Effects of Climate, Sun Exposure, and Dietary Intake on Vitamin D Concentrations in Pregnant Women: A Population-Based Study
Abstract
1. Introduction
2. Materials and Methods
Study Population
3. Data Collection
3.1. Sociodemographic and Pregnancy-Related Characteristics
3.2. Dietary Characteristics
3.3. Sunshine-Related Factors
3.4. Vitamin D Deficiency Assessment
4. Ethical Consideration
5. Statistical Analysis
6. Results
6.1. Characteristics of Study Participants
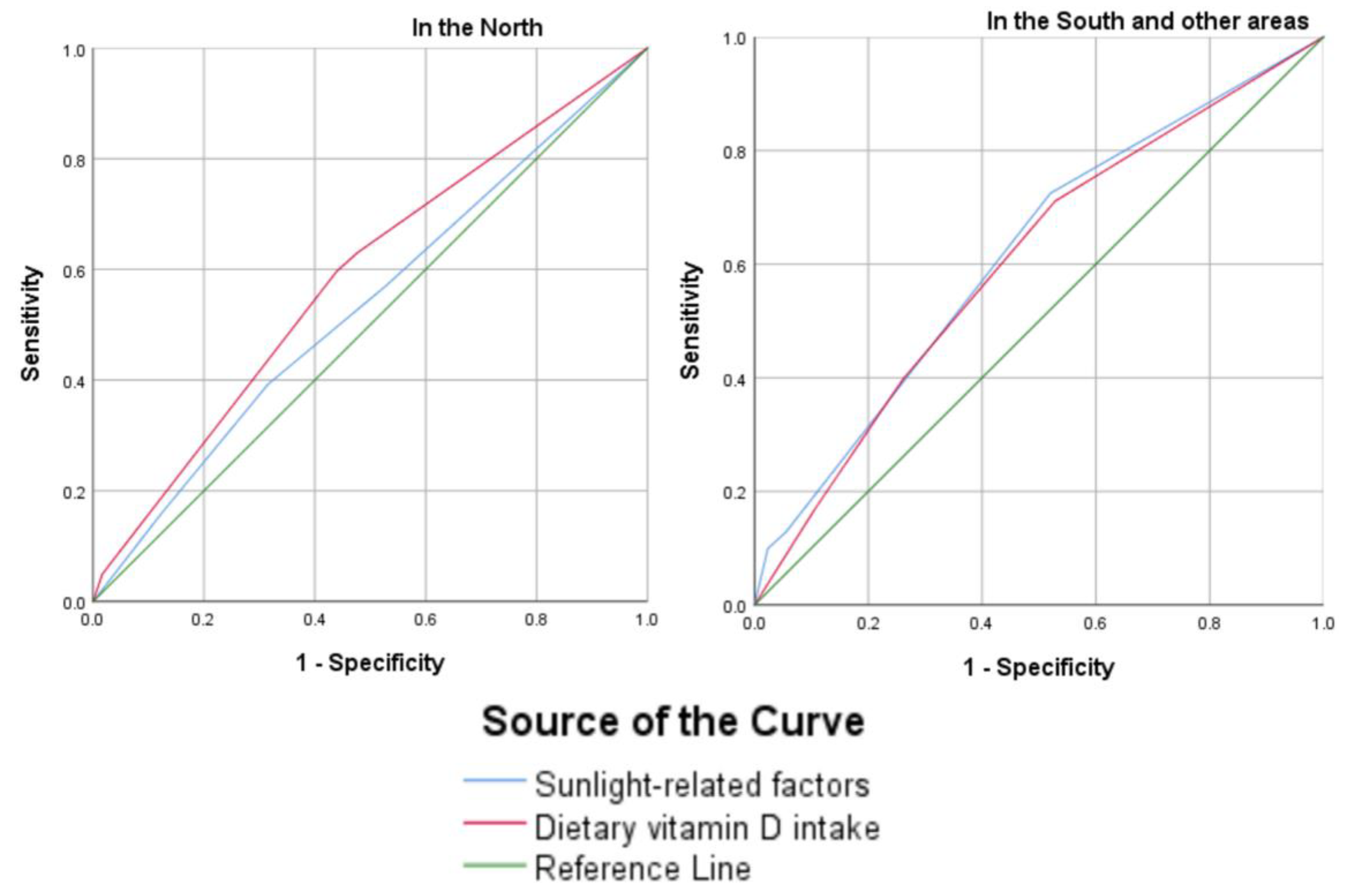
6.2. Associated Factors of Vitamin D Deficiency
7. Discussion
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kiely, M.E.; McCarthy, E.K.; Hennessy, Á. Iron, iodine and vitamin D deficiencies during pregnancy: Epidemiology, risk factors and developmental impacts. Proc. Nutr. Soc. 2021, 80, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Roth, D.E.; Abrams, S.A.; Aloia, J.; Bergeron, G.; Bourassa, M.W.; Brown, K.H.; Calvo, M.S.; Cashman, K.D.; Combs, G.; De-Regil, L.M.; et al. Global prevalence and disease burden of vitamin D deficiency: A roadmap for action in low- and middle-income countries. Ann. N. Y. Acad. Sci. 2018, 1430, 44–79. [Google Scholar] [CrossRef] [PubMed]
- Föcker, M.; Antel, J.; Grasemann, C.; Führer, D.; Timmesfeld, N.; Öztürk, D.; Peters, T.; Hinney, A.; Hebebrand, J.; Libuda, L. Effect of an vitamin D deficiency on depressive symptoms in child and adolescent psychiatric patients—A randomized controlled trial: Study protocol. BMC Psychiatry 2018, 18, 57. [Google Scholar] [CrossRef] [PubMed]
- Woon, F.C.; Chin, Y.S.; Ismail, I.H.; Abdul Latiff, A.H.; Batterham, M.; Chan, Y.M.; On Behalf Of The Micos Research Group. Maternal Vitamin D Levels during Late Pregnancy and Risk of Allergic Diseases and Sensitization during the First Year of Life-A Birth Cohort Study. Nutrients 2020, 12, 2418. [Google Scholar] [CrossRef]
