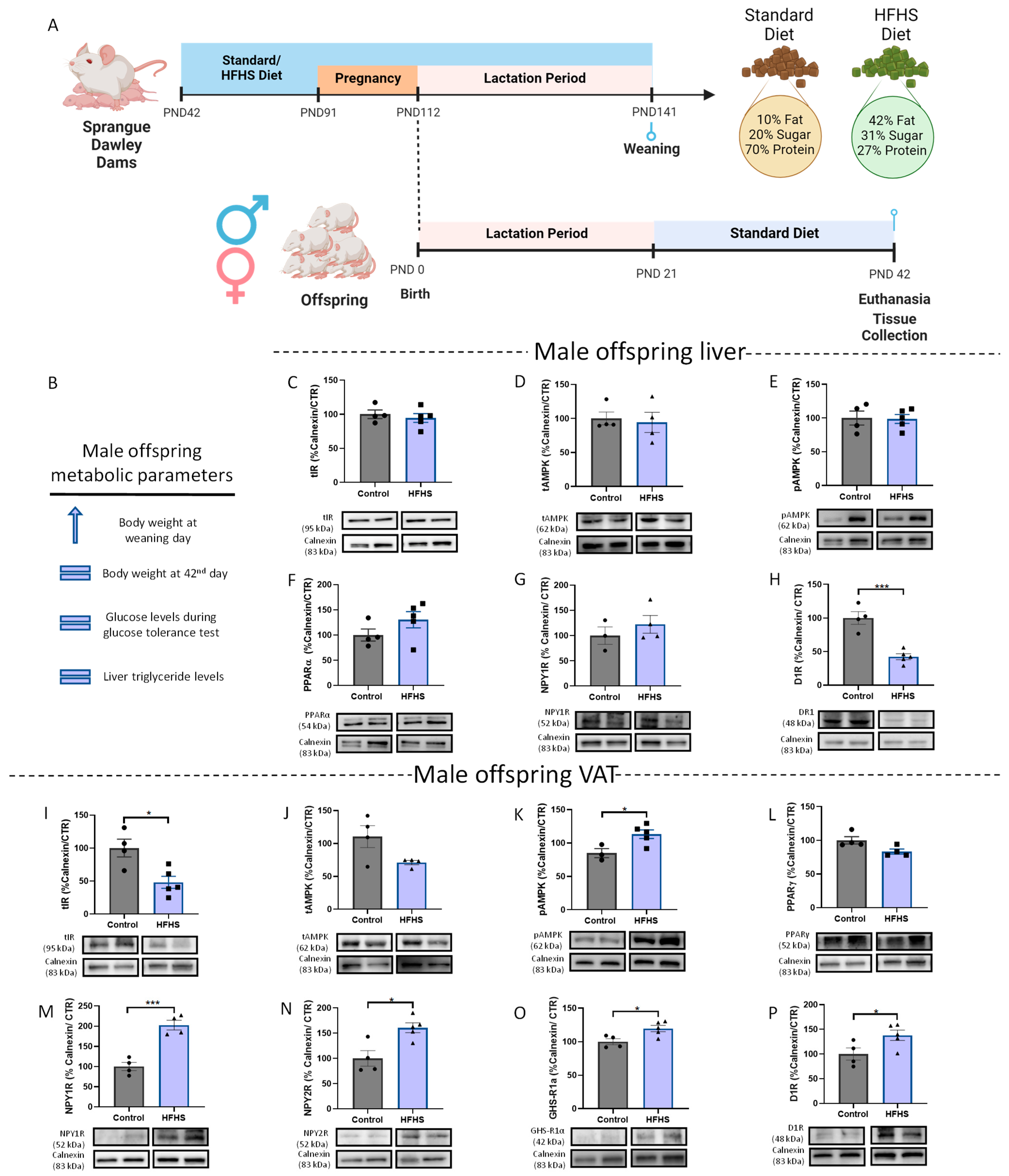
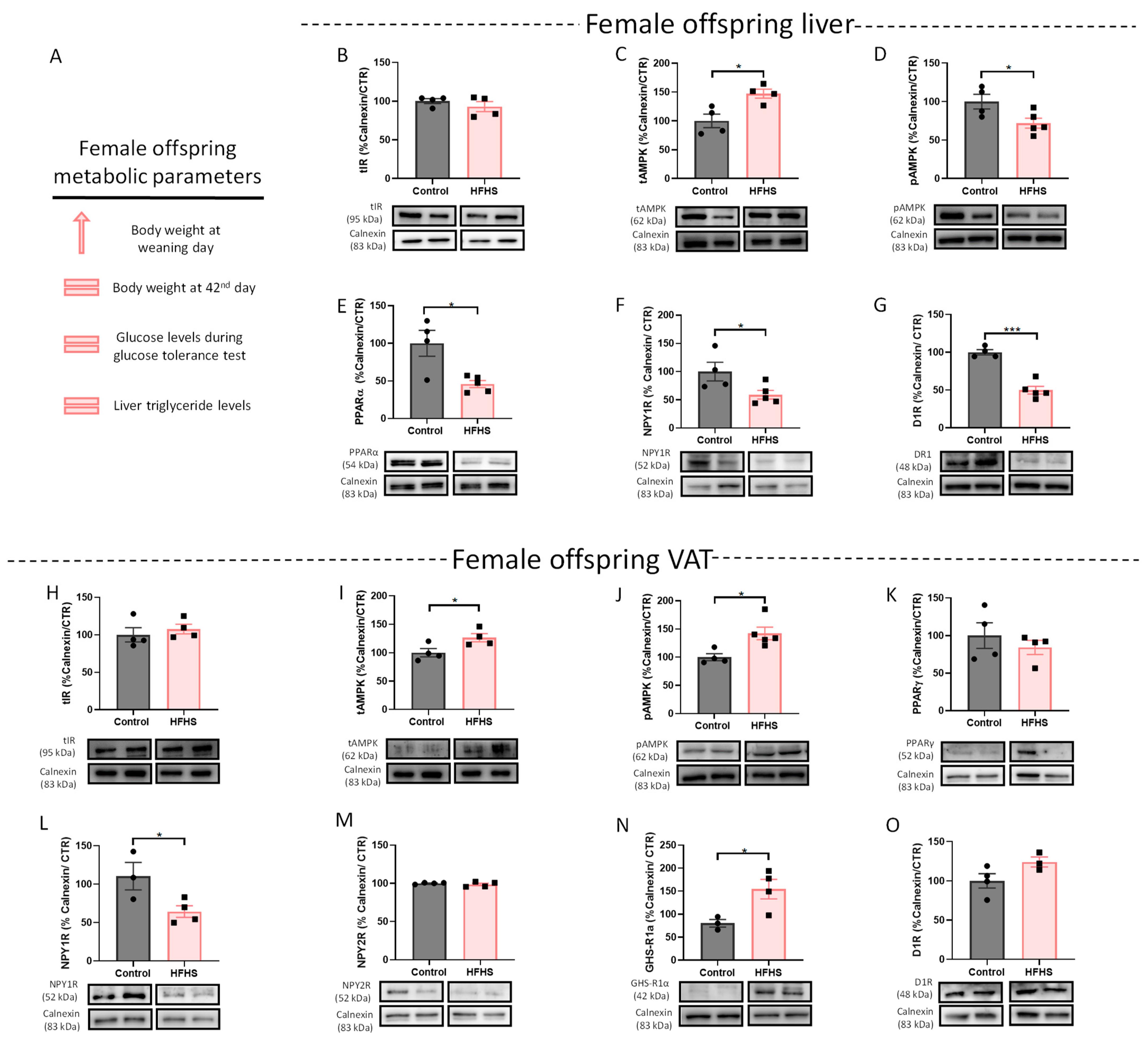
3.1. Liver and VAT Sexual Dysmorphism in the Response to Different Perinatal Obesogenic Environments
3.1.1. Maternal DIO Modulates Energy Balance Mechanisms in the VAT and Liver of Male Offspring
3.1.2. Maternal Obesity Alters Levels of Energy Balance-Regulating Receptors in the VAT of Female Offspring
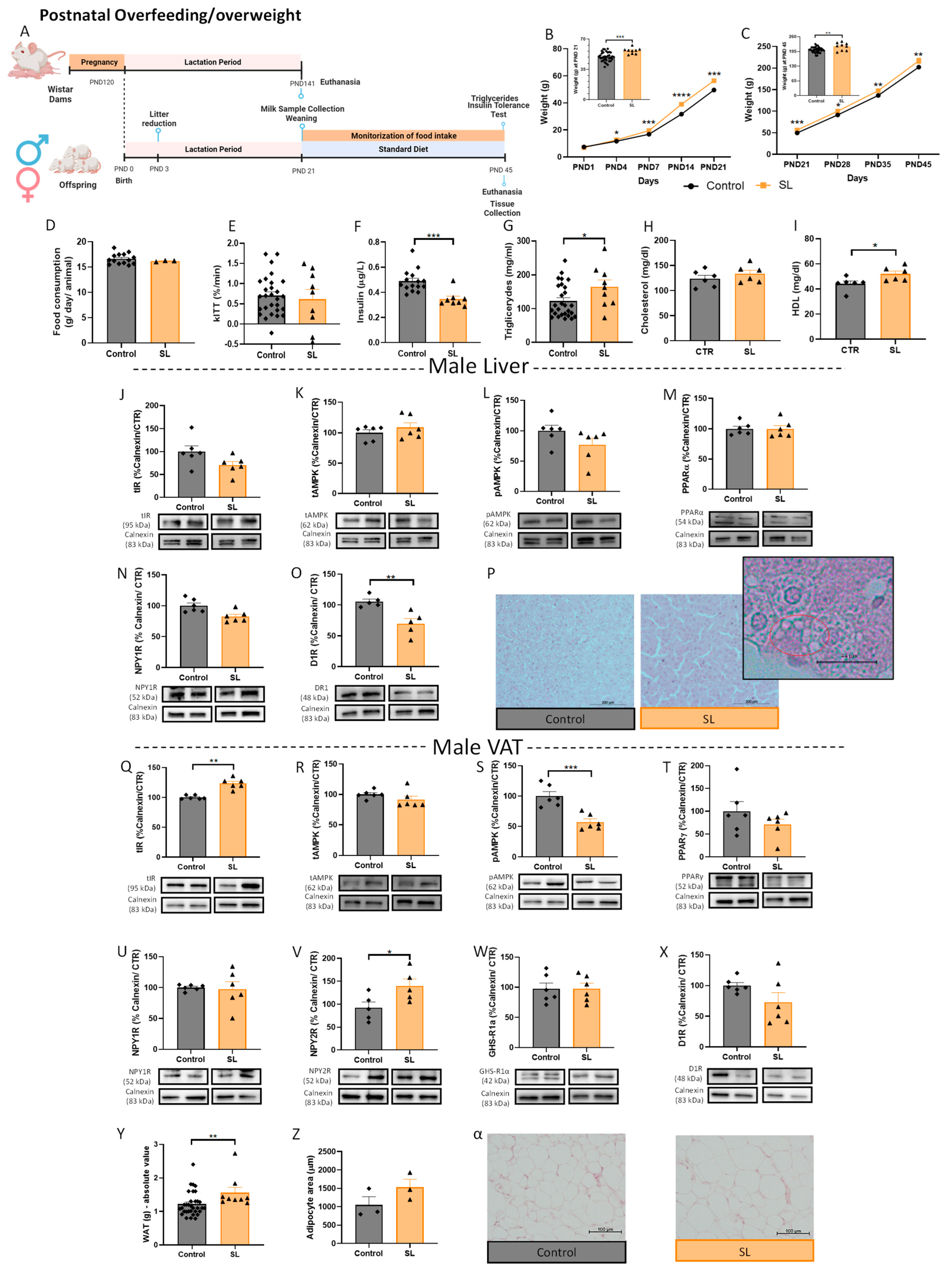
3.1.3. Postnatal Overfeeding Modulates Energy Balance Mechanisms in the VAT and Liver of Male Offspring
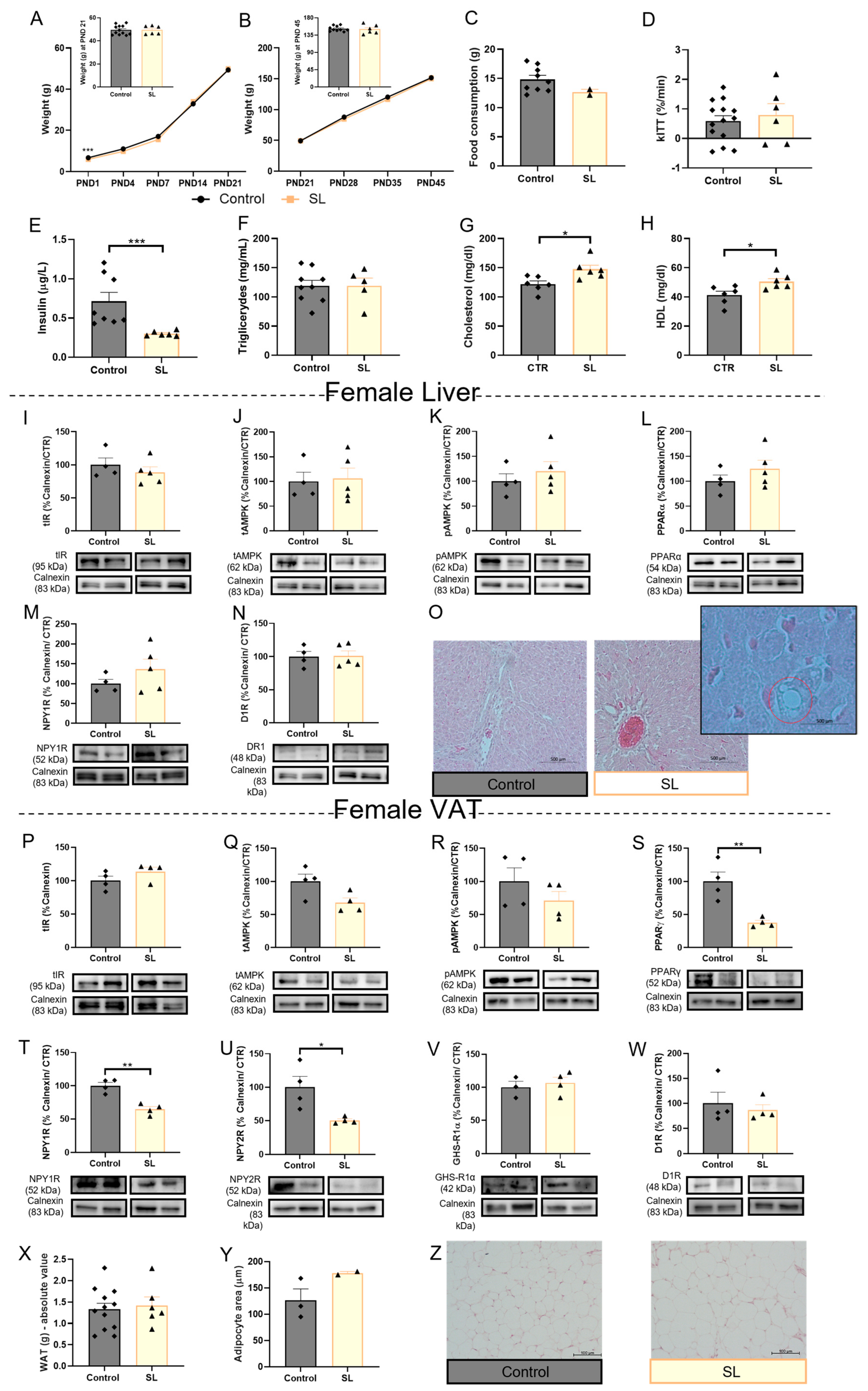
3.1.4. Female Postnatal Overfed Rats Exhibit Similar Metabolic Adaptations to Male Offspring except the Downregulation of VAT Adipogenic Mechanisms
3.2. The Role of Maternal Glycation in Impairing the Neuroendocrine Mechanism of Adaptation to Obesogenic Environments
3.2.1. Glycation Changes Breastmilk Composition
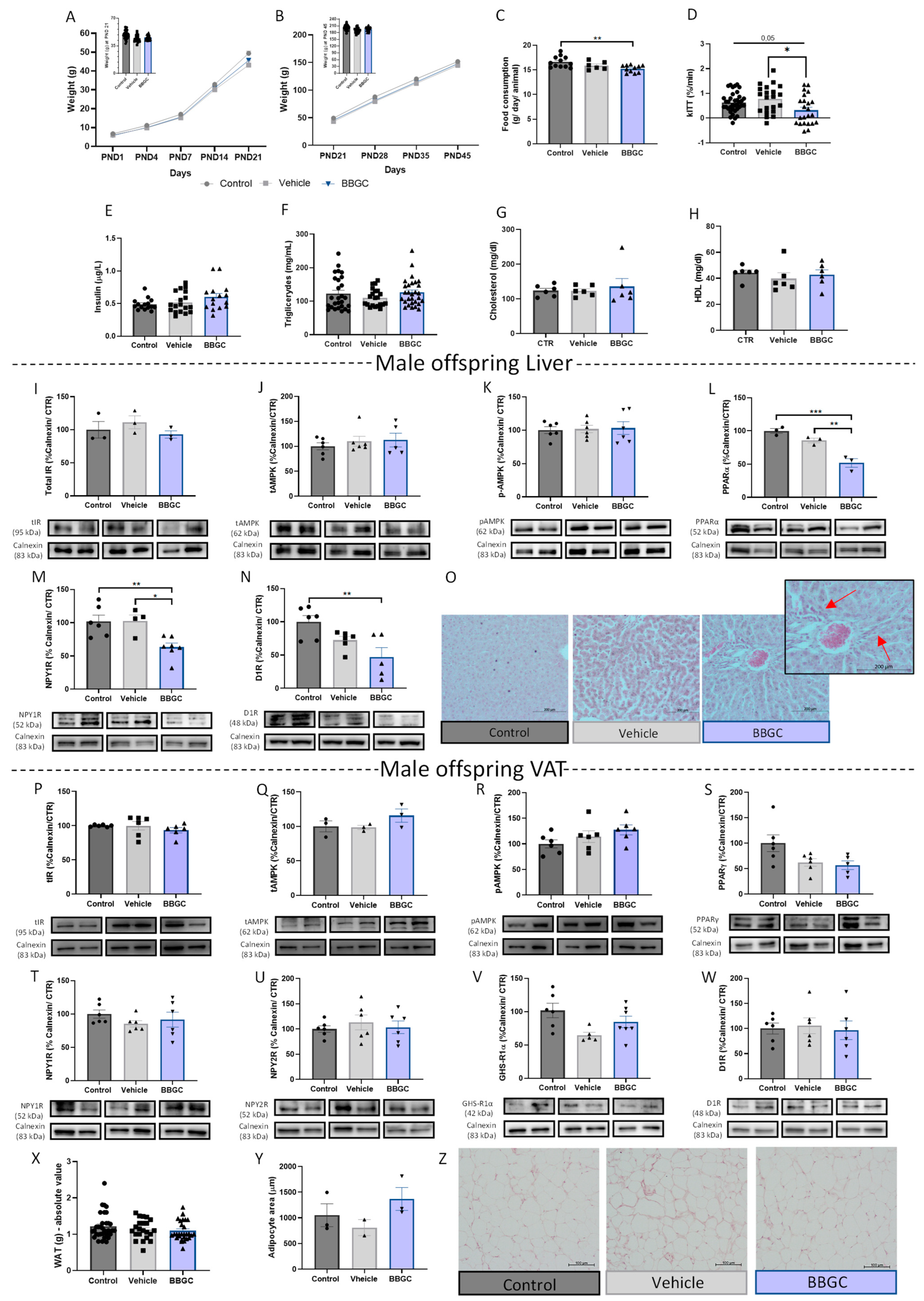
3.2.2. Exposure to Glycotoxins during Lactation Decreases Offspring Food Intake and Impairs Insulin-Dependent Glucose Uptake without Affecting the Insulin Levels of Male Offspring
3.2.3. Exposure to Maternal Glycation Reduces Both NPY and Dopamine Signalling in the Liver from Male Offspring without Affecting the VAT
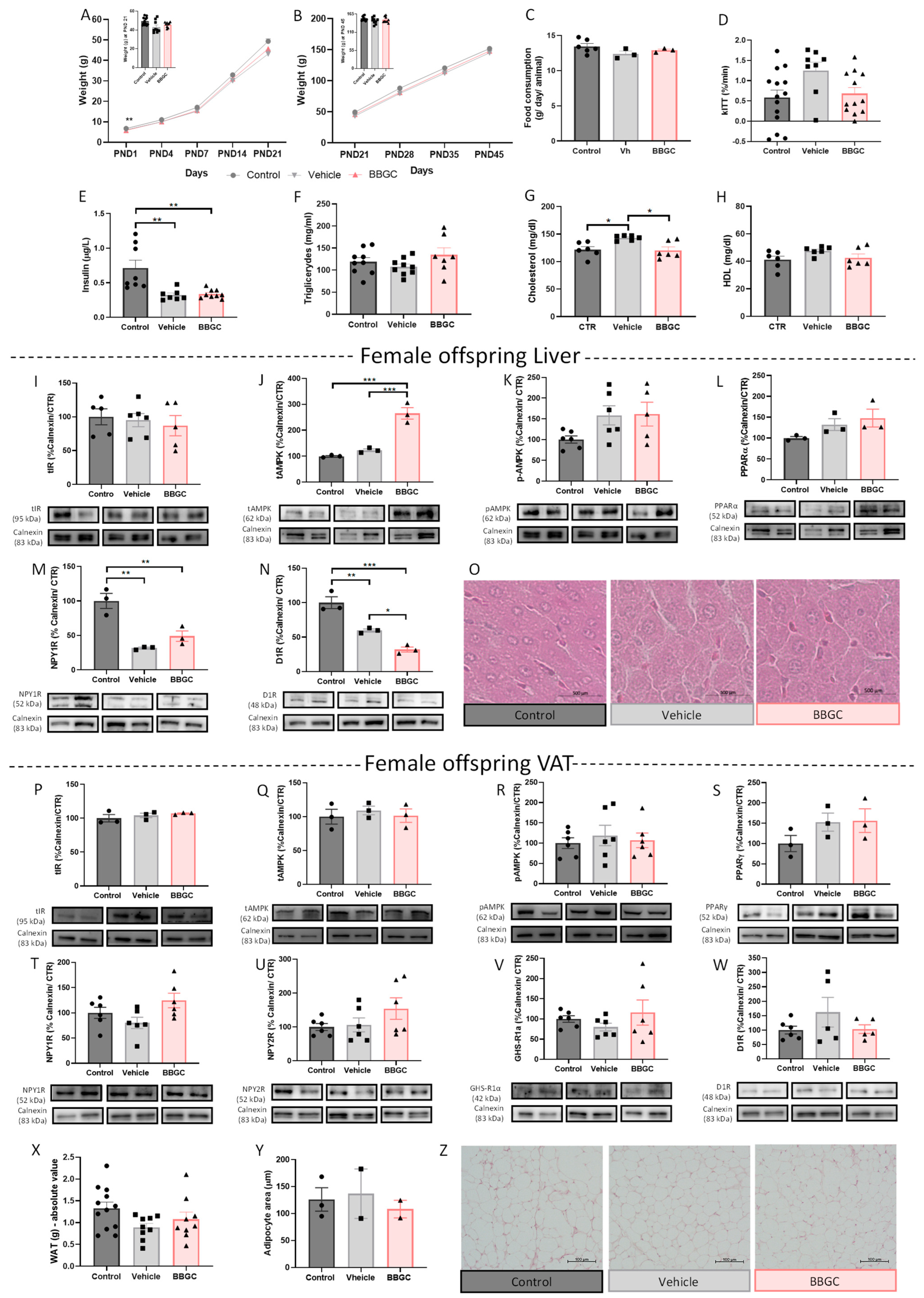
3.2.4. BBGC-Induced Maternal Glycation Does Not Affect Food intake, Metabolic Profile and VAT Mechanisms of Lipid Storage and Energy Expenditure in Female Offspring
3.2.5. Maternal Glycation Inhibits SL-Induced Weight Gain in Male and Impairs VAT Mechanisms of Adaptation to Overfeeding
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet Lond. Engl. 2014, 384, 766–781. [Google Scholar] [CrossRef]
- GBD 2015 Obesity Collaborators; Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Khanna, D.; Khanna, S.; Khanna, P.; Kahar, P.; Patel, B.M. Obesity: A Chronic Low-Grade Inflammation and Its Markers. Cureus 2022, 14, e22711. [Google Scholar] [CrossRef]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef]
- Gravina, G.; Ferrari, F.; Nebbiai, G. The obesity paradox and diabetes. Eat. Weight Disord.-Stud. Anorex. Bulim. Obes. 2021, 26, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Hudish, L.I.; Reusch, J.E.; Sussel, L. β Cell dysfunction during progression of metabolic syndrome to type 2 diabetes. J. Clin. Invest. 2019, 129, 4001–4008. [Google Scholar] [CrossRef]
