Abstract
Among all tree nuts, walnuts contain the highest total polyphenols by weight. This secondary data analysis examined the effect of daily walnut supplementation on the total dietary polyphenols and subclasses and the urinary excretion of total polyphenols in a free-living elderly population. In this 2-year prospective, randomized intervention trial (ID NCT01634841), the dietary polyphenol intake of participants who added walnuts daily to their diets at 15% of daily energy were compared to those in the control group that consumed a walnut-free diet. Dietary polyphenols and subclasses were estimated from 24 h dietary recalls. Phenolic estimates were derived from Phenol-Explorer database version 3.6. Participants in the walnut group compared to the control group had a higher intake of total polyphenols, flavonoids, flavanols, and phenolic acids in mg/d (IQR): 2480 (1955, 3145) vs. 1897 (1369, 2496); 56 (42,84) vs. 29 (15, 54); 174 (90, 298) vs. 140 (61, 277); and 368 (246, 569) vs. 242 (89, 398), respectively. There was a significant inverse association between dietary flavonoid intake and urine polyphenol excretion; less urinary excretion may imply that some of the polyphenols were eliminated via the gut. Nuts had a significant contribution to the total polyphenols in the diet, suggesting that a single food like walnuts added to habitual diet can increase the polyphenol intake in a Western population.
1. Introduction
It is well-recognized that walnuts have a favorable nutrient and fatty acid profile, and their consumption is effective in reducing blood lipids [1,2] and in modifying inflammation and endothelial dysfunction [3,4,5], thus reducing the risk of cardiovascular disease [2,6]. The risk lowering effects of walnuts as demonstrated by supplementing diets with the nuts, are greater than predicted based on the amount and nature of the fat consumed [7]. Evidence suggests that the phenolic phytochemicals found in walnuts and other nuts increase antioxidant defenses and reduce inflammation [7]. A recent review and meta-analysis summarizing the findings from several randomized controlled trials showed that incorporating polyphenol-rich foods impacts blood lipids by increasing high-density lipoprotein (HDL) and lowering low-density lipoprotein (LDL) [8]. Diets rich in polyphenols such as the Mediterranean diet, emphasizing olive oil and walnuts, report decreased blood lipids and inflammatory markers [6,9].
Walnuts are composed of an outer green husk with a hard shell inside the husk containing a walnut kernel covered with a seed coat or pellicle, where most polyphenols reside [10,11]. Among the nuts, walnuts contain the highest concentrations of polyphenols, averaging 2500 gallic acid equivalent (GAE) per 100 g [12]. Current comprehensive analyses of walnut polyphenols using chromatographic, and mass spectrometric techniques have identified hundreds of compounds in the walnut kernel including hydrolysable and condensed tannins, flavonoids, flavanols, phenolic acids, and lignans [13,14]. A study investigated the postprandial effect of walnut intake on the plasma total polyphenols and showed increased concentrations of plasma polyphenols 30 min following ingestion, which reached a peak at 90 min [1]. Therefore, walnuts may contribute to dietary polyphenols and may offer protective health benefits.
Several studies have recently explored dietary compositional changes produced by adding walnuts to the diet. In a randomized parallel design intervention, participants at risk for type 2 diabetes who added walnuts to their habitual diet increased their energy, protein, total fat, and magnesium intake [15] and had a non-significant decrease in sodium, empty calories, and dairy products [16]. In similar studies, individuals randomized to the walnut group showed higher intakes of protein, polyunsaturated fatty acids, both omega-3 and omega-6, but lower intakes of carbohydrates, animal protein and saturated fatty acids [17], and, in a cross-over study, participants who consumed walnuts additionally increased their dietary fiber, calcium, phosphorus, magnesium, and zinc intake [18]. However, no studies have as yet investigated whether the inclusion of walnuts in the diet influences the dietary intake of polyphenols or urinary polyphenol excretion. In this secondary data analyses of the Walnuts and Healthy Aging study (WAHA) [19], we investigated the impact of consuming walnuts on the dietary intake of total polyphenols and their sub-classes (flavonoids, flavanols, and lignans), and on the total urinary polyphenol excretion. Therefore, the aim of the current secondary analysis of data from the WAHA study was to determine whether long-term inclusion of walnuts (Juglans regia L.) in the daily diet increases polyphenol intake and the urinary excretion of phenolic metabolites.
2. Materials and Methods
2.1. Study Design and Participants
The WAHA study was a 2-year parallel group, observer-blinded randomized controlled trial (RCT) examining the effect of the usual diet supplemented with walnuts at 15% (30–60 g/d) of energy compared to a walnut free habitual diet on the aging outcomes in elderly participants [17,20,21,22,23,24,25,26,27]. The parent study was a dual center clinical trial and was carried out in Barcelona, Spain and at Loma Linda University (LLU) in California, USA, from 2014 to 2016. However, only data collected from participants at LLU were used in the current secondary data analyses. The WAHA study was conducted in accordance with the guidelines of the Declaration of Helsinki and was approved by the Ethics Committee of the Loma Linda University Institutional Review Board (IRB 5120066). All participants provided their written informed consent before enrolment. The WAHA study clinical trial (NCT01634841) is registered at www.clinicaltrials.gov (accessed on 23 February 2023).
Detailed information about the WAHA study has been published elsewhere [19,26]. Briefly, candidates for this study were elderly ambulatory men and women aged 63–79 years. Exclusion criteria were inability to undergo neuropsychological testing; previously diagnosed neurodegenerative disease; prior stroke, significant head trauma, or brain surgery; relevant psychiatric illness; major depression; morbid obesity; uncontrolled diabetes; uncontrolled hypertension; prior chemotherapy; allergy to walnuts; habitual consumption of tree nuts (>2 servings/week); or customary use of fish oil, flaxseed oil, and/or soy lecithin. A total of 656 subjects were recruited and assessed for eligibility by the LLU team and 356 met the eligibility criteria and were randomized into the study. Of the total sample of randomized participants, 300 were selected for the current analysis, as shown in the flowchart in Figure 1.