- Balcells, M.E.; García, P.; Tiznado, C.; Villarroel, L.; Scioscia, N.; Carvajal, C.; Zegna-Ratá, F.; Hernández, M.; Meza, P.; González, L.F.; et al. Association of vitamin D deficiency, season of the year, and latent tuberculosis infection among household contacts. PLoS ONE 2017, 12, e0175400. [Google Scholar] [CrossRef]
- Ghasemian, R.; Shamshirian, A.; Heydari, K.; Malekan, M.; Alizadeh-Navaei, R.; Ebrahimzadeh, M.A.; Ebrahimi Warkiani, M.; Jafarpour, H.; Razavi Bazaz, S.; Rezaei Shahmirzadi, A.; et al. The role of vitamin D in the age of COVID-19: A systematic review and meta-analysis. Int. J. Clin. Pract. 2021, 75, e14675. [Google Scholar] [CrossRef]
- Palacios, C.; Gonzalez, L. Is vitamin D deficiency a major global public health problem? J. Steroid Biochem. Mol. Biol. 2014, 144 Pt A, 138–145. [Google Scholar] [CrossRef]
- Heyden, E.L.; Wimalawansa, S.J. Vitamin D: Effects on human reproduction, pregnancy, and fetal well-being. J. Steroid Biochem. Mol. Biol. 2018, 180, 41–50. [Google Scholar] [CrossRef]
- Van der Pligt, P.; Willcox, J.; Szymlek-Gay, E.A.; Murray, E.; Worsley, A.; Daly, R.M. Associations of Maternal Vitamin D Deficiency with Pregnancy and Neonatal Complications in Developing Countries: A Systematic Review. Nutrients 2018, 10, 640. [Google Scholar] [CrossRef]
- Ames, B.N.; Grant, W.B.; Willett, W.C. Does the High Prevalence of Vitamin D Deficiency in African Americans Contribute to Health Disparities? Nutrients 2021, 13, 499. [Google Scholar] [CrossRef]
- Women’s Health. By the Numbers: Health Disparities in Pregnancy. Available online: https://magazine.medlineplus.gov/article/by-the-numbers-health-disparities-in-pregnancy (accessed on 15 January 2023).