- Melo, B.F.; Sacramento, J.F.; Ribeiro, M.J.; Prego, C.S.; Correia, M.C.; Coelho, J.C.; Cunha-Guimaraes, J.P.; Rodrigues, T.; Martins, I.B.; Guarino, M.P.; et al. Evaluating the Impact of Different Hypercaloric Diets on Weight Gain, Insulin Resistance, Glucose Intolerance, and its Comorbidities in Rats. Nutrients 2019, 11, 1197. [Google Scholar] [CrossRef]
- Lai, M.; Chandrasekera, P.C.; Barnard, N.D. You are what you eat, or are you? The challenges of translating high-fat-fed rodents to human obesity and diabetes. Nutr. Diabetes 2014, 4, e135. [Google Scholar] [CrossRef] [PubMed]
- Rohde, K.; Schamarek, I.; Blüher, M. Consequences of Obesity on the Sense of Taste: Taste Buds as Treatment Targets? Diabetes Metab. J. 2020, 44, 509. [Google Scholar] [CrossRef]
- Alamri, B.N.; Shin, K.; Chappe, V.; Anini, Y. The role of ghrelin in the regulation of glucose homeostasis. Horm. Mol. Biol. Clin. Investig. 2016, 26, 3–11. [Google Scholar] [CrossRef]
- Li, R.; Guan, H.; Yang, K. Neuropeptide Y potentiates beta-adrenergic stimulation of lipolysis in 3T3-L1 adipocytes. Regul. Pept. 2012, 178, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Barazzoni, R.; Zanetti, M.; Ferreira, C.; Vinci, P.; Pirulli, A.; Mucci, M.; Dore, F.; Fonda, M.; Ciocchi, B.; Cattin, L.; et al. Relationships between Desacylated and Acylated Ghrelin and Insulin Sensitivity in the Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2007, 92, 3935–3940. [Google Scholar] [CrossRef]
- Loh, K.; Shi, Y.-C.; Bensellam, M.; Lee, K.; Laybutt, D.R.; Herzog, H. Y1 receptor deficiency in β-cells leads to increased adiposity and impaired glucose metabolism. Sci. Rep. 2018, 8, 11835. [Google Scholar] [CrossRef]
- Maffei, A.; Segal, A.M.; Alvarez-Perez, J.C.; Garcia-Ocaña, A.; Harris, P.E. Anti-incretin, Anti-Proliferative action of dopamine on B-Cells. Mol. Endocrinol. 2015, 29, 542–557. [Google Scholar] [CrossRef] [PubMed]
- Bettiga, A.; Fiorio, F.; Di Marco, F.; Trevisani, F.; Romani, A.; Porrini, E.; Salonia, A.; Montorsi, F.; Vago, R. The Modern Western Diet Rich in Advanced Glycation End-Products (AGEs): An Overview of Its Impact on Obesity and Early Progression of Renal Pathology. Nutrients 2019, 11, 1748. [Google Scholar] [CrossRef]