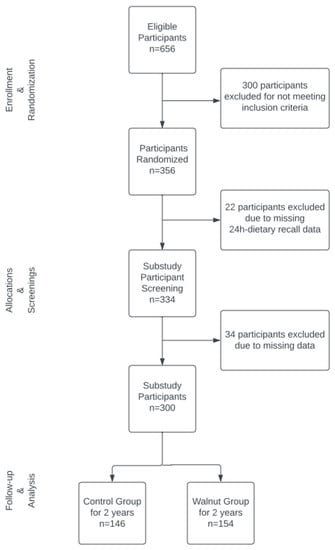
Figure 1.
Participant flowchart at LLU site.
2.2. Sociodemographic, Anthropometric, and Biochemical Outcomes
Demographic data, anthropometric measurements, and dietary and lifestyle habits were collected from the participants at the baseline according to the study protocols [19]. Anthropometric measurements were carried out by trained professionals, and sociodemographic data and lifestyle habits were inputted using self-reported study questionnaires. Blood and spot urine samples were collected at the baseline and at the end of each year of intervention, aliquoted, and stored at −80 °C until analysis. All routine biochemical analyses and the determination of urinary creatinine concentration were performed at the completion of the study in the same laboratory to control for between-assay variability, as previously reported [20].
2.3. Estimation of Dietary Nutrient and Polyphenol Intake
Collection of dietary recall data and nutrient analysis was performed using the Nutrition Data System for Research (software version 2018) developed by the Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN. The 24-h dietary recalls were obtained by telephone or face-to-face interviews using a multiple-pass approach to capture information about the food items, beverages, and dietary supplements consumed during the past 24 h and the nutrient estimates were acquired using the systems’ nutrient database. A total of five unannounced dietary recalls per participant were obtained at random times during the study, and these included at least one weekend day. The dietary intake data were collected by trained research dietitians and conducted at diverse intervals over the 2 year study duration to account for seasonal variations of food intake [17].
The polyphenol content of foods and beverages reported in the 24-h recalls were generated from the Phenol-Explorer database (version 3.6) [28]. The Phenol-Explorer database compiles the total polyphenol content of foods based on analyses performed using the Folin–Ciocalteu (F–C) reagent, whereas chromatography methods are used to estimate polyphenol subclasses. In the current study, the following variables were estimated based on data from Phenol-Explorer: the subclass total flavonoids consisted of flavones, flavonols plus anthocyanins, and the subclass phenolic acids were phenolics obtained either by chromatography or by chromatography after hydrolysis; the subclass flavanols were obtained by chromatography or normal phase HPLC; and the subclass lignans was obtained by chromatography after hydrolysis.
The contribution of food items to the total polyphenols, flavonoids, flavanols, phenolic acids, and lignans were entered into the dietary database in milligrams per 100 g per day. Food items found in the 24-h dietary recalls (24-HDR) and food composition data were matched and the intake of total dietary polyphenols and phenol subclasses was estimated using the following equation: 24-HDRs = Σ Pn × Gn. Here, p is the mg of phenolic compound per 100 g food, and G is the reported portion size of food in grams.
2.4. Urinary Total Polyphenols
Spot urine samples [29] were collected from the WAHA participants at the baseline and at the end of the first and second years of the study. Spot urine samples were collected in the morning at the time participants came in for their fasting blood draw but were not the first void. Samples were processed and stored at −80 °C until use. The total urinary polyphenol concentrations in the spot urine samples were determined using the modified rapid Folin–Ciocalteu (F–C) method, as previously described [30]. Briefly, following solid phase extraction for the removal of interfering substances using Oasis Max cartridges (Waters Corp. Milford, MA, USA), the samples were loaded on 96-well plates (Waters Corp., Milford, MA, USA) for testing using the Folin–Ciocalteu reagent. The Bio Tek Synergy HT spectrometer (Bio Tek, Winooski, VT, USA) was used to measure the resulting absorbance at 765 nm. All analyses were run in triplicate using gallic acid as the standard. Urine creatinine was determined using the Jaffe’ alkaline picrate microplate method as published [30].
2.5. Statistical Analyses
From the LLU cohort, a total of n = 356 subjects were randomized, but only 300 subjects were included in this secondary data analysis. A total of 34 subjects were excluded due to missing data. Dietary polyphenol variables of total polyphenols, total flavonoids, flavanols, phenolic acids, and lignans were energy-adjusted using the residual method and then averaged for each subject. Spot urine polyphenol concentrations in mg GAE/L were adjusted by creatinine concentration to account for urine dilution. Mann–Whitney tests were used for these variables for between-group comparisons. Means and standard deviations (SD) of polyphenol intake by food group were reported.
In the descriptive analysis of urinary polyphenols, the means (SD) by treatment and time were determined. To compare the morning spot urine polyphenol excretion between treatment groups, linear regression mixed models fitted for both variables (mg GAE/L, mg GAE/g Cr) included the treatment, time, treatment × time interaction, age, gender, and BMI as fixed-effects terms and the participants as a random-effects term. To examine the association between spot urine polyphenol excretion at year 2 and the dietary intake of polyphenols and subclasses, a linear regression model was fitted for each combination of urine polyphenol (dependent variable) and log dietary polyphenol (independent variable), while adjusting for age, gender, and BMI. All analyses were performed using R version 4.2.2 with a significance level at p < 0.05.
3. Results
The subject characteristics as observed in Table 1 show that there were more women than men enrolled in the study. In the walnut group, 63% were women and 37% were men, and in the control group, 68% were women and 32% were men.

Table 1.
The subject characteristics of the study by treatment group (n = 300).