- Cashman, K.D. Vitamin D Deficiency: Defining, Prevalence, Causes, and Strategies of Addressing. Calcif. Tissue Int. 2020, 106, 14–29. [Google Scholar] [CrossRef]
- Powers, J.M.; Murphy, J.E.J. Sunlight radiation as a villain and hero: 60 years of illuminating research. Int. J. Radiat. Biol. 2019, 95, 1043–1049. [Google Scholar] [CrossRef]
- Leal, A.; Corrêa, M.P.; Holick, M.F.; Melo, E.V.; Lazaretti-Castro, M. Sun-induced production of vitamin D(3) throughout 1 year in tropical and subtropical regions: Relationship with latitude, cloudiness, UV-B exposure and solar zenith angle. Photochem. Photobiol. Sci 2021, 20, 265–274. [Google Scholar] [CrossRef]
- Benedik, E. Sources of vitamin D for humans. Int. J. Vitam. Nutr. Res. 2022, 92, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Al Anouti, F.; Moukayed, M. Targeted 25-hydroxyvitamin D concentration measurements and vitamin D3 supplementation can have important patient and public health benefits. Eur. J. Clin. Nutr. 2020, 74, 366–376. [Google Scholar] [CrossRef]
- Pérez-López, F.R.; Pilz, S.; Chedraui, P. Vitamin D supplementation during pregnancy: An overview. Curr. Opin. Obstet. Gynecol. 2020, 32, 316–321. [Google Scholar] [CrossRef]
- Geography of Taiwan. Available online: https://en.wikipedia.org/wiki/Geography_of_Taiwan#Climate (accessed on 18 May 2022).
- Pham, T.T.M.; Huang, Y.L.; Chao, J.C.; Chang, J.S.; Chen, Y.C.; Wang, F.F.; Bai, C.H. Plasma 25(OH)D Concentrations and Gestational Diabetes Mellitus among Pregnant Women in Taiwan. Nutrients 2021, 13, 2538. [Google Scholar] [CrossRef] [PubMed]
- Seamans, K.M.; Cashman, K.D. Existing and potentially novel functional markers of vitamin D status: A systematic review. Am. J. Clin. Nutr. 2009, 89, 1997S–2008S. [Google Scholar] [CrossRef]
- Cashman, K.D.; van den Heuvel, E.G.; Schoemaker, R.J.; Prévéraud, D.P.; Macdonald, H.M.; Arcot, J. 25-Hydroxyvitamin D as a Biomarker of Vitamin D Status and Its Modeling to Inform Strategies for Prevention of Vitamin D Deficiency within the Population. Adv. Nutr. 2017, 8, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef]
- Sempos, C.T.; Binkley, N. 25-Hydroxyvitamin D assay standardisation and vitamin D guidelines paralysis. Public Health Nutr. 2020, 23, 1153–1164. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine, S. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Hien, V.T.; Lam, N.T.; Skeaff, C.M.; Todd, J.; McLean, J.M.; Green, T.J. Vitamin D status of pregnant and non-pregnant women of reproductive age living in Hanoi City and the Hai Duong province of Vietnam. Matern. Child Nutr. 2012, 8, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Jing, W.; Ge, S.; Sun, W. Vitamin D status and vitamin D deficiency risk factors among pregnancy of Shanghai in China. BMC Pregnancy Childbirth 2021, 21, 431. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.C.; Chen, H.L.; Tseng, W.T.; Wu, I.C.; Hsu, C.C.; Chang, H.Y.; Chen, Y.I.; Lee, M.M.; Liu, K.; Hsiung, C.A. Circulating 25-hydroxyvitamin D and physical performance in older adults: A nationwide study in Taiwan. Am. J. Clin. Nutr. 2016, 104, 1334–1344. [Google Scholar] [CrossRef]
- Taiwan Geographic Coordinates. Available online: https://www.geodatos.net/en/coordinates/taiwan (accessed on 18 May 2022).
