Supplementation with Natto and Red Yeast Rice Alters Gene Expressions in Cholesterol Metabolism Pathways in ApoE-/- Mice with Concurrent Changes in Gut Microbiota
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Diets
2.2. Biochemical Measurement of Blood Samples
2.3. Atherosclerotic Lesion Measurement
2.4. Gene Expression Analysis
2.5. Gut Microbiome Analysis
2.6. Fecal SCFAs Measurement
2.7. Statistical Analysis
2.7.1. 16S rRNA Sequencing Data Analysis
2.7.2. Additional Statistical Analysis
3. Results
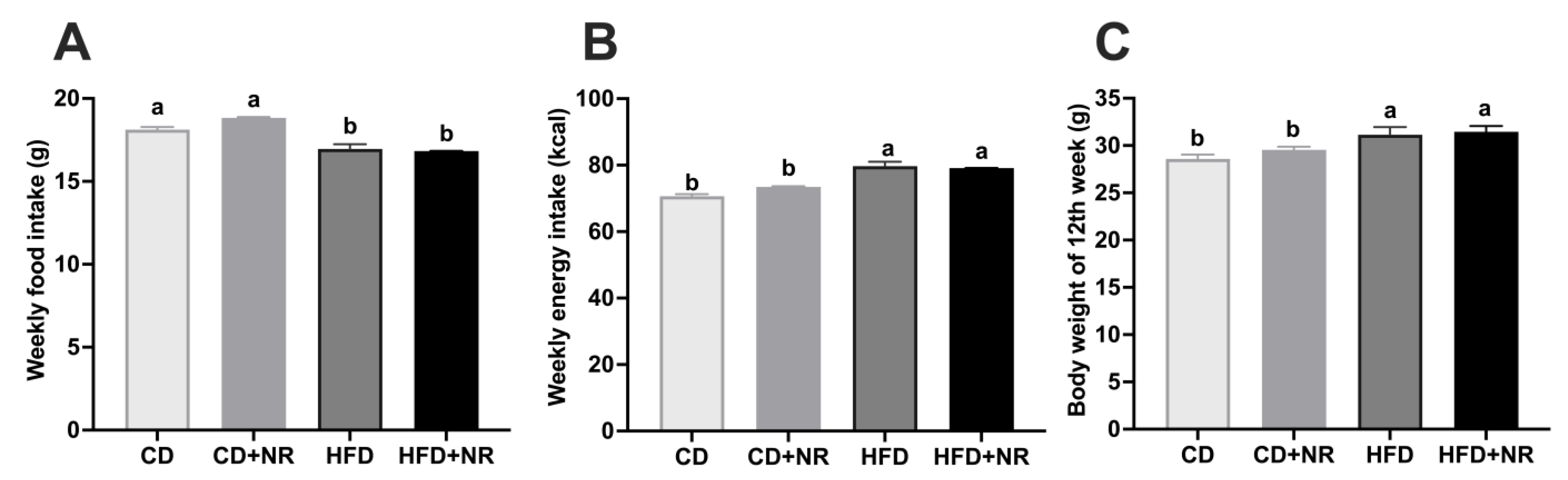
3.1. Effect of Supplementation with NR on Weekly Food and Energy Intaks and Final Body Weights in CD- or HFD-Fed ApoE-/- Mice
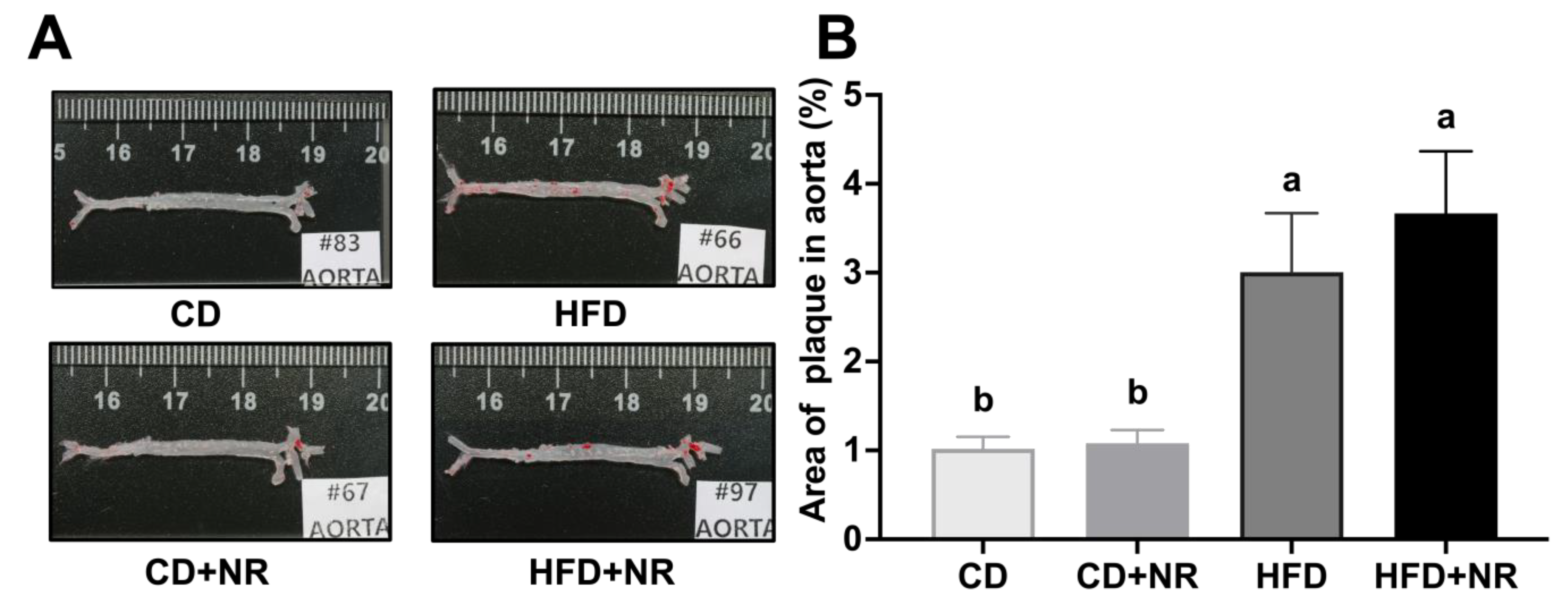
3.2. Effect of Supplementation with NR on Fasting Blood Concentrations of Biochemical Parameters and Atherosclerotic Lesion Development in CD- or HFD-Fed ApoE-/- Mice
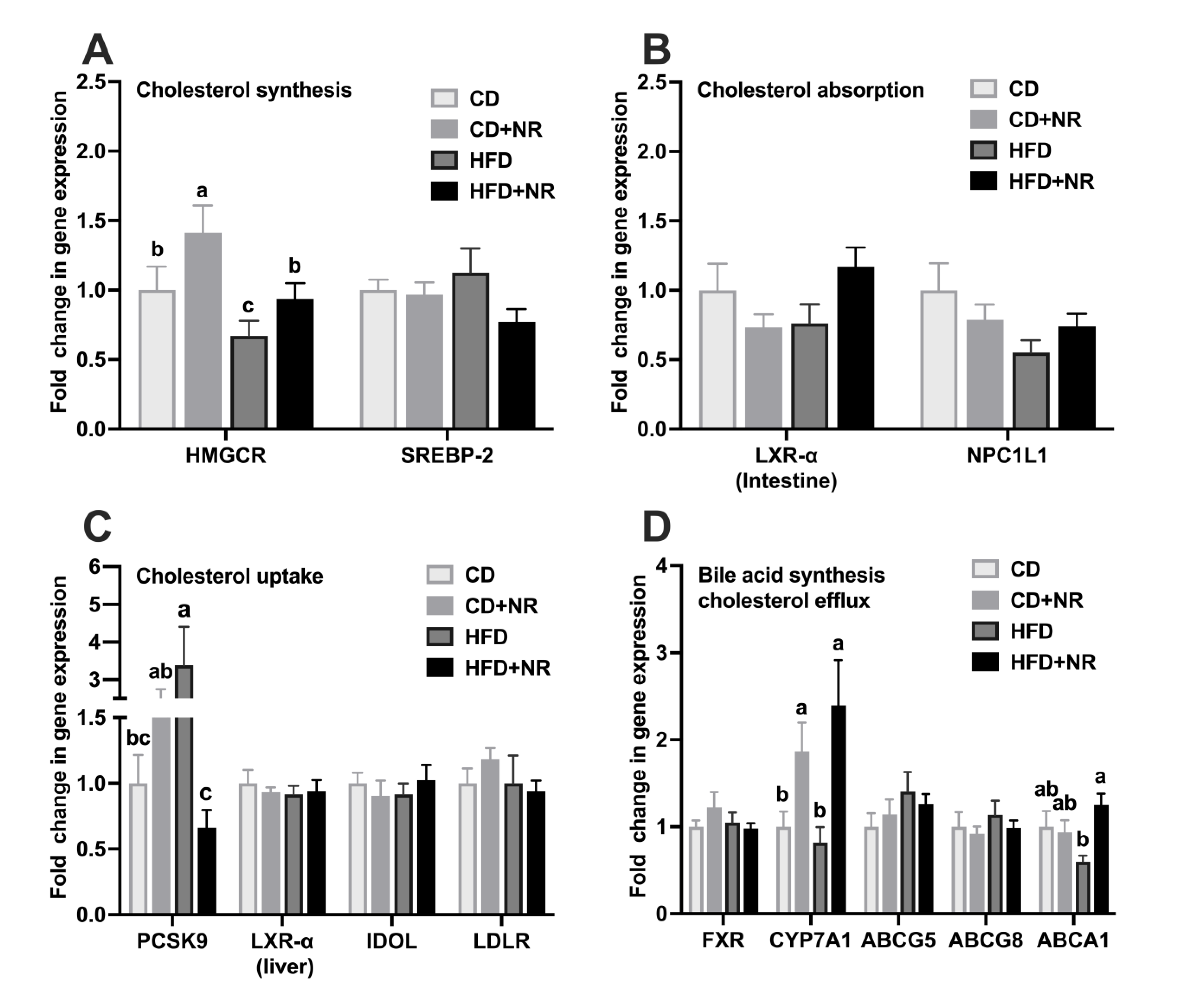
3.3. Effect of Supplementation with NR on Gene Expressions in Cholesterol Metabolism Pathways in CD- or HFD-Fed ApoE-/- Mice
3.4. Effect of Supplementation with NR on Gut Microbial Composition and Diversity in CD- or HFD-Fed ApoE-/- Mice
3.5. Effect of Supplementation with NR on the Relative Abundance of Gut Microbiota at Different Taxa Levels and Concentrations of SCFAs in CD- or HFD-Fed ApoE-/- Mice
3.6. Correlations between the Relative Abundance of Gut Microbiota at Different Taxa Levels with Blood Biochemical Parameters, Gene Expressions in Cholesterol Metabolism, Atherosclerotic Lesions in the Aorta, and Fecal SCFAs Concentrations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Stone, N.J.; Smith, S.C., Jr.; Orringer, C.E.; Rigotti, N.A.; Navar, A.M.; Khan, S.S.; Jones, D.W.; Goldberg, R.; Mora, S.; Blaha, M.; et al. Managing Atherosclerotic Cardiovascular Risk in Young Adults: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 819–836. [Google Scholar] [CrossRef] [PubMed]