Table 2 describes the mean dietary intake of macronutrients by the treatment group over a 2-year period. A total of 1242 sessions were held to collect 24-h dietary recalls from the -participants. A total of five 24-h dietary recalls were collected from most participants (range 1–5 recalls) at random times during the duration of the study. The walnut group had a significantly higher energy intake, total dietary fiber, and total fat intake compared to the control group.

Table 2.
Dietary intake of macronutrients per day by treatment group.
Table 3 describes the mean dietary intake of phenolics by treatment group for a 2 year period. Compared to the control group, participants in the walnut group had a significantly higher mean intake of total polyphenols, flavonoids, flavanols, and phenolic acids in mg/d. There were no significant differences in lignan intake.

Table 3.
Daily intake of energy-adjusted dietary polyphenols 1 by treatment group.
Table 4 shows the contribution of the various food groups to the daily intake of total polyphenols and phenolic subclasses by treatment group. Of the food groups consumed, the mean intake of nuts showed that walnuts significantly (p < 0.001) contributed to the total polyphenol intake in the walnut group in mg/d 632 (182) compared to the control 40 (7). Results of the polyphenol intake by food group also showed that nuts were a significant contributor to all other major subclasses including flavonoids (flavones, flavonols, and anthocyanidins, with the exception of lignan (p = 0.513) compared to the other food categories.

Table 4.
Contribution of food groups by treatment group to the mean daily intake in mg/d of the total polyphenols and polyphenol subclasses.
Table 5 shows the comparison of urinary polyphenol excretion between the control and walnut groups at the baseline and at the end of years 1 and 2. Urinary polyphenols and creatinine were measured in the spot urine samples obtained from the participants at the same clinic visit when the fasting blood samples were drawn. From the baseline, the excretion of polyphenols in the walnut group in the first year approached significance at a 0.066 p value, but not in the second year or when the values were adjusted for urinary creatinine excretion. The values were similar at the baseline and years 1 and 2 in the control group. The results show that there were no significant differences between the intervention groups at any time point.

Table 5.
Comparison of the morning spot urine polyphenol excretion between treatment groups 1.
Table 6 describes the association between dietary and urinary polyphenols in year 2. Results of the linear models showed that there was a significantly negative association between the total urinary polyphenols and the log of total dietary flavonoids (p = 0.0316). There were no significant associations with any other dietary polyphenols.

Table 6.
Association between the spot urine polyphenol excretion at year 2 and the dietary intake of polyphenols and subclasses 1.
4. Discussion
In this sub-study of the WAHA trial, we showed that the daily ingestion of walnuts for 2 years significantly increased the total dietary polyphenols and the subclasses of flavonoids, flavanols and phenolic acids in healthy elderly participants. To our knowledge, this is the first study to show that the inclusion of a single food (i.e., walnuts), with no other changes made to the usual diet, could significantly increase the total polyphenol intake. As expected, those who ate walnuts daily also showed higher intakes of energy, fiber, total fat, and unsaturated fatty acids.
The results of this trial also show that participants in the walnut group consumed significantly higher amounts of total polyphenols and flavonoids (flavones, flavonols, and anthocyanidins), flavanols, and phenolic acids from nuts compared to those in the control group. This finding demonstrates that a single food such as walnuts can increase the intakes of total polyphenols and the polyphenol subclasses except for lignans. The walnut group had a higher intake of total polyphenols from fruits, and the flavanols and lignans from vegetables. The median daily intake of the total polyphenols of the control and walnut groups at 1897 mg/d and 2480 mg/d, respectively, of this elderly cohort residing in California was higher compared to that reported in adults in the U.S. by the National Health and Nutrition Examination Survey (NHANES) at 884 mg per 1000 kcal per day [31]. Like the current study, beverages such as tea, coffee, red wine, and fruit juices, vegetables, and fruits were the main contributors to the total polyphenols, flavonoids, and phenolic acids by NHANES [31] and through a recent systematic review of 91 studies from multiple countries [32]. A study [33] that examined the intake of dietary polyphenols by vegetarian status showed that a coffee intake, being a single food item, was the number one contributor to phenolic intake. Given that the WAHA intervention participants consumed walnuts at ~15% of their energy intake, the total polyphenol content of 2431.52 per 100 g walnuts would have added polyphenols to their diet [4].
While the walnut group showed a higher daily intake of polyphenols, this was not reflected in the urinary excretion of polyphenols tested in the spot urine samples obtained at the baseline, and at the end of either year 1 or year 2. Increased polyphenol metabolites have been identified in urine following the consumption of plant-based foods, suggesting that selected urinary polyphenols could be useful biomarkers to assess the intakes of polyphenol-rich foods and diets [34,35]. Most bioavailable dietary polyphenols have a relatively short half-life, estimated at 1 to 24 h following intake, and studies quantifying polyphenol biomarkers in urine have used 24-h urine collections following the consumption of test foods or diets [36,37,38]. Studies that have used 24-h urine samples were able to capture a wide range of polyphenols and positive relationships between dietary intake and urinary excretion of polyphenols [39,40]. One can conclude that the morning spot void used in our study could have resulted in poor collection of most polyphenols excreted in the urine over a 24-h period. However, similar studies found an increase in the concentration of phenolics in the spot morning urine following the intake of polyphenol-rich foods such fruits and vegetables [41,42]. Therefore, it is unclear why the daily inclusion of walnuts in the diet did not result in consistent increases in the concentration of polyphenols in our fasting spot urine samples collected following the first morning void. It is important to note that the rapid Folin–Ciocalteu (F–C) assay with solid-phase extraction optimized by Medina-Remón et al. [43] was used in this study to determine the total polyphenols in urine was validated in the spot urine samples collected from individuals consuming fruits, vegetables, tea, and red wine [44], and it has not been validated using walnuts.