- Leary, P.F.; Zamfirova, I.; Au, J.; McCracken, W.H. Effect of Latitude on Vitamin D Levels. J. Am. Osteopath Assoc. 2017, 117, 433–439. [Google Scholar] [CrossRef]
- Al Zarooni, A.A.R.; Nagelkerke, N.; Al Marzouqi, F.I.; Al Darmaki, S.H. Risk factors for vitamin D deficiency in Abu Dhabi Emirati population. PLoS ONE 2022, 17, e0264064. [Google Scholar] [CrossRef]
- AlFaris, N.A.; AlKehayez, N.M.; AlMushawah, F.I.; AlNaeem, A.N.; AlAmri, N.D.; AlMudawah, E.S. Vitamin D Deficiency and Associated Risk Factors in Women from Riyadh, Saudi Arabia. Sci. Rep. 2019, 9, 20371. [Google Scholar] [CrossRef]
- Takaoka, N.; Nishida, K.; Sairenchi, T.; Umesawa, M.; Noguchi, R.; Someya, K.; Kobashi, G. Changes in vitamin D status considering hemodilution factors in Japanese pregnant women according to trimester: A longitudinal survey. PLoS ONE 2020, 15, e0239954. [Google Scholar] [CrossRef]
- Perreault, M.; Atkinson, S.A.; Meyre, D.; Fusch, G.; Mottola, M.F. Summer Season and Recommended Vitamin D Intake Support Adequate Vitamin D Status throughout Pregnancy in Healthy Canadian Women and Their Newborns. J. Nutr. 2020, 150, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Savard, C.; Bielecki, A.; Plante, A.S.; Lemieux, S.; Gagnon, C.; Weiler, H.A.; Morisset, A.S. Longitudinal Assessment of Vitamin D Status across Trimesters of Pregnancy. J. Nutr. 2021, 151, 1937–1946. [Google Scholar] [CrossRef]
- Shen, Y.; Pu, L.; Si, S.; Xin, X.; Mo, M.; Shao, B.; Wu, J.; Huang, M.; Wang, S.; Muyiduli, X.; et al. Vitamin D nutrient status during pregnancy and its influencing factors. Clin. Nutr. 2020, 39, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.; Walther, B. Natural Vitamin D Content in Animal Products. Adv. Nutr. 2013, 4, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, L.; De Lorenzo, R.; Giustina, A.; Rovere-Querini, P.; Conte, C. Vitamin D in Osteosarcopenic Obesity. Nutrients 2022, 14, 1816. [Google Scholar] [CrossRef]
- Gallagher, J.C.; Yalamanchili, V.; Smith, L.M. The effect of vitamin D supplementation on serum 25OHD in thin and obese women. J. Steroid Biochem. Mol. Biol. 2013, 136, 195–200. [Google Scholar] [CrossRef]
- Savastano, S.; Barrea, L.; Savanelli, M.C.; Nappi, F.; Di Somma, C.; Orio, F.; Colao, A. Low vitamin D status and obesity: Role of nutritionist. Rev. Endocr. Metab. Disord. 2017, 18, 215–225. [Google Scholar] [CrossRef]
- Yang, Y.; Cai, Z.; Zhang, J. The effect of prepregnancy body mass index on maternal micronutrient status: A meta-analysis. Sci. Rep. 2021, 11, 18100. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, L.M.; Catov, J.M.; Roberts, J.M.; Simhan, H.N. Prepregnancy obesity predicts poor vitamin D status in mothers and their neonates. J. Nutr. 2007, 137, 2437–2442. [Google Scholar] [CrossRef]
| Variables | Total | Non-VDD (1095, 72.9%) | VDD (407, 27.1%) | p |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Maternal age (years) (mean ± SD) | 32.5 ± 4.8 | 32.7 ± 4.8 | 32.1 ± 4.8 | 0.017 |
| Residential area | <0.001 | |||
| North | 501 (33.4) | 312 (28.5) | 189 (46.4) | |
| Central | 371 (24.7) | 260 (23.7) | 111 (27.3) | |