- Siri-Tarino, P.W.; Krauss, R.M. The early years of lipoprotein research: From discovery to clinical application. J. Lipid Res. 2016, 57, 1771–1777. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.Y.; Huang, W.; Yuan, Q.X.; Cheng, J.; Huang, Z.C.; Ouyang, L.J.; Zeng, F.H. Hypolipidaemic effects and mechanisms of the main component of Opuntia dillenii Haw. polysaccharides in high-fat emulsion-induced hyperlipidaemic rats. Food Chem. 2012, 134, 964–971. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, e285–e350. [Google Scholar] [CrossRef]
- Hunter, P.M.; Hegele, R.A. Functional foods and dietary supplements for the management of dyslipidaemia. Nat. Rev. Endocrinol. 2017, 13, 278–288. [Google Scholar] [CrossRef]
- Cao, Z.H.; Green-Johnson, J.M.; Buckley, N.D.; Lin, Q.Y. Bioactivity of soy-based fermented foods: A review. Biotechnol. Adv. 2019, 37, 223–238. [Google Scholar] [CrossRef]
- Urano, T.; Ihara, H.; Umemura, K.; Suzuki, Y.; Oike, M.; Akita, S.; Tsukamoto, Y.; Suzuki, I.; Takada, A. The profibrinolytic enzyme subtilisin NAT purified from Bacillus subtilis Cleaves and inactivates plasminogen activator inhibitor type 1. J. Biol. Chem. 2001, 276, 24690–24696. [Google Scholar] [CrossRef]
- Chen, H.; McGowan, E.M.; Ren, N.; Lal, S.; Nassif, N.; Shad-Kaneez, F.; Qu, X.; Lin, Y. Nattokinase: A Promising Alternative in Prevention and Treatment of Cardiovascular Diseases. Biomark. Insights 2018, 13, 1177271918785130. [Google Scholar] [CrossRef]
- Taniguchi-Fukatsu, A.; Yamanaka-Okumura, H.; Naniwa-Kuroki, Y.; Nishida, Y.; Yamamoto, H.; Taketani, Y.; Takeda, E. Natto and viscous vegetables in a Japanese-style breakfast improved insulin sensitivity, lipid metabolism and oxidative stress in overweight subjects with impaired glucose tolerance. Br. J. Nutr. 2012, 107, 1184–1191. [Google Scholar] [CrossRef]
- Suzuki, H.; Kobayashi, K.; Kosuge, Y.; Sasaoka, Y.; Tajika, Y.; Sugiyama, T.; Ichikawa, T.; Ishihara, K. Nattoesse inhibits the high-fat diet-induced increase in serum lipid levels in ApoE-deficient mice. Int. J. Anal. Bio-Sci. 2017, 5. [Google Scholar]
- Park, K.J.; Kang, J.I.; Kim, T.S.; Yeo, I.H. The antithrombotic and fibrinolytic effect of natto in hypercholesterolemia rats. Prev. Nutr. Food Sci. 2012, 17, 78–82. [Google Scholar] [CrossRef]
