Assessment of the Composition of Breastmilk Substitutes, Commercial Complementary Foods, and Commercial Snack Products Commonly Fed to Infant and Young Children in Lebanon: A Call to Action
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Chemical Analysis of Infant Formulas and Baby Food Products
2.2.1. Fat Content Analysis
2.2.2. Fatty Acid Profile Analysis
2.2.3. Protein Content Analysis
2.2.4. Carbohydrate Content Analysis
2.2.5. Total Sugars
2.2.6. Total Energy Analysis
2.2.7. Ash Content Analysis
2.2.8. Moisture Content Analysis
2.2.9. Chloride Analysis
2.3. Statistical Analysis
- Each 30 mL of milk needs 1 scoop of powdered milk, equal to 4 g. This information was retrieved from the recommendation mentioned on the back of the package.
- Step 1: 100 mL of milk = = 13.3 g
- Step 2: Measured value in g/100 mL =
3. Results
3.1. Total Energy, Macronutrients, Ash, Moisture, and Chloride Composition of Infant Formulas and Baby Food Products
3.1.1. Total Energy
3.1.2. Total Fat
Fatty Acids
3.1.3. Total Carbohydrates
Added Sugars
3.1.4. Protein
3.2. Compliance of the Measured Values of Infant Formulas and Baby Food Products with Codex, EFSA, and Libnor Regulations
3.3. Contributions to Daily Values
3.4. Nutrient Content and Labeling Discrepancies in Infant Formulas and Baby Food Products
4. Discussion
4.1. Comparison of the Composition of Infant Formulas and Baby Food Products with Regional Data
4.2. Comparison of the Composition of Infant Formulas and Baby Food Products with International Data
4.3. Associated Health Risks of Infant Formulas and Baby Food Products
4.4. Nutrient Content and Labeling Discrepancies in Infant Formulas and Baby Food Products
4.5. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- UNICEF. First 1000 Days. The Critical Window to Ensure that Children Survive and Thrive. Available online: https://www.unicef.org/southafrica/media/551/file/ZAF-First-1000-days-brief-2017.pdf (accessed on 20 August 2022).
- UNICEF. The State of Food Security and Nutrition in the World 2022. Available online: https://data.unicef.org/resources/sofi-2022/ (accessed on 20 August 2022).
- Lim, S.L.; Ong, K.C.B.; Chan, Y.H.; Loke, W.C.; Ferguson, M.; Daniels, L. Malnutrition and its impact on cost of hospitalization, length of stay, readmission and 3-year mortality. Clin. Nutr. 2012, 31, 345–350. [Google Scholar] [CrossRef]
- World Health Organization. Breastfeeding. Available online: https://www.who.int/health-topics/breastfeeding#tab=tab_1 (accessed on 20 August 2022).
- Martin, C.R.; Ling, P.; Blackburn, G.L. Review of infant feeding: Key features of breast milk and infant formula. Nutrients 2016, 8, 279. [Google Scholar] [CrossRef]
- Stanislawski, M.A.; Dabelea, D.; Wagner, B.D.; Iszatt, N.; Dahl, C.; Sontag, M.K.; Knight, R.; Lozupone, C.A.; Eggesbø, M. Gut microbiota in the first 2 years of life and the association with body mass index at age 12 in a Norwegian birth cohort. mBio 2018, 9, 1751. [Google Scholar] [CrossRef]
- Bridge, G.; Lomazzi, M.; Bedi, R. A cross-country exploratory study to investigate the labelling, energy, carbohydrate and sugar content of formula milk products marketed for infants. Br. Dent. J. 2020, 228, 198–212. [Google Scholar] [CrossRef]
- Emily, E.S.; Thelma, E.P.; Rita, P. A History of Infant Feeding. J. Perinat. Edu. 2009, 18, 32–39. [Google Scholar]
- World Health Organization. The International Code of Marketing of Breast-Milk Substitutes; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Ching, C.; Zambrano, P.; Nguyen, T.T.; Tharaney, M.; Zafimanjaka, M.G.; Mathisen, R. Old tricks, new opportunities: How companies violate the international code of Marketing of Breast-Milk Substitutes and Undermine Maternal and child health during the COVID-19 pandemic. Int. J. Environ. Res. Public Health 2021, 18, 2381. [Google Scholar] [CrossRef]
- World Health Organization. Infant and Young Child Feeding. Available online: https://www.who.int/news-room/fact-sheets/detail/infant-and-young-child-feeding (accessed on 20 July 2022).