- Matafome, P.; Rodrigues, T.; Sena, C.; Seiça, R. Methylglyoxal in Metabolic Disorders: Facts, Myths, and Promises. Med. Res. Rev. 2017, 37, 368–403. [Google Scholar] [CrossRef]
- Martinez, K.B.; Leone, V.; Chang, E.B. Western diets, gut dysbiosis, and metabolic diseases: Are they linked? Gut Microbes 2017, 8, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Varlamov, O. Western-style diet, sex steroids and metabolism. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2017, 1863, 1147–1155. [Google Scholar] [CrossRef]
- Monteiro-Alfredo, T.; Matafome, P. Gut Metabolism of Sugars: Formation of Glycotoxins and Their Intestinal Absorption. Diabetology 2022, 3, 596–605. [Google Scholar] [CrossRef]
- Rodrigues, T.; Matafome, P.; Sereno, J.; Almeida, J.; Castelhano, J.; Gamas, L.; Neves, C.; Gonçalves, S.; Carvalho, C.; Arslanagic, A.; et al. Methylglyoxal-induced glycation changes adipose tissue vascular architecture, flow and expansion, leading to insulin resistance. Sci. Rep. 2017, 7, 1698. [Google Scholar] [CrossRef]
- Rodrigues, T.; Borges, P.; Mar, L.; Marques, D.; Albano, M.; Eickhoff, H.; Carrêlo, C.; Almeida, B.; Pires, S.; Abrantes, M.; et al. GLP-1 improves adipose tissue glyoxalase activity and capillarization improving insulin sensitivity in type 2 diabetes. Pharmacol. Res. 2020, 161, 105198. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.; Matafome, P.; Santos-Silva, D.; Sena, C.; Seiça, R. Reduction of Methylglyoxal-Induced Glycation by Pyridoxamine Improves Adipose Tissue Microvascular Lesions. J. Diabetes Res. 2013, 2013, 690650. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.C.; Baldock, P.A. Central and peripheral mechanisms of the NPY system in the regulation of bone and adipose tissue. Bone 2012, 50, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Rubí, B.; Maechler, P. Minireview: New Roles for Peripheral Dopamine on Metabolic Control and Tumor Growth: Let’s Seek the Balance. Endocrinology 2010, 151, 5570–5581. [Google Scholar] [CrossRef]
- Borcherding, D.C.; Hugo, E.R.; Idelman, G.; De Silva, A.; Richtand, N.W.; Loftus, J.; Ben-Jonathan, N. Dopamine Receptors in Human Adipocytes: Expression and Functions. PLoS ONE 2011, 6, e25537. [Google Scholar] [CrossRef] [PubMed]
- Tavares, G.; Martins, F.O.; Melo, B.F.; Matafome, P.; Conde, S.V. Peripheral Dopamine Directly Acts on Insulin-Sensitive Tissues to Regulate Insulin Signaling and Metabolic Function. Front. Pharmacol. 2021, 12, 713418. [Google Scholar] [CrossRef]
