Effect of Prolonged and Substantial Weight Loss on Incident Atrial Fibrillation: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
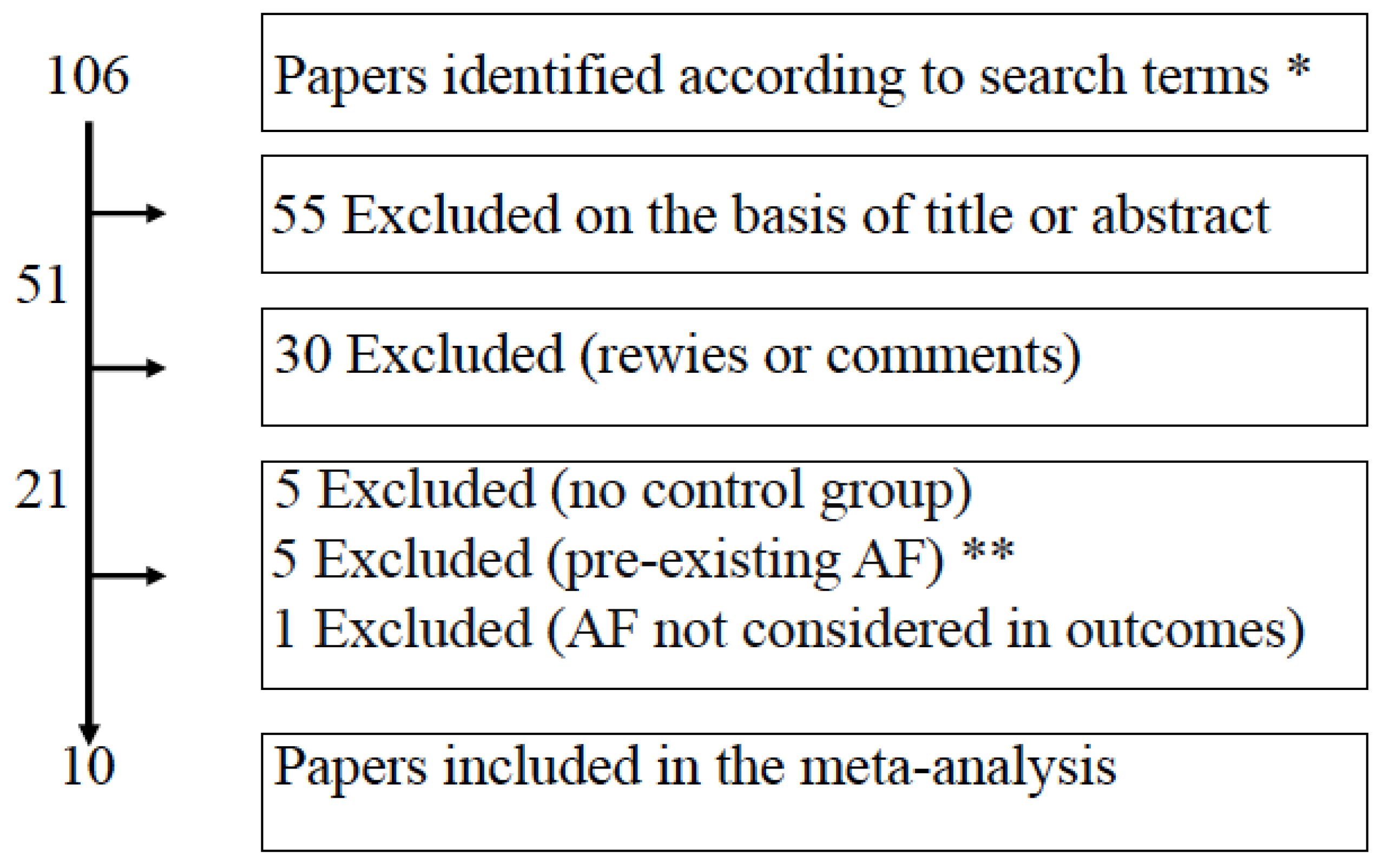
2.1. Search Strategy and Inclusion Criteria
2.2. Data Extraction
2.3. Quality Assessment
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calle, E.E.; Thun, M.J.; Petrelli, J.M.; Rodriguez, C.; Heath, C.W. Body-mass index and mortality in a prospective cohort of U.S. adults. N. Engl. J. Med. 1999, 341, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Wanahita, N.; Messerli, F.H.; Bangalore, S.; Gami, A.S.; Somers, V.K.; Steinberg, J.S. Atrial fibrillation and obesity—Results of a meta-analysis. Am. Heart J. 2008, 155, 310–315. [Google Scholar] [CrossRef]
- Zhuang, J.; Lu, Y.; Tang, K.; Peng, W.; Xu, Y. Influence of body mass index on recurrence and quality of life in atrial fibrillation patients after catheter ablation: A meta-analysis and systematic review. Clin. Cardiol. 2013, 36, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Sen, A.; Schlesinger, S.; Norat, T.; Janszky, I.; Romundstad, P.; Tonstad, S.; Riboli, E.; Vatten, L.J. Body mass index, abdominal fatness, fat mass and the risk of atrial fibril-lation: A systematic review and dose-response meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Abed, H.S.; Samuel, C.S.; Lau, D.H.; Kelly, D.J.; Royce, S.G.; Alasady, M.; Mahajan, R.; Kuklik, P.; Zhang, Y.; Brooks, A.G.; et al. Obesity results in progressive atrial structural and electrical remodeling: Implications for atrial fibrillation. Heart Rhythm. 2013, 10, 90–100. [Google Scholar] [CrossRef]
- Mahajan, R.; Lau, D.H.; Brooks, A.G.; Shipp, N.J.; Manavis, J.; Wood, J.P.; Finnie, J.W.; Samuel, C.S.; Royce, S.G.; Twomey, D.J.; et al. Electrophysiological, electroanatomical, and structural remodeling of the atria as consequences of sustained obesity. J. Am. Coll. Cardiol. 2015, 66, 1–11. [Google Scholar] [CrossRef]
- Pontiroli, A.E.; Morabito, A. Long-term prevention of mortality in morbid obesity through bariatric surgery. A systematic review and meta-analysis of trials performed with gastric banding and gastric bypass. Ann. Surg. 2011, 253, 484–487. [Google Scholar] [CrossRef]
- Pontiroli, A.E.; Ceriani, V.; Tagliabue, E. Compared with Controls, Bariatric Surgery Prevents Long-Term Mortality in Persons with Obesity Only Above Median Age of Cohorts: A Systematic Review and Meta-Analysis. Obes. Surg. 2020, 30, 2487–2496. [Google Scholar] [CrossRef]
- Pontiroli, A.E.; Zakaria, A.S.; Fanchini, M.; Osio, C.; Tagliabue, E.; Micheletto, G.; Saibene, A.; Folli, F. A 23-year study of mortality and development of co-morbidities in patients with obesity undergoing bariatric surgery (laparoscopic gastric banding) in comparison with medical treatment of obesity. Cardiovasc. Diabetol. 2018, 17, 161. [Google Scholar] [CrossRef]
- Sesti, G.; Folli, F.; Perego, L.; Hribal, M.L.; Pontiroli, A.E. Effects of weight loss in metabolically healthy obese subjects after laparoscopic adjustable gastric banding and hypocaloric diet. PLoS ONE 2011, 6, e17737. [Google Scholar] [CrossRef]
