Update of a Genetic Risk Score Predictive of the Plasma Triglyceride Response to an Omega-3 Fatty Acid Supplementation in the FAS Study
Abstract
1. Introduction
2. Materials and Methods
2.1. FAS Study Population
2.2. Study Design
2.3. SNP Genotyping
2.4. GRS Construction
2.5. Statistical Analyses
2.6. Evaluation of the Predictive Performance
3. Results
3.1. Characteristics of Participants
3.2. Association between SNPs and Plasma Lipid Traits
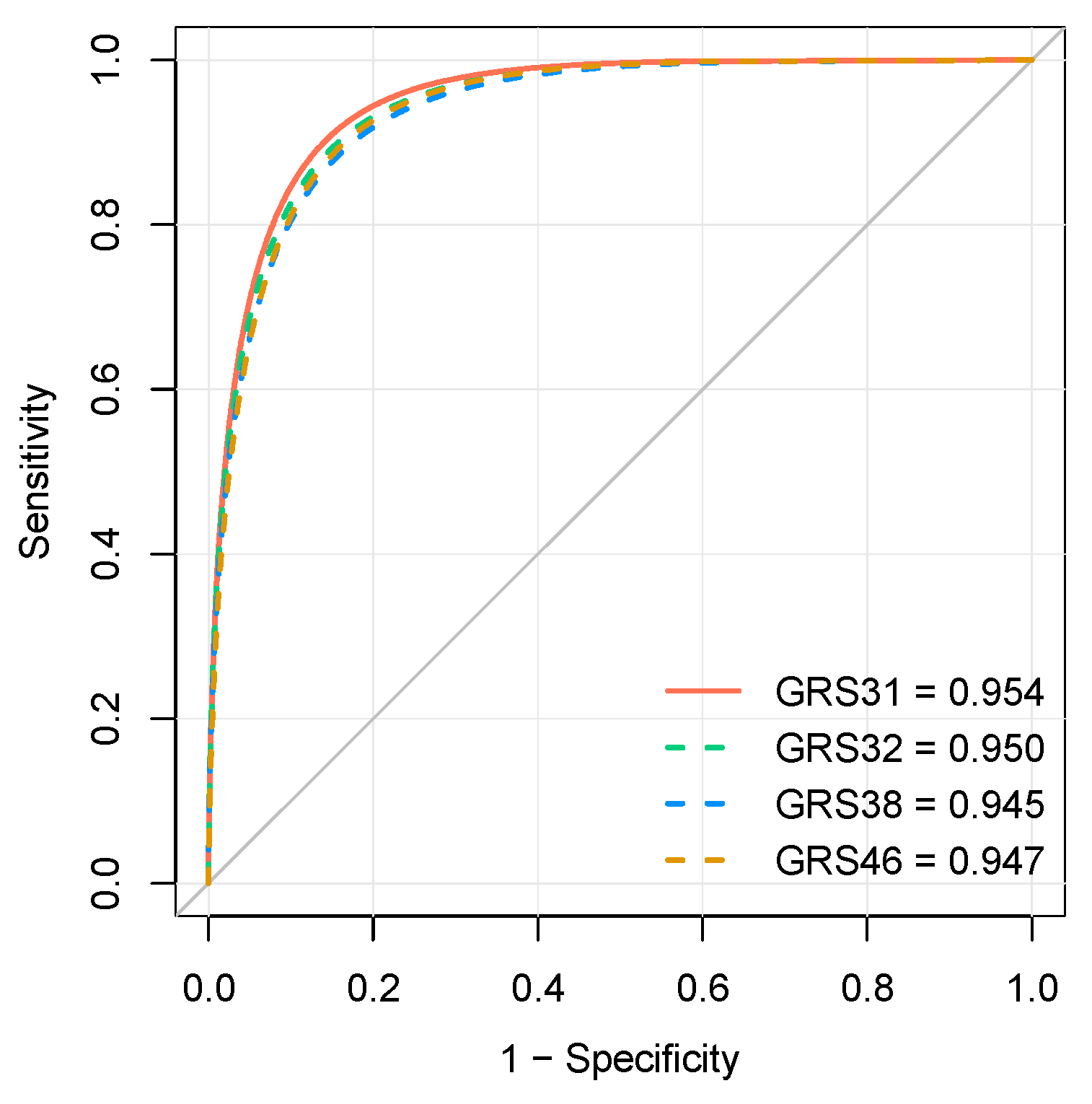
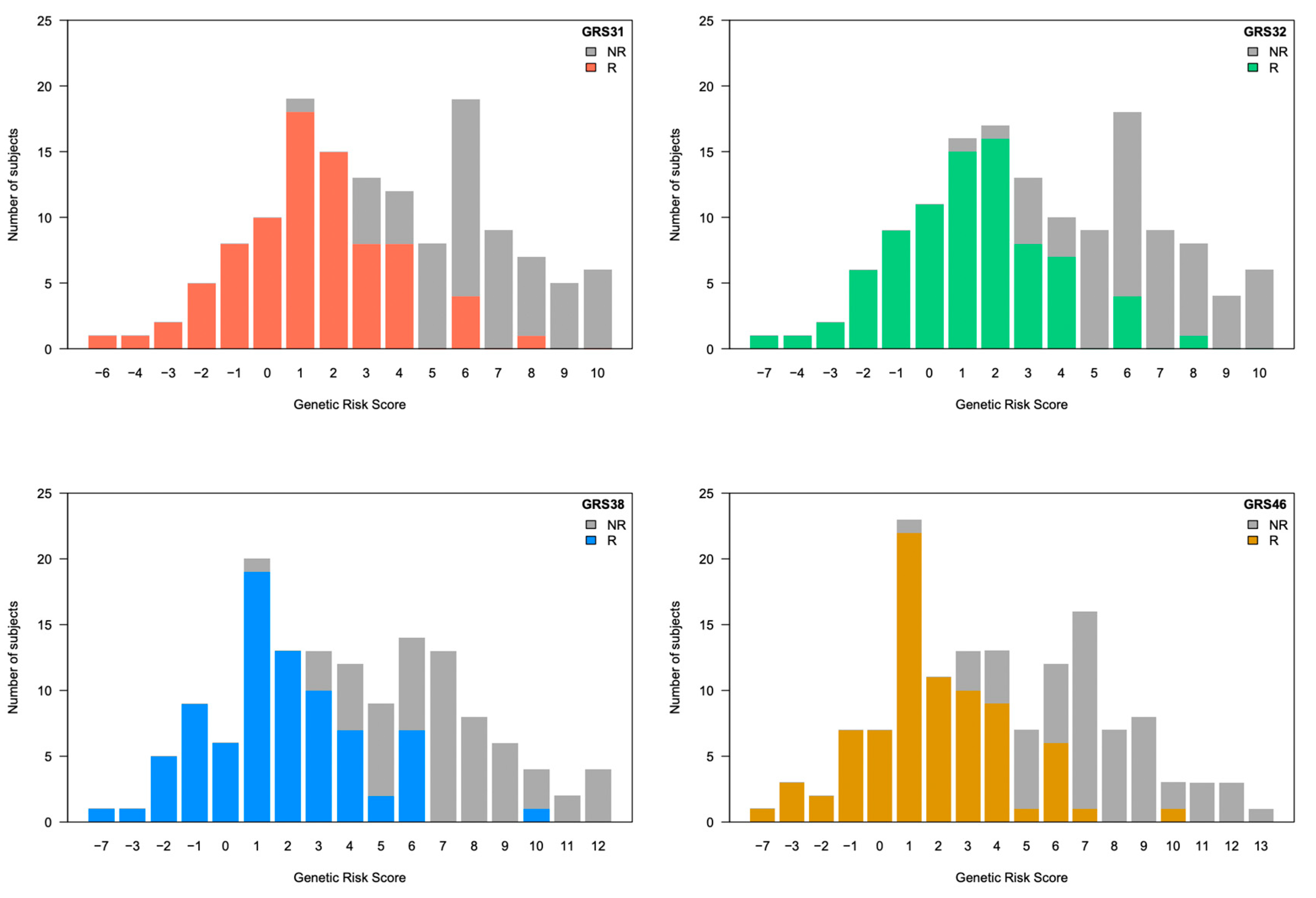
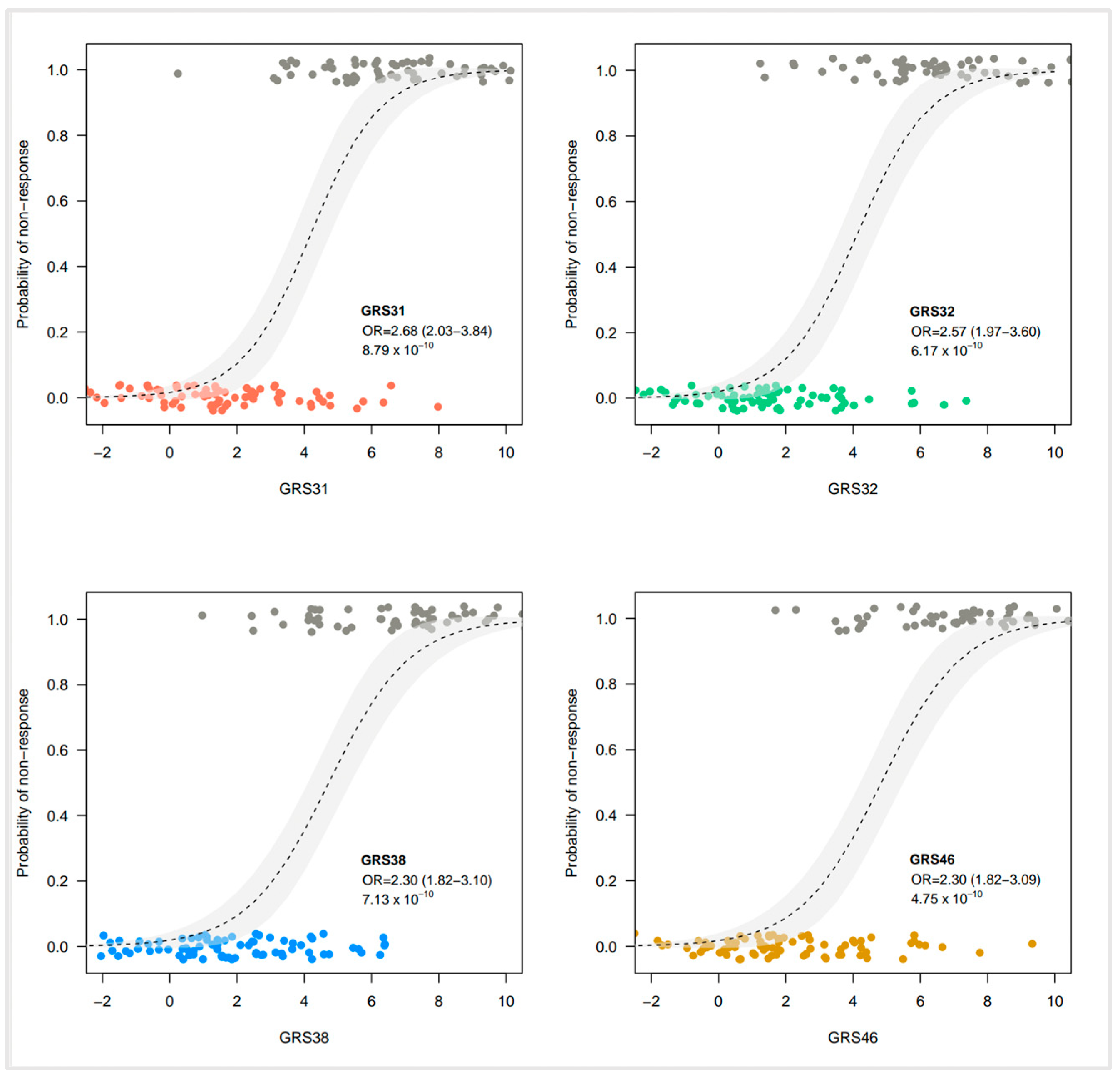
3.3. Evaluation of Genetic Risk Scores
3.4. Comparison of Genetic Risk Scores