In relation to the total polyphenol concentrations in the spot urine samples, our analyses did not show associations either with the dietary total polyphenols or flavanols and phenolic acid subclasses, but disclosed an inverse association with an intake of the flavonoid (flavanones + flavones) category. Studies in which the total urine polyphenols were measured with the F–C assay have shown weak to moderate associations with dietary polyphenol intakes in the 24-h [39], 12-h overnight [33], and morning urine sample collections [43]. In adults prescribed a high vegetable and fruit diet, the fasting spot urine samples collected after the first morning void and tested using liquid chromatography-mass spectroscopy disclosed an inconsistent association between the total urinary polyphenols and total polyphenol intake, while the linear mixed model analysis showed a non-significant inverse association between the total urine polyphenols and polyphenols from fruit [41]. The inverse correlation with fruit ingestion is consistent with our findings, since fruits are rich sources of the flavanone and flavone subclasses. It has been hypothesized that inverse correlations may be due to components of the food matrix that inhibit the intestinal absorption and urinary excretion of polyphenols [45,46]. The observed lack of associations or inverse associations may also be explained by the short half-life of bioavailable polyphenols and their metabolites, which may be absorbed and excreted within a short time period following intake [38,47]. Future studies should utilize 24-h urine collection following the ingestion of walnuts as the best method of capturing the majority of polyphenols. Additionally, reduced urinary excretion may imply that some of the polyphenols were eliminated via the gut, an effect likely to have a favorable impact on the intestinal microbiome [48,49,50,51,52,53].
The quality of phenolic compounds present in walnuts is diverse, ranging from simple phenolic acids and flavonoids to highly polymerized molecules such as tannins. Walnut phenolics are usually found at the highest concentration in the seed coat (also called the pellicle) surrounding the edible kernel and may be bound to other plant components such as carbohydrates and proteins. Consequently, some polyphenolic compounds might not be released in compositional testing studies. Walnuts are distinguished by the predominance of the hydrolysable gallotannins, glansrins (ellagitanins), and ellagic acid and the condensed tannins that are polymers of flavan-3-ol (flavanol) catechin and epicatechin subunits. The Polyphenol-Explorer 3.6 database reports an average amount of total polyphenol assayed by the F–C reagent as 1575 mg/100 g of kernel. Average amounts per 100 g of the total flavonoids, flavanols, and phenolic acids in walnuts are reported as 65 mg, 60 mg, and 449 mg, respectively [28,54,55,56,57].
Aside from the wide diversity and complexity of the phenolic substances found in walnuts, a number of other factors complicate efforts to obtain the exact accounts of their polyphenol composition. The concentration of phenolic compounds from different genomic walnut species and cultivars have been found to vary widely, with mean coefficients of variation of 25% or greater [58]. In addition, the climate, soil characteristics, agricultural practices, storage, and manipulation influence the phenolic content of the nuts [58,59]. Studies have shown substantial differences in the composition depending on the solvent or method (maceration, sonication) employed to extract phenols from walnuts [60]. The results are also influenced by whether the walnut kernel is raw, mildly heated or roasted, or whether it is defatted prior to extraction [4]. Current liquid chromatography techniques coupled with high resolution mass spectrometry and electrospray ionization tandem mass spectrometry have successfully been employed to identify and quantify phenolic compounds in walnuts found in soluble free, soluble esters, or conjugated and insoluble bound forms, thus providing more inclusive phenolic profiles than those reported by Phenol-Explorer [14,61].
It is important to note that the concentration of polyphenol in urine is determined by factors beyond the walnut phenol content and its structural matrix, but is related to human physiology, mainly sex and age, along with factors such as digestive and metabolic efficiency and the gut microbiota [62]. Depending on their structural complexity and solubility, it has been estimated that only 5–10% of the total dietary polyphenols reaching the small intestine are absorbed, with the maximum plasma concentrations attained at 30 min following ingestion. Urinary excretion generally peaks after about 8 h of intake [38,62,63]. Unabsorbed polyphenols reach the large intestine, where they undergo enzymatic action by the microbiota to produce a variety of metabolites. One of the major categories of phenolic compounds in walnuts are ellagitannins [64], which are hydrolyzed to produce ellagic acid and further acted upon by the gut microbiota to produce a series of metabolites known as urolithins [65]. Urolithins are better absorbed than ellagitannins and are thus transported to peripheral tissues or excreted through the urine [5,38,66]. Studies have shown urolithins to be valid biomarkers of walnut consumption [63,67,68] with higher concentrations of the metabolite found 12 h or longer following walnut ingestion [69].
Our study has many strengths. The WAHA study has a significantly long duration of intervention (2 years). The study also includes a relatively large number of participants who demonstrated excellent compliance, with a retention rate of 90%. Moreover, the dietary intake data were extensive, having been acquired using multiple 24-h recalls (up to five recalls) obtained throughout the 2 year period and carefully matched with Phenol-Explorer values to obtain a profile of its polyphenol content.
The main limitation was that the urine samples were obtained from fasting participants following the first morning void, and as such, may not have captured a large enough quantity and diversity of phenolic metabolites excreted in a 24-h urine or in a longer collection period. It is well-known that the half-life of most polyphenol metabolites is relatively short and typically appear in urine within 1 to 24 h following ingestion [36]. Some phenol metabolites produced by microbiota such as urolithins may not be detected or quantified by the F–C reagent assay used in this study. A potential limitation is that despite an open recruitment policy, our study participants included a higher proportion of females than males. Additionally, the diets of the participants in the walnut group showed a higher mean energy and fat intake than the habitual diet group, which was partially mitigated through energy adjustment.
5. Conclusions
A single food such as walnuts eaten daily can increase dietary polyphenol intake. This is important as we now know that polyphenols have significant health benefits, being powerful anti-inflammatory and antioxidant phytochemicals. To reduce the risk for age-related chronic diseases, it may be prudent to include nuts such as walnuts as part of the usual diet to not only benefit from the unsaturated fatty acids and other nutrients that have CVD and neuroprotective effects, but also increase the polyphenol intake, which can synergistically influence the disease risk in a favorable manner.