- Lee, C.L.; Wen, J.Y.; Hsu, Y.W.; Pan, T.M. The blood lipid regulation of Monascus-produced monascin and ankaflavin via the suppression of low-density lipoprotein cholesterol assembly and stimulation of apolipoprotein A1 expression in the liver. J. Microbiol. Immunol. Infect. 2018, 51, 27–37. [Google Scholar] [CrossRef]
- Shanes, J.G. A review of the rationale for additional therapeutic interventions to attain lower LDL-C when statin therapy is not enough. Curr. Atheroscler. Rep. 2012, 14, 33–40. [Google Scholar] [CrossRef]
- Burke, F.M. Red yeast rice for the treatment of dyslipidemia. Curr. Atheroscler. Rep. 2015, 17, 495. [Google Scholar] [CrossRef]
- Ma, J.; Li, Y.; Ye, Q.; Li, J.; Hua, Y.; Ju, D.; Zhang, D.; Cooper, R.; Chang, M. Constituents of red yeast rice, a traditional Chinese food and medicine. J. Agric. Food Chem. 2000, 48, 5220–5225. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Shi, Y.; Grimsgaard, S.; Alraek, T.; Fønnebø, V. Chinese red yeast rice (Monascus purpureus) for primary hyperlipidemia: A meta-analysis of randomized controlled trials. Chin. Med. 2006, 1, 4. [Google Scholar] [CrossRef]
- Heber, D.; Yip, I.; Ashley, J.M.; Elashoff, D.A.; Elashoff, R.M.; Go, V.L. Cholesterol-lowering effects of a proprietary Chinese red-yeast-rice dietary supplement. Am. J. Clin. Nutr. 1999, 69, 231–236. [Google Scholar] [CrossRef]
- Dong, Y.; Cheng, H.; Liu, Y.; Xue, M.; Liang, H. Red yeast rice ameliorates high-fat diet-induced atherosclerosis in Apoe-/- mice in association with improved inflammation and altered gut microbiota composition. Food Funct. 2019, 10, 3880–3889. [Google Scholar] [CrossRef]
- Feng, D.; Sun, J.G.; Sun, R.B.; Ou-Yang, B.C.; Yao, L.; Aa, J.Y.; Zhou, F.; Zhang, J.W.; Zhang, J.; Wang, G.J. Isoflavones and phytosterols contained in Xuezhikang capsules modulate cholesterol homeostasis in high-fat diet mice. Acta Pharmacol. Sin. 2015, 36, 1462–1472. [Google Scholar] [CrossRef]
- Kriaa, A.; Bourgin, M.; Potiron, A.; Mkaouar, H.; Jablaoui, A.; Gérard, P.; Maguin, E.; Rhimi, M. Microbial impact on cholesterol and bile acid metabolism: Current status and future prospects. J. Lipid Res. 2019, 60, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Le Roy, T.; Lecuyer, E.; Chassaing, B.; Rhimi, M.; Lhomme, M.; Boudebbouze, S.; Ichou, F.; Haro Barcelo, J.; Huby, T.; Guerin, M.; et al. The intestinal microbiota regulates host cholesterol homeostasis. BMC Biol. 2019, 17, 94. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Bonder, M.J.; Cenit, M.C.; Tigchelaar, E.F.; Maatman, A.; Dekens, J.A.; Brandsma, E.; Marczynska, J.; Imhann, F.; Weersma, R.K.; et al. The Gut Microbiome Contributes to a Substantial Proportion of the Variation in Blood Lipids. Circ. Res. 2015, 117, 817–824. [Google Scholar] [CrossRef]
- Yang, N.C.; Chou, C.W.; Chen, C.Y.; Hwang, K.L.; Yang, Y.C. Combined nattokinase with red yeast rice but not nattokinase alone has potent effects on blood lipids in human subjects with hyperlipidemia. Asia Pac. J. Clin. Nutr. 2009, 18, 310–317. [Google Scholar]
- Charan, J.; Kantharia, N.D. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013, 4, 303–306. [Google Scholar] [CrossRef]
- Prasad, K. Reduction of serum cholesterol and hypercholesterolemic atherosclerosis in rabbits by secoisolariciresinol diglucoside isolated from flaxseed. Circulation 1999, 99, 1355–1362. [Google Scholar] [CrossRef]
- Nergiz-Unal, R.; Ulug, E.; Kisioglu, B.; Tamer, F.; Bodur, M.; Yalcimin, H.; Yuruk, A.A. Hepatic cholesterol synthesis and lipoprotein levels impaired by dietary fructose and saturated fatty acids in mice: Insight on PCSK9 and CD36. Nutrition 2020, 79–80, 110954. [Google Scholar] [CrossRef]
- Meng, H.; Lee, Y.; Ba, Z.; Peng, J.; Lin, J.; Boyer, A.S.; Fleming, J.A.; Furumoto, E.J.; Roberts, R.F.; Kris-Etherton, P.M.; et al. Consumption of Bifidobacterium animalis subsp. lactis BB-12 impacts upper respiratory tract infection and the function of NK and T cells in healthy adults. Mol. Nutr. Food Res. 2016, 60, 1161–1171. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, H.; Zhu, M.J. A sensitive GC/MS detection method for analyzing microbial metabolites short chain fatty acids in fecal and serum samples. Talanta 2019, 196, 249–254. [Google Scholar] [CrossRef]
- Zhou, J.; Tang, L.; Wang, J.; Wang, J.S. Aflatoxin B1 Disrupts Gut-Microbial Metabolisms of Short-Chain Fatty Acids, Long-Chain Fatty Acids, and Bile Acids in Male F344 Rats. Toxicol. Sci. 2018, 164, 453–464. [Google Scholar] [CrossRef]