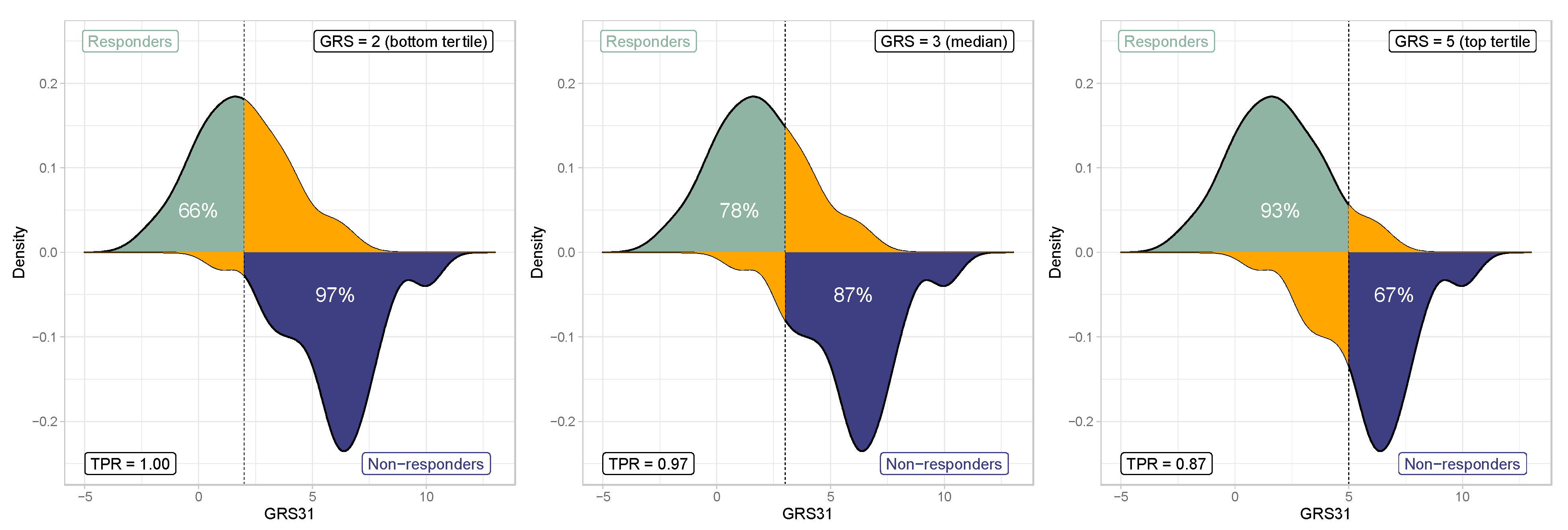
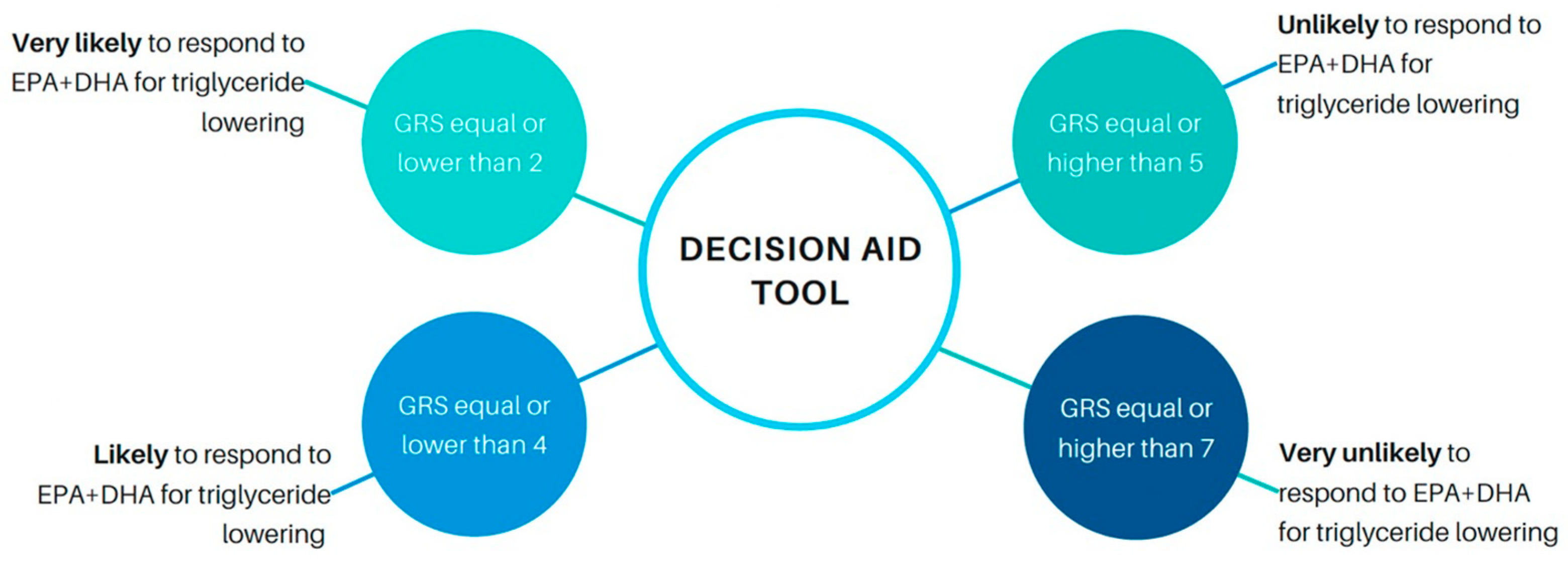
3.5. Genetic Risk Score Threshold Selection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, Y.; Hu, F.B.; Manson, J.E. Marine Omega-3 Supplementation and Cardiovascular Disease: An Updated Meta-Analysis of 13 Randomized Controlled Trials Involving 127,477 Participants. J. Am. Heart Assoc. 2019, 8, e013543. [Google Scholar] [CrossRef]
- Karanchi, H.; Muppidi, V.; Wyne, K. Hypertriglyceridemia. In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2022. Available online: http://www.ncbi.nlm.nih.gov/books/NBK459368/ (accessed on 26 August 2022).