- Caroli, M.; Mele, R.M.; Tomaselli, M.A.; Cammisa, M.; Longo, F.; Attolini, E. Complementary feeding patterns in Europe with a special focus on Italy. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 813–818. [Google Scholar] [CrossRef]
- Baidal, J.A.W.; Locks, L.M.; Cheng, E.R.; Blake-Lamb, T.L.; Perkins, M.E.; Taveras, E.M. Risk factors for childhood obesity in the first 1,000 days: A systematic review. Am. J. Prev. Med. 2016, 50, 761–779. [Google Scholar] [CrossRef]
- Caroli, M.; Vania, A.; Verga, M.C.; Di Mauro, G.; Bergamini, M.; Cuomo, B.; D’Anna, R.; D’Antonio, G.; Dello Iacono, I.; Dessì, A. Recommendations on Complementary Feeding as a Tool for Prevention of Non-Communicable Diseases (NCDs)—Paper Co-Drafted by the SIPPS, FIMP, SIDOHaD, and SINUPE Joint Working Group. Nutrients 2022, 14, 257. [Google Scholar] [CrossRef]
- Antignani, A.; Francavilla, R.; Vania, A.; Leonardi, L.; Di Mauro, C.; Tezza, G.; Cristofori, F.; Dargenio, V.N.; Scotese, I.; Palma, F. Nutritional Assessment of Baby Food Available in Italy. Nutrients 2022, 14, 3722. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Daily Value on the New Nutrition and Supplement Facts Labels. Available online: https://www.fda.gov/food/new-nutrition-facts-label/daily-value-new-nutrition-and-supplement-facts-labels (accessed on 30 October 2022).
- Khans, M.A.; Kissana, A.S. Nutritional evaluation of some commercial baby foods consumed in Pakistan. J. Sci. Food Agric. 1985, 36, 1271–1274. [Google Scholar] [CrossRef]
- Alfaris, N.A.; Alothman, Z.A.; Aldayel, T.S.; Wabaidur, S.M.; Altamimi, J.Z. Evaluation and Comparison of the Nutritional and Mineral Content of Milk Formula in the Saudi Arabia Market. Front. Nutr. 2022, 9, 851229. [Google Scholar] [CrossRef]
- Kotb, M.A.; Farahat, M.F.; El-Daree, H.B. Chemical composition of infant milk formulas sold in Alexandria, Egypt. Can. J. Clin. Nutr. 2016, 4, 4–17. [Google Scholar]
- Saeed, F.; Ullah Khan, A.; Mushtaq, Z.; Afzaal, M.; Niaz, B.; Hussain, M.; Hameed, A.; Ahmad, A.; Anjum, F.M.; Suleria, H.A. Amino acid profile and safety assessment of infant formula available in local market, Pakistan. Int. J. Food Prop. 2021, 24, 533–543. [Google Scholar] [CrossRef]
- Bu-Hamdi, M.A.; Al-Harbi, M.; Anderson, A.K. Assessment of the nutritionally essential minerals and physiochemical properties of infant milk food commercially available in Kuwait. Int. J. Agric. Sci. Food Technol. 2 (1): 001-008 2016, 7, 6–12. [Google Scholar] [CrossRef]
- Youssef, M.S.; Atwa, M.A.; Bassuony, N.I.; Abol Ela, M.P. Chemical And Microbiological Evaluation Of Some Infant Formulas Handling in Egyptian Markets. J. Soil Sci. Agric. Eng. 2006, 31, 5393–5406. [Google Scholar] [CrossRef]
- Bakeet, Z.A.N.; Arzoo, S.; Taha, N.A.A. Fatty acid composition and fat content of some infant formulas commercially available in Sudan and its comparison with fatty acid compositions of mature breast milk from different parts of the world. Int. J. Biosci. (IJB) 2013, 3, 57–64. [Google Scholar] [CrossRef]
- Hayat, L.; Al-Sughayer, M.; Afzal, M. A comparative study of fatty acids in human breast milk and breast milk substitutes in Kuwait. Nutr. Res. 1999, 19, 827–841. [Google Scholar] [CrossRef]
- Shenana, M.E.; El-Alfy, M.B.; Sania, M.A.; Gemiel, D.G. Composition and properties of some market dried infant formulas in comparison with human, cows and buffaloes milks. Egypt. J. Dairy Sci. 2014, 42, 23–36. [Google Scholar]
- Güzel, S.; Keser, A.; Hatun, Ş. Investigating the nutritional value of foods targeting children. Eat. Weight Disord. 2020, 25, 51–58. [Google Scholar] [CrossRef]