Author Contributions
Conceptualization, R.I.A., S.R., J.S. and E.R.; Methodology, R.I.A. and R.S.; Validation, R.S.; Formal analysis, K.O.; Writing—original draft preparation, R.I.A.; Writing—review and editing, R.I.A. and E.H.H.; Supervision, I.N., S.R. and R.S.; Project administration, R.S., I.N., M.C. and J.S.; Funding acquisition, J.S. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by the California Walnut Commission, Folsom, CA, USA. The funding agency had no role in the study design, data collection, analyses, interpretation, or in writing this article. AS-V is recipient of the ISCIII Miguel Servet I fellowship (CP12/03299) and Fondo de Investigación Sanitaria grant—FEDER funds (PI15/01014).
Institutional Review Board Statement
The study protocol was conducted in accordance with the guidelines of the Declaration of Helsinki and approved by the institutional review boards of all centers.
Informed Consent Statement
Informed consent was previously obtained from all subjects involved in the study.
Data Availability Statement
Detailed data relating to the study are available upon request from the corresponding author.
Acknowledgments
The author would like to thank Nabil Razzouk for his encouragement and support, Andrew Mashchak for his excellent technical support of dietary data management, and Aman Kur for the management support.
Conflicts of Interest
Rajaram, Sabaté, and Ros received research funding through their institutions from the California Walnut Commission. Sabaté and Ros were nonpaid members of the Scientific Advisory Council of the CWC. Dr Ros was a paid member of the CWC Health Research Advisory Group and has received personal money from the CWC for presentations. All other authors declare no conflicts of interest.
References
- Torabian, S.; Haddad, E.; Rajaram, S.; Banta, J.; Sabate, J. Acute effect of nut consumption on plasma total polyphenols, antioxidant capacity and lipid peroxidation. J. Hum. Nutr. Diet. 2009, 22, 64–71. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Li, J.; Hu, F.B.; Salas-Salvadó, J.; Tobias, D.K. Effects of walnut consumption on blood lipids and other cardiovascular risk factors: An updated meta-analysis and systematic review of controlled trials. Am. J. Clin. Nutr. 2018, 108, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Ros, E.; Núñez, I.; Pérez-Heras, A.; Serra, M.; Gilabert, R.; Casals, E.; Deulofeu, R. A walnut diet improves endothelial function in hypercholesterolemic subjects: A randomized crossover trial. Circulation 2004, 109, 1609–1614. [Google Scholar] [CrossRef] [PubMed]
- Vinson, J.A.; Cai, Y. Nuts, especially walnuts, have both antioxidant quantity and efficacy and exhibit significant potential health benefits. Food Funct. 2012, 3, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Ros, E.; Izquierdo-Pulido, M.; Sala-Vila, A. Beneficial effects of walnut consumption on human health: Role of micronutrients. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Tresserra-Rimbau, A.; Estruch, R.; Martinez-Gonzalez, M.A.; Medina-Remon, A.; Castaner, O.; Corella, D.; Salas-Salvado, J.; Lamuela-Raventos, R.M. Effects of Polyphenol, Measured by a Biomarker of Total Polyphenols in Urine, on Cardiovascular Risk Factors After a Long-Term Follow-Up in the PREDIMED Study. Oxid. Med. Cell Longev. 2016, 2016, 2572606. [Google Scholar] [CrossRef]
- Sanchez-Gonzalez, C.; Ciudad, C.J.; Noe, V.; Izquierdo-Pulido, M. Health benefits of walnut polyphenols: An exploration beyond their lipid profile. Crit. Rev. Food Sci. Nutr. 2017, 57, 3373–3383. [Google Scholar] [CrossRef]
- Poti, F.; Santi, D.; Spaggiari, G.; Zimetti, F.; Zanotti, I. Polyphenol Health Effects on Cardiovascular and Neurodegenerative Disorders: A Review and Meta-Analysis. Int. J. Mol. Sci. 2019, 20, 351. [Google Scholar] [CrossRef]
- Medina-Remón, A.; Casas, R.; Tressserra-Rimbau, A.; Ros, E.; Martínez-González, M.A.; Fitó, M.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventos, R.M.; Estruch, R. Polyphenol intake from a Mediterranean diet decreases inflammatory biomarkers related to atherosclerosis: A sub study of the PREDIMED trial. Br. J. Clin. Pharmacol. 2017, 83, 114–128. [Google Scholar] [CrossRef]
- Rusu, M.E.; Gheldiu, A.-M.; Mocan, A.; Moldovan, C.; Popa, D.-S.; Tomuta, I.; Vlase, L. Process optimization for improved phenolic compounds recovery from walnut (Juglans regia L.) septum: Phytochemical profile and biological activities. Molecules 2018, 23, 2814. [Google Scholar] [CrossRef]
- Ni, Z.-J.; Zhang, Y.-G.; Chen, S.-X.; Thakur, K.; Wang, S.; Zhang, J.-G.; Shang, Y.-F.; Wei, Z.-J. Exploration of walnut components and their association with health effects. Crit. Rev. Food Sci. Nutr. 2022, 62, 5113–5129. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Yu-Poth, S.; Sabate, J.; Ratcliffe, H.E.; Zhao, G.; Etherton, T.D. Nuts and their bioactive constituents: Effects on serum lipids and other factors that affect disease risk. Am. J. Clin. Nutr. 1999, 70, 504S–511S. [Google Scholar] [CrossRef] [PubMed]