- Yu, J.; Zhu, J.; Deng, J.; Shen, J.; Du, F.; Wu, X.; Chen, Y.; Li, M.; Wen, Q.; Xiao, Z.; et al. Dopamine receptor D1 signaling stimulates lipolysis and browning of white adipocytes. Biochem. Biophys. Res. Commun. 2022, 588, 83–89. [Google Scholar] [CrossRef]
- Park, S.; Fujishita, C.; Komatsu, T.; Kim, S.E.; Chiba, T.; Mori, R.; Shimokawa, I. NPY antagonism reduces adiposity and attenuates age-related imbalance of adipose tissue metabolism. FASEB J. 2014, 28, 5337–5348. [Google Scholar] [CrossRef]
- Sainsbury, A.; Schwarzer, C.; Couzens, M.; Herzog, H. Y2 Receptor Deletion Attenuates the Type 2 Diabetic Syndrome of ob/ob Mice. Diabetes 2002, 51, 3420–3427. [Google Scholar] [CrossRef]
- Yang, K.; Guan, H.; Arany, E.; Hill, D.J.; Cao, X. Neuropeptide Y is produced in visceral adipose tissue and promotes proliferation of adipocyte precursor cells via the Y1 receptor. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2008, 22, 2452–2464. [Google Scholar] [CrossRef]
- Kuo, L.E.; Kitlinska, J.B.; Tilan, J.U.; Li, L.; Baker, S.B.; Johnson, M.D.; Lee, E.W.; Burnett, M.S.; Fricke, S.T.; Kvetnansky, R.; et al. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat. Med. 2007, 13, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Andrews, Z.B.; Erion, D.M.; Beiler, R.; Choi, C.S.; Shulman, G.I.; Horvath, T.L. Uncoupling protein-2 decreases the lipogenic actions of ghrelin. Endocrinology 2010, 151, 2078–2086. [Google Scholar] [CrossRef]
- Muhammad, H.F.L. Obesity as the Sequel of Childhood Stunting: Ghrelin and GHSR Gene Polymorphism Explained. Acta Medica Indones. 2018, 50, 159–164. [Google Scholar]
- Poher, A.-L.; Tschöp, M.H.; Müller, T.D. Ghrelin regulation of glucose metabolism. Peptides 2018, 100, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Saha, P.K.; Ma, X.; Henshaw, I.O.; Shao, L.; Chang, B.H.J.; Buras, E.D.; Tong, Q.; Chan, L.; McGuinness, O.P.; et al. Ablation of ghrelin receptor reduces adiposity and improves insulin sensitivity during aging by regulating fat metabolism in white and brown adipose tissues: GHS-R ablation improves insulin resistance. Aging Cell 2011, 10, 996–1010. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.S.; Kotokorpi, P.; Eccles, S.R.; Barnes, S.K.; Tokarczuk, P.F.; Allen, S.K.; Whitworth, H.S.; Guschina, I.A.; Evans, B.A.J.; Mode, A.; et al. Ghrelin Induces Abdominal Obesity Via GHS-R-Dependent Lipid Retention. Mol. Endocrinol. 2009, 23, 914–924. [Google Scholar] [CrossRef]