- Caslake, M.J.; Miles, E.A.; Kofler, B.M.; Lietz, G.; Curtis, P.; Armah, C.K.; Kimber, A.C.; Grew, J.P.; Farrell, L.; Stannard, J.; et al. Effect of sex and genotype on cardiovascular biomarker response to fish oils: The FINGEN Study. Am. J. Clin. Nutr. 2008, 88, 618–629. [Google Scholar] [CrossRef]
- Rudkowska, I.; Guénard, F.; Julien, P.; Couture, P.; Lemieux, S.; Barbier, O.; Calder, P.C.; Minihane, A.M.; Vohl, M.-C. Genome-wide association study of the plasma triglyceride response to an n-3 polyunsaturated fatty acid supplementation. J. Lipid Res. 2014, 55, 1245–1253. [Google Scholar] [CrossRef]
- Vallée Marcotte, B.; Guénard, F.; Lemieux, S.; Couture, P.; Rudkowska, I.; Calder, P.C.; Minihane, A.M.; Vohl, M.-C. Fine mapping of genome-wide association study signals to identify genetic markers of the plasma triglyceride response to an omega-3 fatty acid supplementation. Am. J. Clin. Nutr. 2019, 109, 176–185. [Google Scholar] [CrossRef]
- Francis, M.; Li, C.; Sun, Y.; Zhou, J.; Li, X.; Brenna, J.T.; Ye, K. Genome-wide association study of fish oil supplementation on lipid traits in 81,246 individuals reveals new gene-diet interaction loci. PLoS Genet. 2021, 17, e1009431. [Google Scholar] [CrossRef]
- Cormier, H.; Rudkowska, I.; Paradis, A.-M.; Thifault, E.; Garneau, V.; Lemieux, S.; Couture, P.; Vohl, M.-C. Association between polymorphisms in the fatty acid desaturase gene cluster and the plasma triacylglycerol response to an n-3 PUFA supplementation. Nutrients 2012, 4, 1026–1041. [Google Scholar] [CrossRef]
- Rudkowska, I.; Paradis, A.-M.; Thifault, E.; Julien, P.; Barbier, O.; Couture, P.; Lemieux, S.; Vohl, M.-C. Differences in metabolomic and transcriptomic profiles between responders and non-responders to an n-3 polyunsaturated fatty acids (PUFAs) supplementation. Genes Nutr. 2013, 8, 411–423. [Google Scholar] [CrossRef]
- Canada, H. Eating Well with Canada’s Food Guide [Guidance]. 5 February 2007. Available online: https://www.canada.ca/en/health-canada/services/canada-food-guide/about/history-food-guide/eating-well-with-canada-food-guide-2007.html (accessed on 26 August 2022).
- Centre de Génomique Clinique Pédiatrique Intégré Génome Québec. (n.d.). Available online: https://www.genomequebec.com/centre-de-genomique-clinique-pediatrique-integre-genome-quebec/ (accessed on 5 September 2022).
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Behl, S.; Hamel, N.; de Ladurantaye, M.; Lepage, S.; Lapointe, R.; Mes-Masson, A.-M.; Foulkes, W.D. Founder BRCA1/BRCA2/PALB2 pathogenic variants in French-Canadian breast cancer cases and controls. Sci. Rep. 2020, 10, 6491. [Google Scholar] [CrossRef]
- Missense Variants Reveal Functional Insights into the Human ARID Family of Gene Regulators—PubMed. (n.d.). Available online: https://pubmed.ncbi.nlm.nih.gov/35257783/ (accessed on 26 August 2022).
- Kanoni, S.; Masca, N.G.D.; Stirrups, K.E.; Varga, T.V.; Warren, H.R.; Scott, R.A.; Southam, L.; Zhang, W.; Yaghootkar, H.; Müller-Nurasyid, M.; et al. Analysis with the exome array identifies multiple new independent variants in lipid loci. Hum. Mol. Genet. 2016, 25, 4094–4106. [Google Scholar] [CrossRef]
- Peloso, G.M.; Auer, P.L.; Bis, J.C.; Voorman, A.; Morrison, A.C.; Stitziel, N.O.; Brody, J.A.; Khetarpal, S.A.; Crosby, J.R.; Fornage, M.; et al. Association of Low-Frequency and Rare Coding-Sequence Variants with Blood Lipids and Coronary Heart Disease in 56,000 Whites and Blacks. Am. J. Hum. Genet. 2014, 94, 223–232. [Google Scholar] [CrossRef]
- PubChem. (n.d.). GJB6—Gap Junction Protein Beta 6 (Human). Available online: https://pubchem.ncbi.nlm.nih.gov/gene/GJB6/human (accessed on 5 September 2022).