- Regueiro, J.; Sánchez-González, C.; Vallverdú-Queralt, A.; Simal-Gándara, J.; Lamuela-Raventós, R.; Izquierdo-Pulido, M. Comprehensive identification of walnut polyphenols by liquid chromatography coupled to linear ion trap–Orbitrap mass spectrometry. Food Chem. 2014, 152, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Shen, D.; Wang, R.; Li, Q.; Mo, R.; Zheng, Y.; Zhou, Y.; Liu, Y. Phenolic profiles, and antioxidant activities of free, esterified and bound phenolic compounds in walnut kernel. Food Chem. 2021, 350, 129217. [Google Scholar] [CrossRef]
- Njike, V.Y.; Costales, V.C.; Petraro, P.; Annam, R.; Yarandi, N.; Katz, D.L. The resulting variation in nutrient intake with the inclusion of walnuts in the diets of adults at risk for type 2 diabetes: A randomized, controlled, crossover trial. Am. J. Health Promot. 2019, 33, 430–438. [Google Scholar] [CrossRef]
- Njike, V.Y.; Yarandi, N.; Petraro, P.; Ayettey, R.G.; Treu, J.A.; Katz, D.L. Inclusion of walnut in the diets of adults at risk for type 2 diabetes and their dietary pattern changes: A randomized, controlled, cross-over trial. BMJ Open Diabetes Res. Care 2016, 4, e000293. [Google Scholar] [CrossRef]
- Bitok, E.; Jaceldo-Siegl, K.; Rajaram, S.; Serra-Mir, M.; Roth, I.; Feitas-Simoes, T.; Ros, E.; Sabaté, J. Favorable nutrient intake and displacement with long-term walnut supplementation among elderly: Results of a randomized trial. Br. J. Nutr. 2017, 118, 201–209. [Google Scholar] [CrossRef]
- Natto, Z.S.; Siapco, G.; Jaceldo-Siegl, K.; Haddad, E.H.; Sabaté, J. Food and Nutrient Displacement by Walnut Supplementation in a Randomized Crossover Study. Nutrients 2022, 14, 1017. [Google Scholar] [CrossRef]
- Rajaram, S.; Valls-Pedret, C.; Cofán, M.; Sabaté, J.; Serra-Mir, M.; Pérez-Heras, A.M.; Arechiga, A.; Casaroli-Marano, R.P.; Alforja, S.; Sala-Vila, A. The Walnuts and Healthy Aging Study (WAHA): Protocol for a nutritional intervention trial with walnuts on brain aging. Front. Aging Neurosci. 2017, 8, 333. [Google Scholar] [CrossRef]
- Sala-Vila, A.; Valls-Pedret, C.; Rajaram, S.; Coll-Padros, N.; Cofan, M.; Serra-Mir, M.; Perez-Heras, A.M.; Roth, I.; Freitas-Simoes, T.M.; Domenech, M.; et al. Effect of a 2-year diet intervention with walnuts on cognitive decline. The Walnuts and Healthy Aging (WAHA) study: A randomized controlled trial. Am. J. Clin. Nutr. 2020, 111, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, S.; Cofan, M.; Sala-Vila, A.; Haddad, E.; Serra-Mir, M.; Bitok, E.; Roth, I.; Freitas-Simoes, T.M.; Kaur, A.; Valls-Pedret, C.; et al. Effects of Walnut Consumption for 2 Years on Lipoprotein Subclasses Among Healthy Elders: Findings from the WAHA Randomized Controlled Trial. Circulation 2021, 144, 1083–1085. [Google Scholar] [CrossRef]
- Domenech, M.; Serra-Mir, M.; Roth, I.; Freitas-Simoes, T.; Valls-Pedret, C.; Cofan, M.; Lopez, A.; Sala-Vila, A.; Calvo, C.; Rajaram, S.; et al. Effect of a Walnut Diet on Office and 24-Hour Ambulatory Blood Pressure in Elderly Individuals. Hypertension 2019, 73, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Ros, E.; Rajaram, S.; Sala-Vila, A.; Serra-Mir, M.; Valls-Pedret, C.; Cofán, M.; Roth, I.; Doménech, M.; Freitas, T.; Calvo, C. Effect of a 1-year walnut supplementation on blood lipids among older individuals: Findings from the walnuts and healthy aging (WAHA) study. FASEB J. 2016, 30, 293.294. [Google Scholar]
- López de las Hazas, M.-C.; Gil-Zamorano, J.; Cofán, M.; Mantilla-Escalante, D.C.; Garcia-Ruiz, A.; del Pozo-Acebo, L.; Pastor, O.; Yañez-Mo, M.; Mazzeo, C.; Serra-Mir, M. One-year dietary supplementation with walnuts modifies exosomal miRNA in elderly subjects. Eur. J. Nutr. 2021, 60, 1999–2011. [Google Scholar] [CrossRef] [PubMed]
- Freitas-Simoes, T.-M.; Cofán, M.; Blasco, M.A.; Soberón, N.; Foronda, M.; Serra-Mir, M.; Roth, I.; Valls-Pedret, C.; Doménech, M.; Ponferrada-Ariza, E. Walnut consumption for two years and leukocyte telomere attrition in Mediterranean elders: Results of a randomized controlled trial. Nutrients 2018, 10, 1907. [Google Scholar] [CrossRef] [PubMed]
- Bitok, E.; Rajaram, S.; Jaceldo-Siegl, K.; Oda, K.; Sala-Vila, A.; Serra-Mir, M.; Ros, E.; Sabate, J. Effects of Long-Term Walnut Supplementation on Body Weight in Free-Living Elderly: Results of a Randomized Controlled Trial. Nutrients 2018, 10, 1317. [Google Scholar] [CrossRef]
- Cofán, M.; Rajaram, S.; Sala-Vila, A.; Valls-Pedret, C.; Serra-Mir, M.; Roth, I.; Freitas-Simoes, T.M.; Bitok, E.; Sabaté, J.; Ros, E.E. Effects of 2-year walnut-supplemented diet on inflammatory biomarkers. J. Am. Coll. Cardiol. 2020, 76, 2282–2284. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Perez-Jimenez, J.; Neveu, V.; Medina-Remon, A.; M’Hiri, N.; García-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013, 2013, bat070. [Google Scholar] [CrossRef]