- Li, Z.; Xu, G.; Qin, Y.; Zhang, C.; Tang, H.; Yin, Y.; Xiang, X.; Li, Y.; Zhao, J.; Mulholland, M.; et al. Ghrelin promotes hepatic lipogenesis by activation of mTOR-PPARγ signaling pathway. Proc. Natl. Acad. Sci. USA 2014, 111, 13163–13168. [Google Scholar] [CrossRef] [PubMed]
- Mercer, R.E.; Chee, M.J.S.; Colmers, W.F. The role of NPY in hypothalamic mediated food intake. Front. Neuroendocrinol. 2011, 32, 398–415. [Google Scholar] [CrossRef]
- Cho, Y.K.; Lee, Y.L.; Jung, C.H. Pathogenesis, Murine Models, and Clinical Implications of Metabolically Healthy Obesity. Int. J. Mol. Sci. 2022, 23, 9614. [Google Scholar] [CrossRef]
- Desai, M.; Ross, M.G. Maternal-infant nutrition and development programming of offspring appetite and obesity. Nutr. Rev. 2020, 78, 25–31. [Google Scholar] [CrossRef]
- Grilo, L.F.; Tocantins, C.; Diniz, M.S.; Gomes, R.M.; Oliveira, P.J.; Matafome, P.; Pereira, S.P. Metabolic Disease Programming: From Mitochondria to Epigenetics, Glucocorticoid Signalling and Beyond. Eur. J. Clin. Invest. 2021, 51, e13625. [Google Scholar] [CrossRef] [PubMed]
- Marousez, L.; Lesage, J.; Eberlé, D. Epigenetics: Linking Early Postnatal Nutrition to Obesity Programming? Nutrients 2019, 11, 2966. [Google Scholar] [CrossRef]
- Ehrenthal, D.B.; Maiden, K.; Rao, A.; West, D.W.; Gidding, S.S.; Bartoshesky, L.; Carterette, B.; Ross, J.; Strobino, D. Independent relation of maternal prenatal factors to early childhood obesity in the offspring. Obstet. Gynecol. 2013, 121, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, R.C. Predicting preschooler obesity at birth: The role of maternal obesity in early pregnancy. Pediatrics 2004, 114, e29–e36. [Google Scholar] [CrossRef]
- Sridhar, S.B.; Darbinian, J.; Ehrlich, S.F.; Markman, M.A.; Gunderson, E.P.; Ferrara, A.; Hedderson, M.M. Maternal gestational weight gain and offspring risk for childhood overweight or obesity. Am. J. Obstet. Gynecol. 2014, 211, 259.e1–8. [Google Scholar] [CrossRef] [PubMed]