- BAZ1B Bromodomain Adjacent to Zinc Finger Domain 1B [Homo Sapiens (Human)]—Gene—NCBI. (n.d.). Available online: https://www.ncbi.nlm.nih.gov/gene/9031 (accessed on 5 September 2022).
- Kong, X.; Zhao, Q.; Xing, X.; Zhang, B.; Zhang, X.; Hong, J.; Yang, W. Genetic Variants Associated with Lipid Profiles in Chinese Patients with Type 2 Diabetes. PLoS ONE 2015, 10, e0135145. [Google Scholar] [CrossRef]
- MAP1A Microtubule Associated Protein 1A [Homo Sapiens (Human)]—Gene—NCBI. (n.d.). Available online: https://www.ncbi.nlm.nih.gov/gene/4130 (accessed on 5 September 2022).
- HAPLN4 Hyaluronan and Proteoglycan Link Protein 4 [Homo Sapiens (Human)]—Gene—NCBI. (n.d.). Available online: https://www.ncbi.nlm.nih.gov/gene/404037 (accessed on 5 September 2022).
- Cadby, G.; Giles, C.; Melton, P.E.; Huynh, K.; Mellett, N.A.; Duong, T.; Nguyen, A.; Cinel, M.; Smith, A.; Olshansky, G.; et al. Comprehensive genetic analysis of the human lipidome identifies loci associated with lipid homeostasis with links to coronary artery disease. Nat. Commun. 2022, 13, 3124. [Google Scholar] [CrossRef]
- PubChem. (n.d.-a). LPL—Lipoprotein Lipase (Human). Available online: https://pubchem.ncbi.nlm.nih.gov/gene/LPL/human (accessed on 27 August 2022).
- Wang, S.; Cheng, Y.; Shi, Y.; Zhao, W.; Gao, L.; Fang, L.; Jin, X.; Han, X.; Sun, Q.; Li, G.; et al. Identification and Characterization of Two Novel Compounds: Heterozygous Variants of Lipoprotein Lipase in Two Pedigrees with Type I Hyperlipoproteinemia. Front. Endocrinol. 2022, 13, 874608. [Google Scholar] [CrossRef]
- Harshfield, E.L.; Fauman, E.B.; Stacey, D.; Paul, D.S.; Ziemek, D.; Ong, R.M.Y.; Danesh, J.; Butterworth, A.S.; Rasheed, A.; Sattar, T.; et al. Genome-wide analysis of blood lipid metabolites in over 5000 South Asians reveals biological insights at cardiometabolic disease loci. BMC Med. 2021, 19, 232. [Google Scholar] [CrossRef]
- PubChem. (n.d.-b). MLXIPL—MLX Interacting Protein Like (Human). Available online: https://pubchem.ncbi.nlm.nih.gov/gene/MLXIPL/human (accessed on 5 September 2022).
- Chambers, J.C.; Zhang, W.; Sehmi, J.; Li, X.; Wass, M.N.; Van der Harst, P.; Holm, H.; Sanna, S.; Kavousi, M.; Baumeister, S.E.; et al. Genome-wide association study identifies loci influencing concentrations of liver enzymes in plasma. Nat. Genet. 2011, 43, 1131–1138. [Google Scholar] [CrossRef]
- PubChem. (n.d.-c). SLC12A3—Solute Carrier Family 12 Member 3 (Human). Available online: https://pubchem.ncbi.nlm.nih.gov/gene/SLC12A3/human (accessed on 5 September 2022).
- An, C.; Liang, J.; Zhang, K.; Su, X. Genetic variants of SLC12A3 modulate serum lipid profiles in a group of Mongolian pedigree population. Lipids Health Dis. 2018, 17, 83. [Google Scholar] [CrossRef]
- An, C.; Zhang, K.; Su, X. SLC12A3 variants modulate LDL cholesterol levels in the Mongolian population. Lipids Health Dis. 2017, 16, 29. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, T.; Tan, X.; Lei, S.; Huang, L.; Yang, L. Polymorphisms in PCSK9, LDLR, BCMO1, SLC12A3, and KCNJ1 Are Associated with Serum Lipid Profile in Chinese Han Population. Int. J. Environ. Res. Public Health 2019, 16, 3207. [Google Scholar] [CrossRef]
- ABCA6 ATP Binding Cassette Subfamily A Member 6 [Homo Sapiens (Human)]—Gene—NCBI. (n.d.). Available online: https://www.ncbi.nlm.nih.gov/gene/23460 (accessed on 5 September 2022).