| South and east | 291 (19.4) | 254 (23.2) | 37 (9.1) | |
| Eastern and outlying islands | 339 (22.6) | 269 (24.6) | 70 (17.2) | |
| Education level * | 0.291 | |||
| High school and below | 237 (15.9) | 182 (16.8) | 55 (13.5) | |
| College, university | 1025 (68.7) | 740 (68.1) | 285 (70.0) | |
| Postgraduate studies | 231 (15.5) | 164 (15.1) | 67 (16.5) | |
| Household monthly income | 0.465 | |||
| Less than NT$30,000 | 212 (14.4) | 162 (15.1) | 50 (12.5) | |
| NT$30,000–59,999 | 634 (43.0) | 464 (43.2) | 170 (42.4) | |
| NT$60,000–99,999 | 443 (30.1) | 318 (29.6) | 125 (31.2) | |
| More than NT$100,000 | 185 (12.6) | 129 (12.0) | 56 (14.0) | |
| Religion | 0.242 | |||
| None | 689 (45.9) | 488 (44.6) | 201 (49.4) | |
| Buddhism | 281 (18.7) | 205 (18.7) | 76 (18.7) | |
| Taoism | 345 (23.0) | 265 (24.2) | 80 (19.7) | |
| Other (Yiguandao, Christian, Catholic, Muslim) | 187 (12.5) | 137 (12.5) | 50 (12.3) | |
| Gravidity * | 0.061 | |||
| 1 | 694 (46.3) | 487 (44.6) | 207 (51.0) | |
| 2 | 498 (33.2) | 366 (33.6) | 132 (32.5) | |
| 3 | 199 (13.3) | 158 (14.5) | 41 (10.1) | |
| ≥4 | 107 (7.1) | 81 (7.4) | 26 (6.4) | |
| The ordinal of current pregnancy (parity) * | 0.010 | |||
| 1st child | 824 (55.0) | 577 (52.9) | 247 (60.7) | |
| 2nd child | 527 (35.2) | 395 (36.2) | 132 (32.4) | |
| ≥3rd child | 146 (9.8) | 118 (10.8) | 28 (6.9) | |
| Number of fetuses in this pregnancy | 0.972 | |||
| ≥2 | 33 (2.2) | 24 (2.2) | 9 (2.2) | |
| Gestational age | <0.001 | |||
| 1st trimester (less than 17 weeks) | 375 (25.0) | 235 (21.5) | 140 (34.4) | |
| 2nd trimester (17 weeks to less than 29 weeks) | 485 (32.3) | 357 (32.6) | 128 (31.4) | |
| 3rd trimester (more than 29 weeks) | 642 (42.7) | 503 (45.9) | 139 (34.2) | |
| Pre-pregnancy BMI * | 0.098 | |||
| Normal (18.5 ≤ BMI < 25.0) | 141 (9.4) | 99 (9.1) | 42 (10.3) | |
| Underweight (<18.5) | 1018 (68.1) | 730 (67.0) | 288 (70.9) | |
| Overweight/obese (≥25.0) | 336 (22.5) | 260 (23.9) | 76 (18.7) | |
| Dairy products * | 0.546 | |||
| Enough | 1213 (81.2) | 888 (81.6) | 325 (80.2) | |
| Egg * | 0.034 | |||
| Enough | 1397 (93.6) | 1027 (94.4) | 370 (91.4) | |
| Red meat * | <0.001 | |||
| Enough | 1390 (93.1) | 1029 (94.6) | 361 (89.1) | |
| Nut fruits * | 0.514 | |||
| Enough | 875 (58.6) | 643 (59.2) | 232 (57.3) | |
| Fat (%) (mean ± SD) | 35.8 ± 9.0 | 36.1 ± 9.1 | 34.9 ± 8.9 | 0.023 |
| Protein (%) (mean ± SD) | 15.3 ± 3.7 | 15.3 ± 3.7 | 15.0 ± 3.7 | 0.147 |
| Carbohydrate (%) (mean ± SD) | 49.8 ± 9.8 | 49.4 ± 9.9 | 50.8 ± 9.5 | 0.013 |
| Vitamin D content (g) (median, IQR) | 2.8 (7.7) | 2.8 (9.6) | 2.5 (4.8) | 0.031 |
| Vitamin supplements * | <0.001 | |||
| Vitamin D and/or Calcium | 698 (47.7) | 560 (52.3) | 138 (35.0) | |
| Sun exposure | 0.001 | |||
| Yes | 1046 (69.6) | 789 (72.1) | 257 (63.1) | |
| Protective methods for sunshine (mean ± SD) | 1.6 ± 1.3 | 1.6 ± 1.3 | 1.6 ± 1.3 | 0.504 |
| Remained indoors during pregnancy | 0.018 | |||
| Yes | 228 (15.3) | 152 (14.0) | 76 (19.0) | |
| Season of blood draw | 0.004 | |||
| Sunny months | 927 (61.7) | 700 (63.9) | 227 (55.8) |