- Habbout, A.; Li, N.; Rochette, L.; Vergely, C. Postnatal overfeeding in rodents by litter size reduction induces major short- and long-term pathophysiological consequences. J. Nutr. 2013, 143, 553–562. [Google Scholar] [CrossRef]
- Bayol, S.A.; Farrington, S.J.; Stickland, N.C. A maternal “junk food” diet in pregnancy and lactation promotes an exacerbated taste for “junk food” and a greater propensity for obesity in rat offspring. Br. J. Nutr. 2007, 98, 843–851. [Google Scholar] [CrossRef]
- Francisco, F.A.; Barella, L.F.; Silveira, S.d.S.; Saavedra, L.P.J.; Prates, K.V.; Alves, V.S.; Franco, C.C.d.S.; Miranda, R.A.; Ribeiro, T.A.; Tófolo, L.P.; et al. Methylglyoxal treatment in lactating mothers leads to type 2 diabetes phenotype in male rat offspring at adulthood. Eur. J. Nutr. 2018, 57, 477–486. [Google Scholar] [CrossRef]
- Stevanović-Silva, J.; Beleza, J.; Coxito, P.; Rocha, H.; Gaspar, T.B.; Gärtner, F.; Correia, R.; Fernandes, R.; Oliveira, P.J.; Ascensão, A.; et al. Exercise performed during pregnancy positively modulates liver metabolism and promotes mitochondrial biogenesis of female offspring in a rat model of diet-induced gestational diabetes. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166526. [Google Scholar] [CrossRef]
- Stevanović-Silva, J.; Beleza, J.; Coxito, P.; Pereira, S.; Rocha, H.; Gaspar, T.B.; Gärtner, F.; Correia, R.; Martins, M.J.; Guimarães, T.; et al. Maternal high-fat high-sucrose diet and gestational exercise modulate hepatic fat accumulation and liver mitochondrial respiratory capacity in mothers and male offspring. Metabolism. 2021, 116, 154704. [Google Scholar] [CrossRef]
- Lutz, T.A. Considering our methods: Methodological issues with rodent models of appetite and obesity research. Physiol. Behav. 2018, 192, 182–187. [Google Scholar] [CrossRef]
- Matafome, P. Another Player in the Field: Involvement of Glycotoxins and Glycosative Stress in Insulin Secretion and Resistance. Diabetology 2020, 1, 24–36. [Google Scholar] [CrossRef]
- Neves, C.; Rodrigues, T.; Sereno, J.; Simões, C.; Castelhano, J.; Gonçalves, J.; Bento, G.; Gonçalves, S.; Seiça, R.; Domingues, M.R.; et al. Dietary Glycotoxins Impair Hepatic Lipidemic Profile in Diet-Induced Obese Rats Causing Hepatic Oxidative Stress and Insulin Resistance. Oxid. Med. Cell. Longev. 2019, 2019, 6362910. [Google Scholar] [CrossRef] [PubMed]