- Zand, N.; Chowdhry, B.Z.; Pollard, L.V.; Pullen, F.S.; Snowden, M.J.; Zotor, F.B. Commercial ‘ready-to-feed’infant foods in the UK: Macro-nutrient content and composition. Matern. Child Nutr. 2015, 11, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Zunin, P.; Boggia, R.; Turrini, F.; Leardi, R. Total and “free” lipids in commercial infant formulas: Fatty acid composition and their stability to oxidation. Food Chem. 2015, 173, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, M.A.; Araújo, W.M.C.; Borgo, L.A.; de Rodrigues Alencar, E. Lipid profile of different infant formulas for infants. PLoS ONE 2017, 12, e0177812. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Hernández, S.; Esteban-Muñoz, A.; Giménez-Martínez, R.; Aguilar-Cordero, M.J.; Miralles-Buraglia, B.; Olalla-Herrera, M. A comparison of changes in the fatty acid profile of human milk of Spanish lactating women during the first month of lactation using gas chromatography-mass spectrometry. A comparison with infant formulas. Nutrients 2019, 11, 3055. [Google Scholar] [CrossRef]
- Martínez, M.Á.; Castro, I.; Rovira, J.; Ares, S.; Rodríguez, J.M.; Cunha, S.C.; Casal, S.; Fernandes, J.O.; Schuhmacher, M.; Nadal, M. Early-life intake of major trace elements, bisphenol A, tetrabromobisphenol A and fatty acids: Comparing human milk and commercial infant formulas. Environ. Res. 2019, 169, 246–255. [Google Scholar] [CrossRef]
- Satchithanandam, S.; Fritsche, J.; Rader, J.I. Gas chromatographic analysis of infant formulas for total fatty acids, including trans fatty acids. J. AOAC Int. 2002, 85, 86–94. [Google Scholar] [CrossRef]
- Kpaibé, A.P.S.; Kouassi, Y.A.K.; N’goran Jean Simon, T.; Koko, A.; N’bra, S.K.; Aké, M. Short Research Article Fat Content and Fatty Acids Profile in Follow-on Formulas Commercialized in Côte d’Ivoire. Food Sci. Nutr. 2019, 3. [Google Scholar] [CrossRef]
- Elliott, C.D. Sweet and salty: Nutritional content and analysis of baby and toddler foods. J. Public Health 2011, 33, 63–70. [Google Scholar] [CrossRef]
- Garro-Mellado, L.; Guerra-Hernández, E.; García-Villanova, B. Sugar Content and Sources in Commercial Infant Cereals in Spain. Children 2022, 9, 115. [Google Scholar] [CrossRef]
- Codex Alimentarius Standard for Infant Formula and Formulas for Special Medical Purposes Intended for Infants. 2020. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B156-1987%252FCXS_156e.pdf. (accessed on 3 August 2022).
- Koletzko, B.; Baker, S.; Cleghorn, G.; Neto, U.F.; Gopalan, S.; Hernell, O.; Hock, Q.S.; Jirapinyo, P.; Lonnerdal, B.; Pencharz, P. Global standard for the composition of infant formula: Recommendations of an ESPGHAN coordinated international expert group. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 584–599. [Google Scholar] [CrossRef]
- Awad, R.; Kowash, M.; Hussein, I.; Salami, A.; Abdo, M.; Al-Halabi, M. Sugar content in infant formula: Accuracy of labeling and conformity to guidelines. Int. J. Paediatr. Dent. 2022. [CrossRef] [PubMed]
- World Health Organization. Guideline: Sugars intake for Adults and Children. Available online: https://www.who.int/publications/i/item/9789241549028 (accessed on 29 October 2022).
- World Health Organization. Noncommunicable Diseases: Childhood Overweight and Obesity. 2020. Available online: https://globalnutritionreport.org/resources/nutrition-profiles/asia/western-asia/saudi-arabia/#:~:text=Saudi%20Arabia%20has%20shown%20limited,women%20and%207.5%25%20for%20men (accessed on 17 February 2023).