| Variables | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Age | 0.96 | 0.93–0.98 | 0.005 | 0.95 | 0.93–0.98 | <0.001 |
| Residential area | ||||||
| North | 1.00 | |||||
| Central | 0.68 | 0.50–0.94 | 0.021 | 0.66 | 0.48–0.90 | 0.010 |
| South and east | 0.22 | 0.14–0.33 | <0.001 | 0.20 | 0.13–0.31 | <0.001 |
| Eastern and outlying Islands | 0.36 | 0.25–0.52 | <0.001 | 0.33 | 0.23–0.47 | <0.001 |
| The ordinal of current pregnancy (parity) | ||||||
| 1st child | 1.00 | |||||
| 2nd child | 0.83 | 0.62–1.10 | 0.203 | |||
| ≥3rd child | 0.69 | 0.42–1.12 | 0.141 | |||
| Gestational age | ||||||
| 1st trimester (less than 17 weeks) | 1.00 | 1.00 | ||||
| 2nd trimester (17 weeks to less than 29 weeks) | 0.73 | 0.52–1.01 | 0.054 | 0.72 | 0.52–0.99 | 0.046 |
| 3rd trimester (more than 29 weeks) | 0.61 | 0.44–0.84 | 0.002 | 0.60 | 0.44–0.83 | 0.002 |
| Pre-pregnancy BMI | ||||||
| Normal (18.5 ≤ BMI < 25.0) | 1.00 | |||||
| Underweight (<18.5) | 1.04 | 0.67–1.59 | 0.850 | |||
| Overweight/obese (≥25.0) | 0.87 | 0.63–1.20 | 0.397 | |||
| Egg intake | ||||||
| Not enough | 1.00 | |||||
| Enough | 0.72 | 0.43–1.23 | 0.236 | |||
| Red meat intake | ||||||
| Not enough | 1.00 | 1.00 | ||||
| Enough | 0.54 | 0.34–0.86 | 0.010 | 0.50 | 0.32–0.78 | 0.002 |
| Fat (%) | 0.99 | 0.98–1.01 | 0.711 | |||
| Vitamin D content | ||||||
| ≤median | 1.00 | 1.00 | ||||
| >median | 0.80 | 0.62–1.03 | 0.091 | 0.78 | 0.60–1.00 | 0.057 |
| Vitamin supplements | ||||||
| No relevant supplements | 1.00 | 1.00 | ||||
| Vitamin D and/or calcium | 0.47 | 0.36–0.62 | <0.001 | 0.51 | 0.39–0.66 | <0.001 |
| Sun exposure | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 0.77 | 0.59–1.01 | 0.064 | 0.75 | 0.57–0.98 | 0.034 |
| Remained indoors during pregnancy | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.33 | 0.95–1.87 | 0.089 | 1.35 | 0.97–1.88 | 0.071 |
| Season of blood draw | ||||||
| Rainy months | 1.00 | |||||
| Sunny months | 0.57 | 0.44–0.75 | <0.001 | 0.59 | 0.46–0.77 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-L.; Pham, T.T.M.; Chen, Y.-C.; Chang, J.-S.; Chao, J.C.-J.; Bai, C.-H. Effects of Climate, Sun Exposure, and Dietary Intake on Vitamin D Concentrations in Pregnant Women: A Population-Based Study. Nutrients 2023, 15, 1182. https://doi.org/10.3390/nu15051182
Huang Y-L, Pham TTM, Chen Y-C, Chang J-S, Chao JC-J, Bai C-H. Effects of Climate, Sun Exposure, and Dietary Intake on Vitamin D Concentrations in Pregnant Women: A Population-Based Study. Nutrients. 2023; 15(5):1182. https://doi.org/10.3390/nu15051182
Chicago/Turabian StyleHuang, Ya-Li, Thu T. M. Pham, Yi-Chun Chen, Jung-Su Chang, Jane C.-J. Chao, and Chyi-Huey Bai. 2023. "Effects of Climate, Sun Exposure, and Dietary Intake on Vitamin D Concentrations in Pregnant Women: A Population-Based Study" Nutrients 15, no. 5: 1182. https://doi.org/10.3390/nu15051182
APA StyleHuang, Y.-L., Pham, T. T. M., Chen, Y.-C., Chang, J.-S., Chao, J. C.-J., & Bai, C.-H. (2023). Effects of Climate, Sun Exposure, and Dietary Intake on Vitamin D Concentrations in Pregnant Women: A Population-Based Study. Nutrients, 15(5), 1182. https://doi.org/10.3390/nu15051182