- Pontiroli, A.E.; Ceriani, V.; Tagliabue, E.; Zakaria, A.S.; Veronelli, A.; Folli, F.; Zanoni, I. Bariatric surgery, compared to medical treatment, reduces morbidity at all ages but does not reduce mortality in patients aged <43 years, especially if diabetes mellitus is present: A post hoc analysis of two retrospective cohort studies. Acta Diabetol. 2020, 57, 323–333. [Google Scholar]
- García de la Torre, N.; Rubio, M.A.; Bordiú, E.; Cabrerizo, L.; Aparicio, E.; Hernández, C.; Sánchez-Pernaute, A.; Díez-Valladares, L.; Torres, A.J.; Puente, M.; et al. Effects of weight loss after bariatric surgery for morbid obesity on vascular endothelial growth factor-A, adipocytokines, and insulin. J. Clin. Endocrinol. Metab. 2008, 93, 4276–4281. [Google Scholar] [CrossRef] [PubMed]
- Veronelli, A.; Laneri, M.; Ranieri, R.; Koprivec, D.; Vardaro, D.; Paganelli, M.; Folli, F.; Pontiroli, A.E. White blood cells in obesity and diabetes: Effects of weight loss and normalization of glucose metabolism. Diabetes Care 2004, 27, 2501–2502. [Google Scholar] [CrossRef] [PubMed]
- Boido, A.; Ceriani, V.; Cetta, F.; Lombardi, F.; Pontiroli, A.E. Bariatric surgery and prevention of cardiovascular events and mortality in morbid obesity: Mechanisms of action and choice of surgery. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Raaijmakers, L.C.; Pouwels, S.; Thomassen, S.E.; Nienhuijs, S.W. Quality of life and bariatric surgery: A systematic review of short- and long-term results and comparison with community norms. Eur. J. Clin. Nutr. 2017, 71, 441–449. [Google Scholar] [CrossRef]
- Wasmund, S.L.; Owan, T.; Yanowitz, F.G.; Adams, T.D.; Hunt, S.C.; Hamdan, M.H.; Litwin, S.E. Improved heart rate recovery after marked weight loss induced by gastric bypass surgery: Two-year follow up in the Utah Obesity Study. Heart Rhythm. 2011, 8, 84–90. [Google Scholar] [CrossRef]
- Pontiroli, A.E. Heart rate and sympathetic activity after obesity surgery. Heart Rhythm. 2011, 8, e1. [Google Scholar] [CrossRef]
- Aldaas, O.M.; Lupercio, F.; Han, F.T.; Hoffmayer, K.S.; Krummen, D.; Ho, G.; Raissi, F.; Birgersdotter-Green, U.; Feld, G.K.; Hsu, J.C. Meta-analysis of Effect of Modest (≥10%) Weight Loss in Management of Overweight and Obese Patients with Atrial Fibrillation. Am. J. Cardiol. 2019, 124, 1568–1574. [Google Scholar] [CrossRef]
- Park, D.Y.; An, S.; Murthi, M.; Kattoor, A.J.; Kaur, A.; Ravi, V.; Huang, H.D.; Vij, A. Effect of weight loss on recurrence of atrial fibrillation after ablative therapy: A systematic review and meta-analysis. J. Interv. Card. Electrophysiol. 2022, 64, 763–771. [Google Scholar] [CrossRef]
- Chokesuwattanaskul, R.; Thongprayoon, C.; Bathini, T.; Sharma, K.; Watthanasuntorn, K.; Lertjitbanjong, P.; Pachariyanon, P.; Prechawat, S.; Mao, M.A.; Torres-Ortiz, A.; et al. Incident atrial fibrillation in patients undergoing bariatric surgery: A systematic review and meta-analysis. Intern. Med. J. 2020, 50, 810–817. [Google Scholar] [CrossRef]