- Tavares, G.; Marques, D.; Barra, C.; Rosendo-Silva, D.; Costa, A.; Rodrigues, T.; Gasparini, P.; Melo, B.F.; Sacramento, J.F.; Seiça, R.; et al. Dopamine D2 receptor agonist, bromocriptine, remodels adipose tissue dopaminergic signalling and upregulates catabolic pathways, improving metabolic profile in type 2 diabetes. Mol. Metab. 2021, 51, 101241. [Google Scholar] [CrossRef]
- Zhang, L.; Macia, L.; Turner, N.; Enriquez, R.F.; Riepler, S.J.; Nguyen, A.D.; Lin, S.; Lee, N.J.; Shi, Y.C.; Yulyaningsih, E.; et al. Peripheral neuropeptide Y Y1 receptors regulate lipid oxidation and fat accretion. Int. J. Obes. 2010, 34, 357–373. [Google Scholar] [CrossRef] [PubMed]
- Lustig, R.H.; Collier, D.; Kassotis, C.; Roepke, T.A.; Kim, M.J.; Blanc, E.; Barouki, R.; Bansal, A.; Cave, M.C.; Chatterjee, S.; et al. Obesity I: Overview and molecular and biochemical mechanisms. Biochem. Pharmacol. 2022, 199, 115012. [Google Scholar] [CrossRef]
- Chang, E.; Varghese, M.; Singer, K. Gender and Sex Differences in Adipose Tissue. Curr. Diab. Rep. 2018, 18, 69. [Google Scholar] [CrossRef] [PubMed]
- Björntorp, P. How should obesity be defined? J. Intern. Med. 1990, 227, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Waterland, R.A.; Garza, C. Early Postnatal Nutrition Determines Adult Pancreatic Glucose-Responsive Insulin Secretion and Islet Gene Expression in Rats. J. Nutr. 2002, 132, 357–364. [Google Scholar] [CrossRef]
- Wang, Y.; Nakajima, T.; Gonzalez, F.J.; Tanaka, N. PPARs as Metabolic Regulators in the Liver: Lessons from Liver-Specific PPAR-Null Mice. Int. J. Mol. Sci. 2020, 21, 2061. [Google Scholar] [CrossRef]
- Qin, Y.-Y.; Huang, X.-R.; Zhang, J.; Wu, W.; Chen, J.; Wan, S.; Yu, X.-Y.; Lan, H.-Y. Neuropeptide Y attenuates cardiac remodeling and deterioration of function following myocardial infarction. Mol. Ther. 2022, 30, 881–897. [Google Scholar] [CrossRef]
- Tan, R.-Z.; Li, J.-C.; Zhu, B.-W.; Huang, X.-R.; Wang, H.-L.; Jia, J.; Zhong, X.; Liu, J.; Wang, L.; Lan, H.-Y. Neuropeptide Y protects kidney from acute kidney injury by inactivating M1 macrophages via the Y1R-NF-κB-Mincle-dependent mechanism. Int. J. Biol. Sci. 2023, 19, 521–536. [Google Scholar] [CrossRef] [PubMed]
- Leppert, B.; Junge, K.M.; Röder, S.; Borte, M.; Stangl, G.I.; Wright, R.J.; Hilbert, A.; Lehmann, I.; Trump, S. Early maternal perceived stress and children’s BMI: Longitudinal impact and influencing factors. BMC Public Health 2018, 18, 1211. [Google Scholar] [CrossRef] [PubMed]