- Wei, J.; Carroll, R.J.; Harden, K.K.; Wu, G. Comparisons of treatment means when factors do not interact in two-factorial studies. Amino Acids 2012, 42, 2031–2035. [Google Scholar] [CrossRef]
- Oh, H.G.; Kang, Y.R.; Lee, H.Y.; Kim, J.H.; Shin, E.H.; Lee, B.G.; Park, S.H.; Moon, D.I.; Kim, O.J.; Lee, I.A.; et al. Ameliorative effects of Monascus pilosus-fermented black soybean (Glycine max L. Merrill) on high-fat diet-induced obesity. J. Med. Food 2014, 17, 972–978. [Google Scholar] [CrossRef]
- Huang, C.H.; Chen, C.L.; Chang, S.H.; Tsai, G.J. Evaluation of Antiobesity Activity of Soybean Meal Products Fermented by Lactobacillus plantarum FPS 2520 and Bacillus subtilis N1 in Rats Fed with High-Fat Diet. J. Med. Food 2020, 23, 667–675. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.J.; Shin, H.W.; Jung, S.; Ha, J.H. Effect of Soybean and Soybean Koji on Obesity and Dyslipidemia in Rats Fed a High-Fat Diet: A Comparative Study. Int. J. Environ. Res. Public Health 2021, 18, 6032. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, W.G.; Liu, L.T. Red yeast rice prevents atherosclerosis through regulating inflammatory signaling pathways. Chin. J. Integr. Med. 2017, 23, 689–695. [Google Scholar] [CrossRef]
- Lee, H.S.; Lee, Y.J.; Chung, Y.H.; Nam, Y.; Kim, S.T.; Park, E.S.; Hong, S.M.; Yang, Y.K.; Kim, H.C.; Jeong, J.H. Beneficial Effects of Red Yeast Rice on High-Fat Diet-Induced Obesity, Hyperlipidemia, and Fatty Liver in Mice. J. Med. Food 2015, 18, 1095–1102. [Google Scholar] [CrossRef]
- Ference, B.A.; Robinson, J.G.; Brook, R.D.; Catapano, A.L.; Chapman, M.J.; Neff, D.R.; Voros, S.; Giugliano, R.P.; Smith, G.D.; Fazio, S.; et al. Variation in PCSK9 and HMGCR and Risk of Cardiovascular Disease and Diabetes. N. Engl. J. Med. 2016, 375, 2144–2153. [Google Scholar] [CrossRef]
- Wang, J.; Xiao, Q.; Wang, L.; Wang, Y.; Wang, D.; Ding, H. Role of ABCA1 in Cardiovascular Disease. J. Pers. Med. 2022, 12, 1010. [Google Scholar] [CrossRef]
- Chambers, K.F.; Day, P.E.; Aboufarrag, H.T.; Kroon, P.A. Polyphenol Effects on Cholesterol Metabolism via Bile Acid Biosynthesis, CYP7A1: A Review. Nutrients 2019, 11, 2588. [Google Scholar] [CrossRef]