- Van Leeuwen, E.M.; Karssen, L.C.; Deelen, J.; Isaacs, A.; Medina-Gomez, C.; Mbarek, H.; Kanterakis, A.; Trompet, S.; Postmus, I.; Verweij, N.; et al. Genome of the Netherlands population-specific imputations identify an ABCA6 variant associated with cholesterol levels. Nat. Commun. 2015, 6, 6065. [Google Scholar] [CrossRef]
- Mens, M.M.J.; Maas, S.C.E.; Klap, J.; Weverling, G.J.; Klatser, P.; Brakenhoff, J.P.J.; van Meurs, J.B.J.; Uitterlinden, A.G.; Ikram, M.A.; Kavousi, M.; et al. Multi-Omics Analysis Reveals MicroRNAs Associated with Cardiometabolic Traits. Front. Genet. 2020, 11, 110. [Google Scholar] [CrossRef] [PubMed]
- Keathley, J.; Garneau, V.; Marcil, V.; Mutch, D.M.; Robitaille, J.; Rudkowska, I.; Sofian, G.M.; Desroches, S.; Vohl, M.-C. Nutrigenetics, omega-3 and plasma lipids/lipoproteins/apolipoproteins with evidence evaluation using the GRADE approach: A systematic review. BMJ Open 2022, 12, e054417. [Google Scholar] [CrossRef] [PubMed]
| SNP | CHR | BP | Nearest Gene (Location) | Major/Minor Allele | MAF (%) | HWE P | Phenotype |
|---|---|---|---|---|---|---|---|
| rs115675705 | 6 | 34,094,919 | GRM4 (intron) | T/C | 1.4 | 1 | HDL |
| rs117788606 | 7 | 72,921,771 | BAZ1B (intron) | A/G | 0.7 | 1 | TG |
| rs11983997 | 7 | 72,939,244 | BAZ1B (upstream) | C/G | 20.0 | 0.2 | TG |
| rs80189144 | 7 | 72,939,939 | BAZ1B (upstream) | T/C | 13.2 | 0.1 | TG |
| rs799157 | 7 | 73,020,301 | MLXIPL (synonymous) | C/T | 3.9 | 1 | LDL |
| rs117860853 | 8 | 19,722,204 | LPL (upstream) | G/A | 1.8 | 1 | HDL |
| rs142084074 | 8 | 19,768,150 | LOC107986921 (intron) | G/A | 0.7 | 1 | TG |
| rs144018203 | 11 | 116,916,060 | SIK3 (intron) | G/C | 0.7 | 1 | HDL.TG |
| rs112803755 | 13 | 20,790,451 | GJB6 (downstream) | A/G | 1.4 | 1 | TG |
| rs55707100 | 15 | 43,820,717 | MAP1A (missense) | C/T | 4.6 | 1 | TG |
| rs148931404 | 16 | 56,914,455 | SLC12A3 (intron) | C/T | 1.1 | 1 | HDL |
| rs147438979 | 17 | 42,061,277 | PYY (intron) | G/C | 1.8 | 1 | HDL |
| rs77542162 | 17 | 67,081,278 | ABCA6 (missense) | T/C | 0.4 | 1 | LDL.TC |
| rs141844019 | 19 | 19,365,178 | HAPLN4 (downstream) | G/A | 1.1 | 1 | TG |
| rs112952132 | 19 | 45,198,060 | LOC107985305 (intron) | G/A | 0.7 | 1 | LDL |
| MAF (%) | |||||
|---|---|---|---|---|---|
| SNP | R | NR | OR | (95% CI) | GRS |
| rs115675705 | 0.01 | 0.02 | 0.73 | (0.10–5.22) | 1 |
| rs117788606 | 0.01 | 0.01 | 0.74 | (0.05–11.9) | 1 |
| rs11983997 | 0.18 | 0.23 | 0.73 | (0.41–1.32) | 1 |
| rs80189144 | 0.12 | 0.14 | 0.84 | (0.42–1.68) | 1 |
| rs799157 | 0.03 | 0.05 | 0.59 | (0.18–2.00) | 1 |
| rs117860853 | 0.01 | 0.03 | 0.48 | (0.08–2.91) | 1 |
| rs142084074 | 0.01 | 0.01 | 0.74 | (0.05–11.9) | 1 |
| rs144018203 | 0.01 | 0.01 | 0.73 | (0.04–11.7) | 1 |
| rs112803755 | 0.01 | 0.02 | 0.74 | (0.10–5.31) | 1 |
| rs55707100 | 0.06 | 0.03 | 1.71 | (0.51–5.68) | −1 |