- Koo, W.W.; Hockman, E.M.; Dow, M. Palm olein in the fat blend of infant formulas: Effect on the intestinal absorption of calcium and fat, and bone mineralization. J. Am. Coll. Nutr. 2006, 25, 117–122. [Google Scholar] [CrossRef] [PubMed]
- de Queiroz Leite, M.E.; Lasekan, J.; Baggs, G.; Ribeiro, T.; Menezes-Filho, J.; Pontes, M.; Druzian, J.; Barreto, D.L.; de Souza, C.O.; Mattos, Â. Calcium and fat metabolic balance, and gastrointestinal tolerance in term infants fed milk-based formulas with and without palm olein and palm kernel oils: A randomized blinded crossover study. BMC Pediatr. 2013, 13, 215. [Google Scholar] [CrossRef] [PubMed]
- Padial-Jaudenes, M.; Castanys-Munoz, E.; Ramirez, M.; Lasekan, J. Physiological impact of palm olein or palm oil in infant formulas: A review of clinical evidence. Nutrients 2020, 12, 3676. [Google Scholar] [CrossRef]
- Lasekan, J.B.; Hustead, D.S.; Masor, M.; Murray, R. Impact of palm olein in infant formulas on stool consistency and frequency: A meta-analysis of randomized clinical trials. Food Nutr. Res. 2017, 61, 1330104. [Google Scholar] [CrossRef] [PubMed]
- Ebbesson, S.O.; Voruganti, V.S.; Higgins, P.B.; Fabsitz, R.R.; Ebbesson, L.O.; Laston, S.; Harris, W.S.; Kennish, J.; Umans, B.D.; Wang, H. Fatty acids linked to cardiovascular mortality are associated with risk factors. Int. J. Circumpolar Health 2015, 74, 28055. [Google Scholar] [CrossRef] [PubMed]
- Pearce, J.; Langley-Evans, S.C. The types of food introduced during complementary feeding and risk of childhood obesity: A systematic review. Int. J. Obes. 2013, 37, 477–485. [Google Scholar] [CrossRef]
- National Institutes of Health. Office of Dietary Supplements—Daily Values (DVs). Available online: https://ods.od.nih.gov/HealthInformation/dailyvalues.aspx (accessed on 20 February 2023).