- Shi, Y.C.; Pan, T.M. Antioxidant and pancreas-protective effect of red mold fermented products on streptozotocin-induced diabetic rats. J. Sci. Food Agric. 2010, 90, 2519–2525. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, W.; Zhong, Y.; Lu, B.; Shao, J.; Jiang, S.; Gu, P. Xuezhikang attenuated the functional and morphological impairment of pancreatic islets in diabetic mice via the inhibition of oxidative stress. J. Cardiovasc. Pharmacol. 2014, 63, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Chen, J.; Yi, K.; Peng, L.; Xie, J.; Gou, X.; Peng, T.; Tang, L. Phlorizin ameliorates obesity-associated endotoxemia and insulin resistance in high-fat diet-fed mice by targeting the gut microbiota and intestinal barrier integrity. Gut Microbes 2020, 12, 1842990. [Google Scholar] [CrossRef]
- Du, Y.; Li, D.X.; Lu, D.Y.; Zhang, R.; Zheng, X.X.; Xu, B.J.; Zhao, Y.L.; Ji, S.; Guo, M.Z.; Wang, L.; et al. Morus alba L. water extract changes gut microbiota and fecal metabolome in mice induced by high-fat and high-sucrose diet plus low-dose streptozotocin. Phytother. Res. 2022, 36, 1241–1257. [Google Scholar] [CrossRef]
- Zhou, W.; Yang, T.; Xu, W.; Huang, Y.; Ran, L.; Yan, Y.; Mi, J.; Lu, L.; Sun, Y.; Zeng, X.; et al. The polysaccharides from the fruits of Lycium barbarum L. confer anti-diabetic effect by regulating gut microbiota and intestinal barrier. Carbohydr. Polym. 2022, 291, 119626. [Google Scholar] [CrossRef]


| Variables | CD | CD + NR | HFD | HFD + NR | P-Diet | P-NR | P-Diet*NR |
|---|---|---|---|---|---|---|---|
| TC, mmol/L | 22.5 ± 1.5 b | 24.9 ± 1.8 b | 40.2 ± 2.3 a | 37.0 ± 4.5 a | <0.0001 | 0.89 | 0.33 |
| HDL-C, mmol/L | 1.0 ± 0.1 b | 1.1 ± 0.0 b | 1.3 ± 0.1 a | 1.4 ± 0.1 a | <0.0001 | 0.07 | 0.97 |
| non-HDL-C, mmol/L | 21.5 ± 1.5 b | 23.7 ± 1.8 b | 38.8 ± 2.3 a | 35.6 ± 4.4 a | <0.0001 | 0.87 | 0.33 |
| TC: HDL-C | 21.0 ± 2.1 b | 21.9 ± 1.3 b | 29.2 ± 2.9 a | 26.3 ± 3.0 a | 0.0139 | 0.68 | 0.44 |
| LDL-C: HDL-C | 7.1 ± 0.8 b | 7.6 ± 0.5 b | 10.4 ± 1.1 a | 9.0 ± 1.1 a | 0.0315 | 0.20 | 0.26 |
| TG, mmol/L | 1.2 ± 0.2 | 1.3 ± 0.2 | 1.6 ± 0.2 | 1.5 ± 0.1 | 0.21 | 0.87 | 0.76 |