| rs148931404 | 0.01 | 0.01 | 1.46 | (0.13–16.3) | −1 |
| rs147438979 | 0.02 | 0.01 | 2.96 | (0.33–26.9) | −1 |
| rs77542162 | 0.00 | 0.01 | - | - | - |
| rs141844019 | 0.01 | 0.01 | 1.49 | (0.13–16.6) | −1 |
| rs112952132 | 0.01 | 0.01 | 0.73 | (0.04–11.7) | 1 |
| p-Value (Treatment-by-Visit Interaction Term) | ||||
|---|---|---|---|---|
| SNP | TG | LDL-C | HDL-C | TC |
| rs115675705 | 0.96 | 0.21 | 0.04 * | 0.11 |
| rs11983997 | 0.43 | 0.25 | 0.02 * | 0.02 * |
| rs80189144 | 0.90 | 0.51 | 0.01 * | 0.06 |
| rs799157 | 0.29 | 0.52 | 0.37 | 0.43 |
| rs117860853 | 0.35 | 0.81 | 0.73 | 0.86 |
| rs144018203 | 0.58 | 0.33 | 0.14 | 0.10 |
| rs112803755 | 0.98 | 0.19 | 0.48 | 0.11 |
| rs55707100 | 0.67 | 0.60 | 0.65 | 0.66 |
| rs148931404 | 0.53 | 0.14 | 0.79 | 0.17 |
| rs147438979 | 0.86 | 0.43 | 0.46 | 0.73 |
| rs77542162 | 0.06 | 0.34 | 0.93 | 0.19 |
| rs112952132 | 0.72 | 0.33 | 0.71 | 0.30 |
| rs117788606 | 0.70 | 0.33 | 0.92 | 0.41 |
| rs141844019 | 0.87 | 0.09 | 0.001 * | 0.02 * |
| rs142084074 | 0.84 | 0.11 | 0.001 * | 0.02 * |
| GRS31 | GRS32 | GRS38 | GRS46 | |||||
|---|---|---|---|---|---|---|---|---|
| Train | Test | Train | Test | Train | Test | Train | Test | |
| Accuracy | 0.93 | 0.77 | 0.93 | 0.77 | 0.91 | 0.76 | 0.90 | 0.77 |
| AUC-ROC | 0.97 | 0.87 | 0.97 | 0.86 | 0.97 | 0.84 | 0.97 | 0.85 |
| Sensitivity | 0.93 | 0.73 | 0.93 | 0.73 | 0.90 | 0.70 | 0.86 | 0.73 |
| Specificity | 0.92 | 0.80 | 0.92 | 0.80 | 0.92 | 0.80 | 0.92 | 0.80 |
| McFadden | 0.66 | 0.48 | 0.64 | 0.48 | 0.61 | 0.45 | 0.61 | 0.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gauthier, E.; de Toro-Martín, J.; Vallée-Marcotte, B.; Lemieux, S.; Rudkowska, I.; Couture, P.; Vohl, M.-C. Update of a Genetic Risk Score Predictive of the Plasma Triglyceride Response to an Omega-3 Fatty Acid Supplementation in the FAS Study. Nutrients 2023, 15, 1156. https://doi.org/10.3390/nu15051156
Gauthier E, de Toro-Martín J, Vallée-Marcotte B, Lemieux S, Rudkowska I, Couture P, Vohl M-C. Update of a Genetic Risk Score Predictive of the Plasma Triglyceride Response to an Omega-3 Fatty Acid Supplementation in the FAS Study. Nutrients. 2023; 15(5):1156. https://doi.org/10.3390/nu15051156
Chicago/Turabian StyleGauthier, Ellie, Juan de Toro-Martín, Bastien Vallée-Marcotte, Simone Lemieux, Iwona Rudkowska, Patrick Couture, and Marie-Claude Vohl. 2023. "Update of a Genetic Risk Score Predictive of the Plasma Triglyceride Response to an Omega-3 Fatty Acid Supplementation in the FAS Study" Nutrients 15, no. 5: 1156. https://doi.org/10.3390/nu15051156
APA StyleGauthier, E., de Toro-Martín, J., Vallée-Marcotte, B., Lemieux, S., Rudkowska, I., Couture, P., & Vohl, M.-C. (2023). Update of a Genetic Risk Score Predictive of the Plasma Triglyceride Response to an Omega-3 Fatty Acid Supplementation in the FAS Study. Nutrients, 15(5), 1156. https://doi.org/10.3390/nu15051156