- World Health Organization. WHO Calls on Countries to Reduce Sugars Intake among Adults and Children. Available online: https://www.who.int/news/item/04-03-2015-who-calls-on-countries-to-reduce-sugars-intake-among-adults-and-children (accessed on 20 February 2023).
- Food and Agriculture Organization. Fats and Fatty Acids in Human Nutrition. Report of an Expert Consultation. 2008. Available online: https://www.fao.org/3/i1953e/i1953e00.pdf (accessed on 20 February 2023).

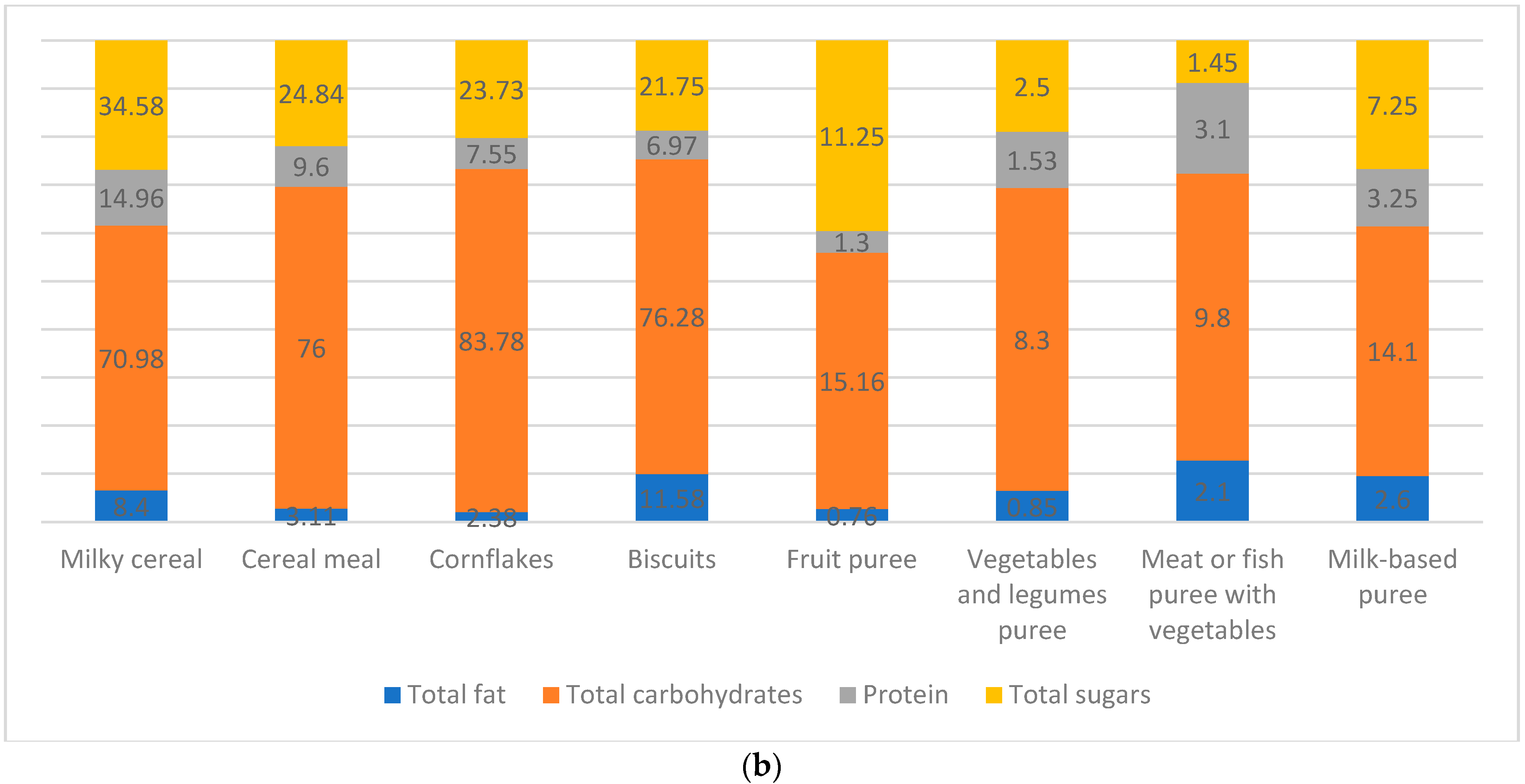

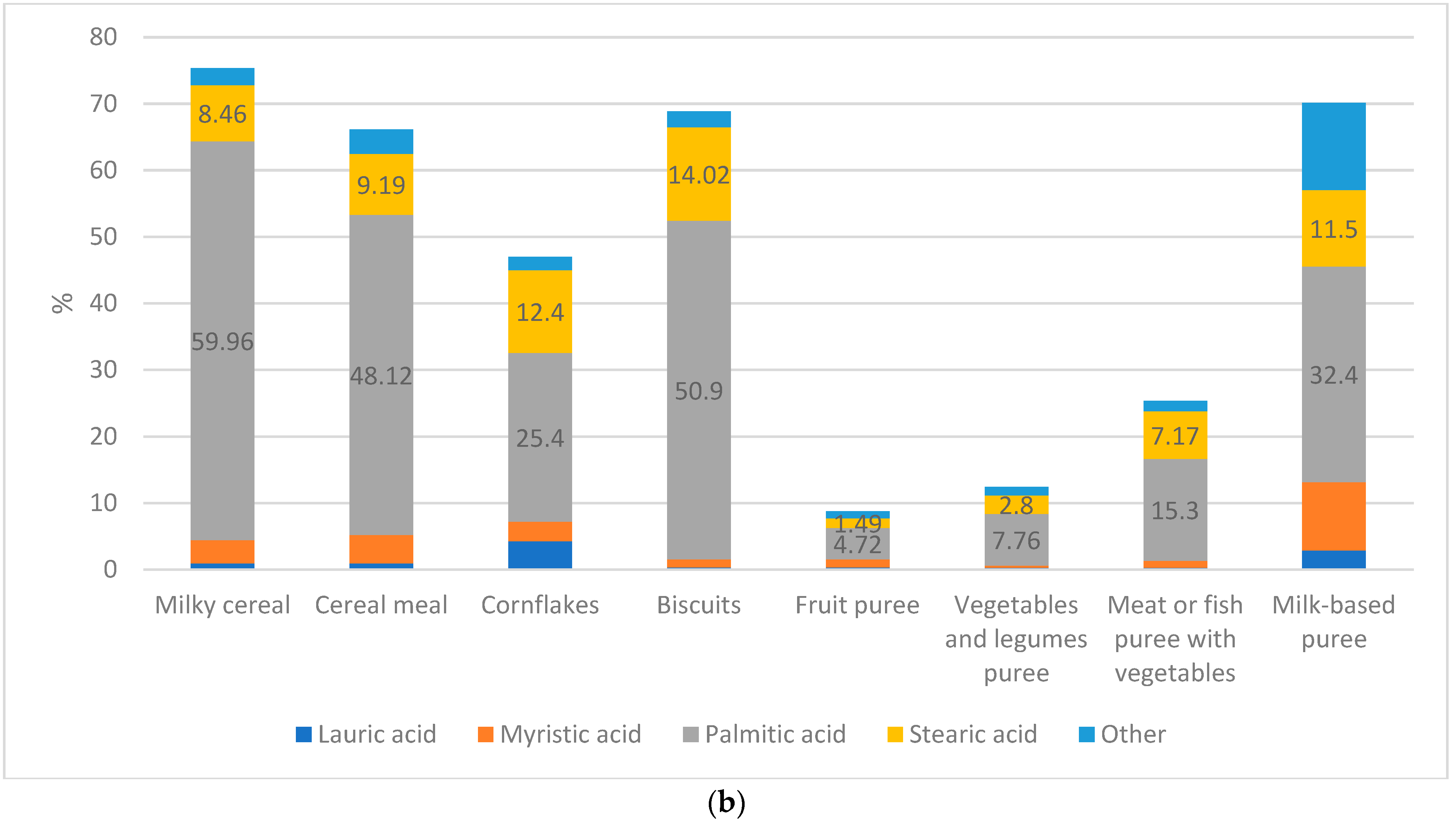

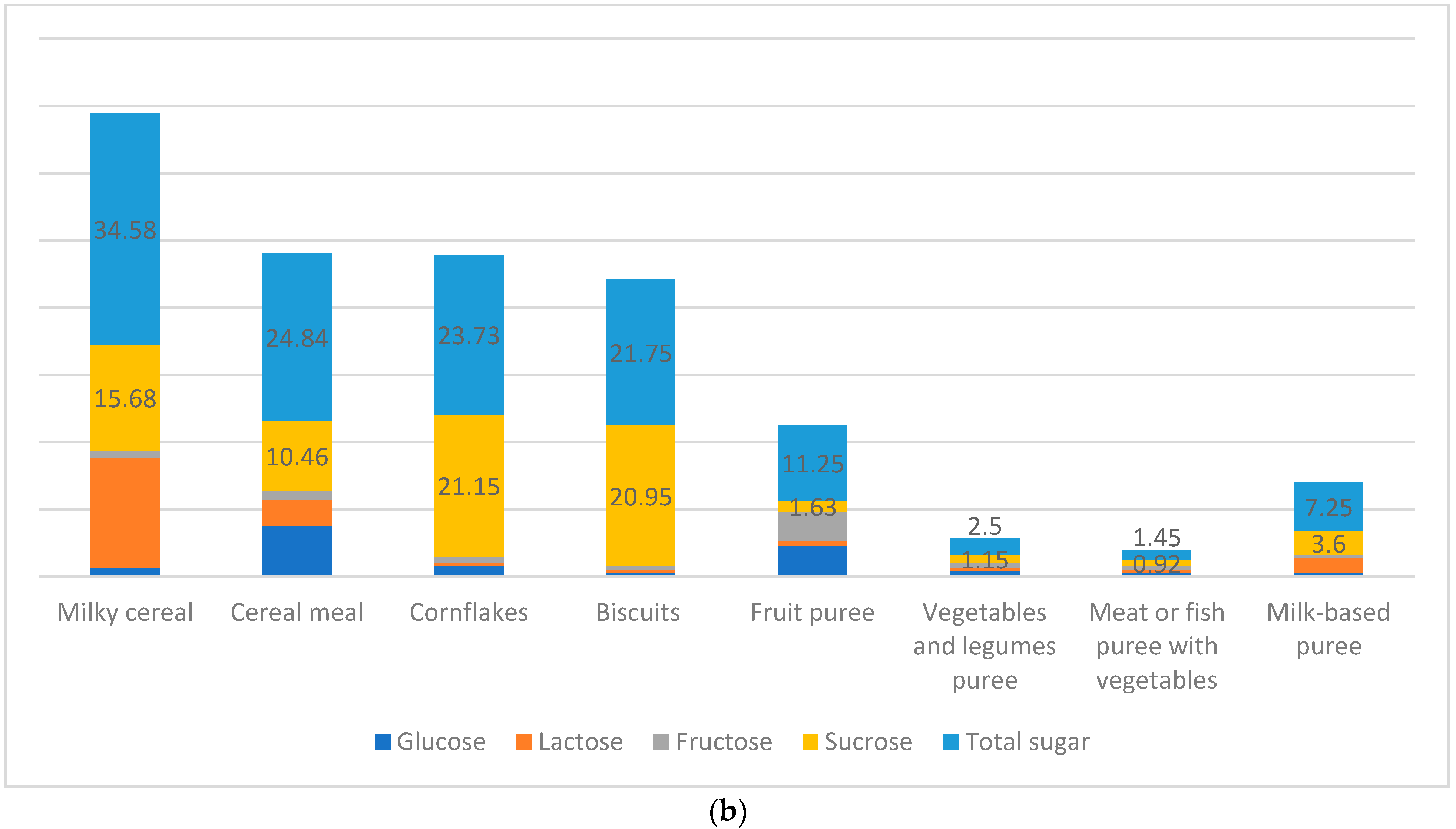
| Carbohydrates | Codex ^/ EFSA +/Libnor Regulations | Protein | Codex/ EFSA/Libnor Regulations | Total Calories from Protein | Codex/ EFSA/Libnor Regulations | Total Fat | Codex/ EFSA/Libnor Regulations | Total Calories from Fat | Codex/ EFSA/Libnor Regulations | |
|---|---|---|---|---|---|---|---|---|---|---|
| Follow-up formulas | - | - | 3.65 g/100kcal * | Exceeding EFSA | - | - | - | - | - | - |
| Growing-up formulas | 14.3 g/100kcal * | Exceeding EFSA | 3.07 g/100kcal * | Exceeding EFSA | - | - | - | - | - | - |
| Extra-care formulas | - | - | 2.69 g/100kcal * | Exceeding EFSA | - | - | 4.06 g/100kcal * | Below Codex | - | - |
| Milky cereal | - | - | 14.96 | Below Libnor | - | - | 8.4 g/100g | Below Libnor | 75.6 Kcal ** | Below Codex |
| Cereal meal | - | - | - | - | - | - | 3.11 g/100g | Below Libnor | 28 Kcal ** | Below Codex |
| Cornflakes | - | - | 7.55 g/100kcal * | Below Libnor | - | - | 2.38 g/100g | Below Libnor | 21.42 Kcal ** | Below Codex |
| Biscuits | - | - | 6.97 g/100kcal * | Below Libnor | - | - | - | - | - | - |