| LDL-C, mmol/L | 7.6 ± 0.6 | 8.6 ± 0.7 | 14.4 ± 0.8 | 12.6 ± 1.6 | 0.24 | 0.34 | 0.40 |
| VLDL-C, mmol/L | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.21 | 0.85 | 0.76 |
| Glucose, mmol/L | 4.3 ± 0.2 c | 5.0 ± 0.1 bc | 6.9 ± 0.5 a | 5.6 ± 0.2 b | <0.0001 | 0.31 | 0.0011 |
| PCSK9, ng/mL | 3.1 ± 0.5 b | 3.0 ± 0.5 b | 12.1 ± 2.4 a | 16.0 ± 5.8 a | <0.0001 | 0.74 | 0.77 |
| Gut Microbiota | CD | CD + NR | HFD | HFD + NR | P-diet | P-NR | P-diet*NR |
|---|---|---|---|---|---|---|---|
| Phylum | |||||||
| Firmicutes | 56.65 ± 1.48 b | 54.33 ± 3.04 b | 67.34 ± 4.49 a | 60.27 ± 5.84 a | 0.0451 | 0.25 | 0.56 |
| Bacteroidetes | 23.10 ± 1.91 a | 20.41 ± 1.78 a | 12.61 ± 2.94 b | 14.22 ± 2.35 b | 0.0008 | 0.81 | 0.35 |
| Desulfobacterota | 5.46 ± 0.81 | 9.95 ± 1.70 | 9.42 ± 1.79 | 9.82 ± 2.30 | 0.28 | 0.17 | 0.25 |
| Actinobacteria | 4.13 ± 1.02 ab | 5.87 ± 0.55 a | 5.40 ± 0.86 a | 2.08 ± 0.31 b | 0.10 | 0.29 | 0.0016 |
| Verrucomicrobia | 4.75 ± 1.42 | 2.07 ± 0.57 | 1.05 ± 0.44 | 7.60 ± 3.92 | 0.36 | 0.86 | 0.82 |
| Deferribacteres | 0.98 ± 0.57 | 1.20 ± 0.37 | 2.16 ± 0.63 | 2.10 ± 0.60 | 0.05 | 0.33 | 0.22 |
| Proteobacteria | 0.21 ± 0.05 a | 0.41 ± 0.10 a | 0.25 ± 0.11 b | 0.36 ± 0.29 b | 0.0476 | 0.66 | 0.30 |
| Firmicutes: Bacteroidetes | 2.43 ± 0.18 b | 2.91 ± 0.31 b | 9.78 ± 2.67 a | 5.47 ± 1.66 a | 0.0018 | 0.29 | 0.13 |
| Genus | |||||||
| Faecalibaculum | 33.58 ± 4.40 b | 26.11 ± 4.31 b | 42.22 ± 4.63 a | 38.32 ± 5.49 a | 0.0332 | 0.24 | 0.71 |
| Muribaculaceae | 15.68 ± 1.59 a | 14.96 ± 1.01 a | 7.68 ± 1.24 b | 10.88 ± 1.63 b | 0.0001 | 0.38 | 0.17 |
| Lactobacillus | 4.01 ± 1.31 | 4.62 ± 1.22 | 4.80 ± 1.82 | 2.72 ± 0.72 | 0.83 | 0.89 | 0.25 |
| Dubosiella | 1.87 ± 0.43 | 4.76 ± 2.05 | 4.62 ± 1.35 | 2.28 ± 0.48 | 0.23 | 0.66 | 0.42 |
| Coriobacteriaceae_UCG-002 | 3.46 ± 0.90 ab | 5.41 ± 0.55 a | 5.18 ± 0.85 a | 1.96 ± 0.28 b | 0.22 | 0.37 | 0.0007 |
| Akkermansia | 4.75 ± 1.42 | 2.07 ± 0.57 | 1.05 ± 0.44 | 7.60 ± 3.92 | 0.36 | 0.93 | 0.76 |
| Clostridia_UCG-014 | 4.91 ± 1.58 | 4.37 ± 1.27 | 0.14 ± 0.01 | 0.28 ± 0.06 | 0.35 | 0.32 | 0.87 |
| Mucispirillum | 0.98 ± 0.57 | 1.20 ± 0.37 | 2.16 ± 0.63 | 2.10 ± 0.60 | 0.05 | 0.33 | 0.22 |