- Valls-Pedret, C.; Lamuela-Raventós, R.M.; Medina-Remon, A.; Quintana, M.; Corella, D.; Pinto, X.; Martínez-González, M.Á.; Estruch, R.; Ros, E. Polyphenol-rich foods in the Mediterranean diet are associated with better cognitive function in elderly subjects at high cardiovascular risk. J. Alzheimer’s Dis. 2012, 29, 773–782. [Google Scholar] [CrossRef]
- Medina-Remón, A.; Barrionuevo-González, A.; Zamora-Ros, R.; Andres-Lacueva, C.; Estruch, R.; Martínez-González, M.-Á.; Diez-Espino, J.; Lamuela-Raventos, R.M. Rapid Folin–Ciocalteu method using microtiter 96-well plate cartridges for solid phase extraction to assess urinary total phenolic compounds, as a biomarker of total polyphenols intake. Anal. Chim. Acta 2009, 634, 54–60. [Google Scholar] [CrossRef]
- Huang, Q.; Braffett, B.H.; Simmens, S.J.; Young, H.A.; Ogden, C.L. Dietary polyphenol intake in US adults and 10-year trends: 2007–2016. J. Acad. Nutr. Diet. 2020, 120, 1821–1833. [Google Scholar] [CrossRef] [PubMed]
- Del Bo’, C.; Bernardi, S.; Marino, M.; Porrini, M.; Tucci, M.; Guglielmetti, S.; Cherubini, A.; Carrieri, B.; Kirkup, B.; Kroon, P. Systematic review on polyphenol intake and health outcomes: Is there sufficient evidence to define a health-promoting polyphenol-rich dietary pattern? Nutrients 2019, 11, 1355. [Google Scholar] [PubMed]
- Burkholder-Cooley, N.; Rajaram, S.; Haddad, E.; Fraser, G.E.; Jaceldo-Siegl, K. Comparison of polyphenol intakes according to distinct dietary patterns and food sources in the Adventist Health Study-2 cohort. Br. J. Nutr. 2016, 115, 2162–2169. [Google Scholar] [CrossRef] [PubMed]
- Clarke, E.D.; Rollo, M.E.; Pezdirc, K.; Collins, C.E.; Haslam, R.L. Urinary biomarkers of dietary intake: A review. Nutr. Rev. 2020, 78, 364–381. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Gonthier, M.-P.; Manach, C.; Morand, C.; Mennen, L.; Rémésy, C.; Scalbert, A. Polyphenol levels in human urine after intake of six different polyphenol-rich beverages. Br. J. Nutr. 2005, 94, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P.; Abd El Mohsen, M.M.; Minihane, A.M.; Mathers, J.C. Biomarkers of the intake of dietary polyphenols: Strengths, limitations, and application in nutrition research. Br. J. Nutr 2008, 99, 12–22. [Google Scholar] [CrossRef]
- Santhakumar, A.B.; Battino, M.; Alvarez-Suarez, J.M. Dietary polyphenols: Structures, bioavailability, and protective effects against atherosclerosis. Food Chem. Toxicol. 2018, 113, 49–65. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Rabassa, M.; Cherubini, A.; Urpi-Sarda, M.; Llorach, R.; Bandinelli, S.; Ferrucci, L.; Andres-Lacueva, C. Comparison of 24-h volume and creatinine-corrected total urinary polyphenol as a biomarker of total dietary polyphenols in the Invecchiare InCHIANTI study. Anal. Chim. Acta 2011, 704, 110–115. [Google Scholar] [CrossRef]
- Burkholder-Cooley, N.M.; Rajaram, S.S.; Haddad, E.H.; Oda, K.; Fraser, G.E.; Jaceldo-Siegl, K. Validating polyphenol intake estimates from a food-frequency questionnaire by using repeated 24-h dietary recalls and a unique method-of-triads approach with 2 biomarkers. Am. J. Clin. Nutr. 2017, 105, 685–694. [Google Scholar] [CrossRef]
- Clarke, E.D.; Rollo, M.E.; Collins, C.E.; Wood, L.; Callister, R.; Philo, M.; Kroon, P.A.; Haslam, R.L. The relationship between dietary polyphenol intakes and urinary polyphenol concentrations in adults prescribed a high vegetable and fruit diet. Nutrients 2020, 12, 3431. [Google Scholar] [CrossRef]
- Mennen, L.I.; Sapinho, D.; Ito, H.; Bertrais, S.; Galan, P.; Hercberg, S.; Scalbert, A. Urinary flavonoids and phenolic acids as biomarkers of intake for polyphenol-rich foods. Br. J. Nutr. 2006, 96, 191–198. [Google Scholar] [CrossRef]
- Medina-Remon, A.; Tresserra-Rimbau, A.; Arranz, S.; Estruch, R.; Lamuela-Raventos, R.M. Polyphenols excreted in urine as biomarkers of total polyphenol intake. Bioanalysis 2012, 4, 2705–2713. [Google Scholar] [CrossRef] [PubMed]
- Tresserra-Rimbau, A.; Medina-Remón, A.; Pérez-Jiménez, J.; Martínez-González, M.; Covas, M.I.; Corella, D.; Salas-Salvadó, J.; Gómez-Gracia, E.; Lapetra, J.; Arós, F. Dietary intake and major food sources of polyphenols in a Spanish population at high cardiovascular risk: The PREDIMED study. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 953–959. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary intake, and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef]
- Rechner, A.R.; Kuhnle, G.; Hu, H.; Roedig-Penman, A.; van den Braak, M.H.; Moore, K.P.; Rice-Evans, C.A. The metabolism of dietary polyphenols and the relevance to circulating levels of conjugated metabolites. Free Radic. Res. 2002, 36, 1229–1241. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Munoz, C.; Vaillant, F. Metabolic fate of ellagitannins: Implications for health, and research perspectives for innovative functional foods. Crit. Rev. Food Sci. Nutr. 2014, 54, 1584–1598. [Google Scholar] [CrossRef] [PubMed]