| Fruit puree | - | - | 1.3 g/100kcal * | Below Libnor | - | - | 0.76 g/100g | Below Libnor | 6.84 Kcal ** | Below Codex |
| Vegetable and legume puree | - | - | 1.53 g/100kcal | Below Libnor | - | - | 0.85 g/100g | Below Libnor | 7.65 Kcal ** | Below Codex |
| Meat or fish puree with vegetables | - | - | 3.1 g/100kcal * | Below Libnor | 12.4 Kcal *** | Exceeding Codex | 2.1 g/100g | Below Libnor | - | - |
| Milk-based puree | - | - | 3.25 g/100kcal * | Below Libnor | - | - | 2.6 g/100g | Below Libnor | - | - |
| Percentage of Contribution to the Daily Value | Percentage of Real Intake per Day According to the Daily Intake (Label) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Fat | SFA | TFA | Total Carbs | Added Sugar | Protein | Total Fat | SFA | TFA | Total Carbs | Added Sugar | Protein | |
| Infant formulas (100 mL) +^ | ||||||||||||
| Starting formulas (0–6 months) | 9.5 | NA | NA | 8.9 | NA | 17.5 | 71.3 | NA | NA | 66.8 | NA | 131 |
| Follow-up formulas (6–12 months) | 7.3 | NA | NA | 8.7 | NA | 20.6 | 49.3 | NA | NA | 58.7 | NA | 139.05 |
| Growing-up formulas (1–3 years) | 5.2 | 15.1 | 8.2 | 5.8 | 28.8 | 14.5 | 28.1 | 81.5 | 44.3 | 31.3 | 155.5 | 78.3 |
| Extra-care formulas (1–3 years) | 8.7 | NA | NA | 8.9 | NA | 19.1 | 66.9 | NA | NA | 68.5 | NA | 147.07 |
| Baby food products (100 g) +^ | ||||||||||||
| Milky cereals | 21.5 | 63.3 | 1.68 | 74.7 | 138.3 | 136 | 9.4 | 27.8 | 0.7 | 32.8 | 60.8 | 59.8 |
| Cereal meal | 7.9 | 20.5 | 1.92 | 80 | 99.3 | 87.2 | 3.5 | 9.02 | 0.8 | 35.2 | 43.7 | 38.3 |
| Cornflakes | 6.1 | 11.2 | 0.9 | 88.2 | 94.9 | 68.6 | 1.8 | 3.3 | 0.27 | 26.4 | 28.5 | 20.6 |
| Biscuits | 29.7 | 79.7 | 4.6 | 80.3 | 87 | 63.3 | 4.75 | 12.7 | 0.7 | 12.8 | 13.9 | 10.1 |