| Alistipes | 2.78 ± 0.44 a | 2.83 ± 0.40 a | 0.60 ± 0.23 b | 0.89 ± 0.26 b | 0.0229 | 0.73 | 0.90 |
| Bacteroides | 3.07 ± 0.43 a | 1.20 ± 0.23 b | 1.44 ± 0.30 b | 0.64 ± 0.15 c | 0.0006 | 0.0001 | 0.96 |
| Alloprevotella | 0.85 ± 0.12 a | 1.38 ± 0.34 a | 1.06 ± 0.68 b | 0.45 ± 0.29 b | 0.0006 | 0.51 | 0.23 |
| [Eubacterium]_coprostanoligenes_group | 0.31 ± 0.09 b | 0.20 ± 0.05 b | 1.77 ± 0.53 a | 0.67 ± 0.28 a | 0.0406 | 0.10 | 0.33 |
| Lactococcus | 0.82 ± 0.32 a | 0.35 ± 0.05 b | 0.88 ± 0.16 a | 0.33 ± 0.09 b | 0.98 | 0.0013 | 0.34 |
| Blautia | 0.23 ± 0.14 | 0.27 ± 0.13 | 0.96 ± 0.17 | 0.85 ± 0.26 | 0.05 | 0.81 | 0.59 |
| Lachnospiraceae_NK4A136_group | 0.75 ± 0.18 a | 0.70 ± 0.11 a | 0.24 ± 0.05 b | 0.54 ± 0.15 b | 0.0044 | 0.08 | 0.30 |
| Parasutterella | 0.18 ± 0.05 | 0.41 ± 0.10 | 0.24 ± 0.11 | 0.34 ± 0.29 | 0.23 | 0.85 | 0.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Liu, W.; Lv, Y.; Liu, K.; Wang, Y.; Meng, S.; Kang, T.; Bao, Y.; Meng, H. Supplementation with Natto and Red Yeast Rice Alters Gene Expressions in Cholesterol Metabolism Pathways in ApoE-/- Mice with Concurrent Changes in Gut Microbiota. Nutrients 2023, 15, 973. https://doi.org/10.3390/nu15040973
Zhou H, Liu W, Lv Y, Liu K, Wang Y, Meng S, Kang T, Bao Y, Meng H. Supplementation with Natto and Red Yeast Rice Alters Gene Expressions in Cholesterol Metabolism Pathways in ApoE-/- Mice with Concurrent Changes in Gut Microbiota. Nutrients. 2023; 15(4):973. https://doi.org/10.3390/nu15040973
Chicago/Turabian StyleZhou, Haiyan, Wenjing Liu, Yiqian Lv, Ke Liu, Yin Wang, Shuangli Meng, Tong Kang, Yuechao Bao, and Huicui Meng. 2023. "Supplementation with Natto and Red Yeast Rice Alters Gene Expressions in Cholesterol Metabolism Pathways in ApoE-/- Mice with Concurrent Changes in Gut Microbiota" Nutrients 15, no. 4: 973. https://doi.org/10.3390/nu15040973
APA StyleZhou, H., Liu, W., Lv, Y., Liu, K., Wang, Y., Meng, S., Kang, T., Bao, Y., & Meng, H. (2023). Supplementation with Natto and Red Yeast Rice Alters Gene Expressions in Cholesterol Metabolism Pathways in ApoE-/- Mice with Concurrent Changes in Gut Microbiota. Nutrients, 15(4), 973. https://doi.org/10.3390/nu15040973