- García-Mantrana, I.; Calatayud, M.; Romo-Vaquero, M.; Espín, J.C.; Selma, M.V.; Collado, M.C. Urolithin metabotypes can determine the modulation of gut microbiota in healthy individuals by tracking walnuts consumption over three days. Nutrients 2019, 11, 2483. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Martín, A.; Selma, M.V.; Tomás-Barberán, F.A.; González-Sarrías, A.; Espín, J.C. Where to investigate the puzzle of polyphenols and health? Postbiotics and gut microbiota are associated with human metabotypes. Mol. Nutr. Food Res. 2020, 64, 1900952. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak-Drozd, K.; Oniszczuk, T.; Stasiak, M.; Oniszczuk, A. Beneficial effects of phenolic compounds on gut microbiota and metabolic syndrome. Int. J. Mol. Sci. 2021, 22, 3715. [Google Scholar] [CrossRef]
- Hidalgo, M.; Oruna-Concha, M.J.; Kolida, S.; Walton, G.E.; Kallithraka, S.; Spencer, J.P.; de Pascual-Teresa, S. Metabolism of anthocyanins by human gut microflora and their influence on gut bacterial growth. J. Agric. Food Chem. 2012, 60, 3882–3890. [Google Scholar] [CrossRef] [PubMed]
- Aravind, S.M.; Wichienchot, S.; Tsao, R.; Ramakrishnan, S.; Chakkaravarthi, S. Role of dietary polyphenols on gut microbiota, their metabolites and health benefits. Food Res. Int. 2021, 142, 110189. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Gebhardt, S.; Prior, R.L. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J. Nutr. 2004, 134, 613–617. [Google Scholar] [PubMed]
- Colaric, M.; Veberic, R.; Solar, A.; Hudina, M.; Stampar, F. Phenolic acids, syringaldehyde, and juglone in fruits of different cultivars of Juglans regia L. J. Agric. Food Chem. 2005, 53, 6390–6396. [Google Scholar] [CrossRef]
- Harnly, J.M.; Doherty, R.F.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Bhagwat, S.; Gebhardt, S. Flavonoid content of US fruits, vegetables, and nuts. J. Agric. Food Chem. 2006, 54, 9966–9977. [Google Scholar] [CrossRef]
- Li, L.; Tsao, R.; Yang, R.; Liu, C.; Zhu, H.; Young, J.C. Polyphenolic profiles and antioxidant activities of heartnut (Juglans ailanthifolia var. cordiformis) and Persian walnut (Juglans regia L.). J. Agric. Food Chem. 2006, 54, 8033–8040. [Google Scholar] [CrossRef]
- Trandafir, I.; Cosmulescu, S. Total phenolic content, antioxidant capacity and individual phenolic compounds of defatted kernel from different cultivars of walnut. Erwerbs-Obstbau 2020, 62, 309–314. [Google Scholar] [CrossRef]
- Slatnar, A.; Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R.; Solar, A. Identification, and quantification of phenolic compounds in kernels, oil, and bagasse pellets of common walnut (Juglans regia L.). Food Res. Int. 2015, 67, 255–263. [Google Scholar] [CrossRef]
- Labuckas, D.O.; Maestri, D.M.; Perello, M.; Martínez, M.L.; Lamarque, A.L. Phenolics from walnut (Juglans regia L.) kernels: Antioxidant activity and interactions with proteins. Food Chem. 2008, 107, 607–612. [Google Scholar] [CrossRef]
- Woźniak, M.; Waśkiewicz, A.; Ratajczak, I. The Content of Phenolic Compounds and Mineral Elements in Edible Nuts. Molecules 2022, 27, 4326. [Google Scholar] [CrossRef] [PubMed]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly) phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Ito, H.; Yoshida, T. Antioxidative polyphenols from walnuts (Juglans regia L.). Phytochemistry 2003, 63, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Aloy, M.; Hulshof, P.J.M.; Estruel-Amades, S.; Oste, M.C.J.; Lankinen, M.; Geleijnse, J.M.; de Goede, J.; Ulaszewska, M.; Mattivi, F.; Bakker, S.J.L.; et al. Biomarkers of food intake for nuts and vegetable oils: An extensive literature search. Genes Nutr. 2019, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Alkhaldy, A.; Edwards, C.A.; Combet, E. The urinary phenolic acid profile varies between younger and older adults after a polyphenol-rich meal despite limited differences in in vitro colonic catabolism. Eur. J. Nutr. 2019, 58, 1095–1111. [Google Scholar] [CrossRef]
- Cerdá, B.; Tomás-Barberán, F.A.; Espín, J.C. Metabolism of antioxidant and chemopreventive ellagitannins from strawberries, raspberries, walnuts, and oak-aged wine in humans: Identification of biomarkers and individual variability. J. Agric. Food Chem. 2005, 53, 227–235. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; González-Sarrías, A.; García-Villalba, R.; Núñez-Sánchez, M.A.; Selma, M.V.; García-Conesa, M.T.; Espín, J.C. Urolithins, the rescue of “old” metabolites to understand a “new” concept: Metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis, and host health status. Mol. Nutr. Food Res. 2017, 61, 1500901. [Google Scholar] [CrossRef]
- Haddad, E.H.; Gaban-Chong, N.; Oda, K.; Sabaté, J. Effect of a walnut meal on postprandial oxidative stress and antioxidants in healthy individuals. Nutr. J. 2014, 13, 4. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).