| Fruit puree | 1.9 | 0.66 | 0.06 | 15.9 | 45 | 11.8 | 6.8 | 2.4 | 0.2 | 57.4 | 162.4 | 42.5 |
| Vegetable and legume puree | 2.2 | 1.05 | 0.51 | 8.7 | 10 | 13.9 | 7.9 | 3.8 | 1.8 | 31.4 | 36.1 | 50.1 |
| Meat or fish puree with vegetables | 5.4 | 5.32 | 0.93 | 10.3 | 5.8 | 28.2 | 19.5 | 19.2 | 3.3 | 37.2 | 20.9 | 101.8 |
| Milk-based puree | 6.6 | 18.2 | 3.9 | 14.8 | 29 | 29.5 | 23.8 | 65.7 | 14.07 | 53.4 | 104.7 | 106.5 |
| Energy | Total Fat | Saturated Fatty Acids | Monounsaturated Fatty Acids | Polyunsaturated Fatty Acids | Trans Fatty Acids | carbohydrates | Total Sugar | Glucose | Lactose | Fructose | Sucrose | Protein | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Starting formulas | 12.6% * | 5% * | 33.1% * | 21.55% * | 15.4% * | NL | 7.43% * | NL | 0.5% | 1.31% | NL | NL | 0.95% * |
| Follow-up formulas | 11.86% | 4.8% * | 39.6% * | NL | NL | NL | 5.6% * | NL | NL | 8.94% * | NL | NL | 2.14% |
| Growing-up formulas | 1.7% | 5.04% * | 37.4% * | 33.77% * | 13.45% * | 0.65% | 11.98% * | 8.35% | 0.66% | 8.65% * | NL | NL | 0.47% |
| Extra-care formulas | 17.9% * | 6.04% * | 31.84% * | 19.12% * | 14.36% * | 0.25% | 8.74% * | 2.14% | 2% * | 6.82% * | NL | NL | 0.62% |
| Milky cereal | 3.8% | 0.52% | 36.34% * | NL | NL | NL | 2.26% | 2.16% * | NL | NL | NL | NL | 0% |
| Cereal Meal | 7.3% * | 0.33% | 45.26% * | NL | NL | NL | 3.93% | 2.32% | NL | NL | NL | NL | 0.58% * |
| Cornflakes | 1.9% | 0.69% * | 19.49 * | NL | 24.63% | 0.15 | 1.62% * | 1.66% | NL | NL | NL | NL | 0.63% * |
| Biscuits | 3.12% | 1.3% * | 35.13 * | NL | NL | NL | 2.88% * | 0.49% | NL | NL | NL | NL | 0.51% |
| Fruit puree | 8.23 * | 0.2% | 18.39% | NL | NL | NL | 1.87% * | 1.19% | NL | NL | NL | NL | 0.46% * |