- Jašarević, E.; Howard, C.D.; Misic, A.M.; Beiting, D.P.; Bale, T.L. Stress during pregnancy alters temporal and spatial dynamics of the maternal and offspring microbiome in a sex-specific manner. Sci. Rep. 2017, 7, 44182. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Hatlebakk, J.G.; Hausken, T. Diet in Irritable Bowel Syndrome (IBS): Interaction with Gut Microbiota and Gut Hormones. Nutrients 2019, 11, 1824. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, D.; Rocha, M.; Amaro, A.; Ferreira-Junior, M.D.; Cavalcante, K.V.N.; Monteiro-Alfredo, T.; Barra, C.; Rosendo-Silva, D.; Saavedra, L.P.J.; Magalhães, J.; et al. Exposure to Obesogenic Environments during Perinatal Development Modulates Offspring Energy Balance Pathways in Adipose Tissue and Liver of Rodent Models. Nutrients 2023, 15, 1281. https://doi.org/10.3390/nu15051281
Sousa D, Rocha M, Amaro A, Ferreira-Junior MD, Cavalcante KVN, Monteiro-Alfredo T, Barra C, Rosendo-Silva D, Saavedra LPJ, Magalhães J, et al. Exposure to Obesogenic Environments during Perinatal Development Modulates Offspring Energy Balance Pathways in Adipose Tissue and Liver of Rodent Models. Nutrients. 2023; 15(5):1281. https://doi.org/10.3390/nu15051281
Chicago/Turabian StyleSousa, Diana, Mariana Rocha, Andreia Amaro, Marcos Divino Ferreira-Junior, Keilah Valéria Naves Cavalcante, Tamaeh Monteiro-Alfredo, Cátia Barra, Daniela Rosendo-Silva, Lucas Paulo Jacinto Saavedra, José Magalhães, and et al. 2023. "Exposure to Obesogenic Environments during Perinatal Development Modulates Offspring Energy Balance Pathways in Adipose Tissue and Liver of Rodent Models" Nutrients 15, no. 5: 1281. https://doi.org/10.3390/nu15051281
APA StyleSousa, D., Rocha, M., Amaro, A., Ferreira-Junior, M. D., Cavalcante, K. V. N., Monteiro-Alfredo, T., Barra, C., Rosendo-Silva, D., Saavedra, L. P. J., Magalhães, J., Caseiro, A., Freitas Mathias, P. C. d., Pereira, S. P., Oliveira, P. J., Gomes, R. M., & Matafome, P. (2023). Exposure to Obesogenic Environments during Perinatal Development Modulates Offspring Energy Balance Pathways in Adipose Tissue and Liver of Rodent Models. Nutrients, 15(5), 1281. https://doi.org/10.3390/nu15051281