| Vegetable and legume puree | 4.4% | 0.17% | 2.58% | NL | NL | NL | 1.5% * | 0.03% | NL | NL | NL | NL | 0.25% * |
| Meat or fish puree with vegetables | 5.52% * | 0 | 8.76% * | NL | NL | NL | 1.77% * | 0.35% | NL | NL | NL | NL | 0.3% |
| Milk-based puree | 8.5% | 0.95% | 15.73% | NL | NL | NL | 0.25% | 0.6% | NL | NL | NL | NL | 0.6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoteit, M.; Ibrahim, C.; Nohra, J.; Sacre, Y.; Hanna-Wakim, L.; Al-Jawaldeh, A. Assessment of the Composition of Breastmilk Substitutes, Commercial Complementary Foods, and Commercial Snack Products Commonly Fed to Infant and Young Children in Lebanon: A Call to Action. Nutrients 2023, 15, 1200. https://doi.org/10.3390/nu15051200
Hoteit M, Ibrahim C, Nohra J, Sacre Y, Hanna-Wakim L, Al-Jawaldeh A. Assessment of the Composition of Breastmilk Substitutes, Commercial Complementary Foods, and Commercial Snack Products Commonly Fed to Infant and Young Children in Lebanon: A Call to Action. Nutrients. 2023; 15(5):1200. https://doi.org/10.3390/nu15051200
Chicago/Turabian StyleHoteit, Maha, Carla Ibrahim, Joanna Nohra, Yonna Sacre, Lara Hanna-Wakim, and Ayoub Al-Jawaldeh. 2023. "Assessment of the Composition of Breastmilk Substitutes, Commercial Complementary Foods, and Commercial Snack Products Commonly Fed to Infant and Young Children in Lebanon: A Call to Action" Nutrients 15, no. 5: 1200. https://doi.org/10.3390/nu15051200
APA StyleHoteit, M., Ibrahim, C., Nohra, J., Sacre, Y., Hanna-Wakim, L., & Al-Jawaldeh, A. (2023). Assessment of the Composition of Breastmilk Substitutes, Commercial Complementary Foods, and Commercial Snack Products Commonly Fed to Infant and Young Children in Lebanon: A Call to Action. Nutrients, 15(5), 1200. https://doi.org/10.3390/nu15051200