- Van Veldhuisen, S.L.; Gorter, T.M.; van Woerden, G.; de Boer, R.A.; Rienstra, M.; Hazebroek, E.J.; van Veldhuisen, D.J. Bariatric surgery and cardiovascular disease: A systematic review and meta-analysis. Eur. Heart J. 2022, 43, 1955–1969. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Medina-Inojosa, J.R.; Lopez-Jimenez, F.; Miranda, W.R.; Collazo-Clavell, M.L.; Sarr, M.G.; Chamberlain, A.M.; Hodge, D.O.; Bailey, K.R.; Wang, Y.; et al. The Long-Term Impact of Bariatric Surgery on Development of Atrial Fibrillation and Cardiovascular Events in Obese Patients: An Historical Cohort Study. Front. Cardiovasc. Med. 2021, 8, 647118. [Google Scholar] [CrossRef]
- Doumouras, A.G.; Wong, J.A.; Paterson, J.M.; Lee, Y.; Sivapathasundaram, B.; Tarride, J.E.; Thabane, L.; Hong, D.; Yusuf, S.; Anvari, M. Bariatric Surgery and Cardiovascular Outcomes in Patients with Obesity and Cardiovascular Disease: A Population-Based Retrospective Cohort Study. Circulation 2021, 143, 1468–1480. [Google Scholar] [CrossRef]
- Moher, D.A.; Tetzlaff, J.; Altman, D.G. PRISMA group preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Jamaly, S.; Carlsson, L.; Peltonen, M.; Jacobson, P.; Sjöström, L.; Karason, K. Bariatric Surgery and the Risk of New-Onset Atrial Fibrillation in Swedish Obese Subjects. J. Am. Coll. Cardiol. 2016, 68, 2497–2504. [Google Scholar] [CrossRef]
- Lynch, K.T.; Mehaffey, J.H.; Hawkins, R.B.; Hassinger, T.E.; Hallowell, P.T.; Kirby, J.L. Bariatric surgery reduces incidence of atrial fibrillation: A propensity score-matched analysis. Surg. Obes. Relat. Dis. 2019, 15, 279–285. [Google Scholar] [CrossRef]
- Aminian, A.; Zajichek, A.; Arterburn, D.E.; Wolski, K.E.; Brethauer, S.A.; Schauer, P.R.; Kattan, M.W.; Nissen, S.E. Association of metabolic surgery with major adverse cardiovascular outcomes in patients with type 2 diabetes and obesity. JAMA 2019, 322, 1271–1282. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Subramanian, A.; Adderley, N.; Gokhale, K.; Singhal, R.; Bellary, S.; Nirantharakumar, K.; Tahrani, A.A. Impact of bariatric surgery on cardiovascular outcomes and mortality: A population-based cohort study. Br. J. Surg. 2020, 107, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Srivatsa, U.N.; Malhotra, P.; Zhang, X.J.; Beri, N.; Xing, G.; Brunson, A.; Ali, M.; Fan, D.; Pezeshkian, N.; Chiamvimonvat, N.; et al. Bariatric surgery to aLleviate OCcurrence of Atrial Fibrillation Hospitalization-BLOC-AF. Heart Rhythm O2 2020, 1, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Moussa, O.; Ardissino, M.; Eichhorn, C.; Reddy, R.K.; Khan, O.; Ziprin, P.; Darzi, A.; Collins, P.; Purkayastha, S. Atrial fibrillation and obesity: Long-term incidence and outcomes after bariatric surgery. Eur. J. Prev. Cardiol. 2021, 28, e22–e24. [Google Scholar] [CrossRef]
- Höskuldsdóttir, G.; Sattar, N.; Miftaraj, M.; Näslund, I.; Ottosson, J.; Franzén, S.; Svensson, A.M.; Eliasson, B. Potential Effects of Bariatric Surgery on the Incidence of Heart Failure and Atrial Fibrillation in Patients With Type 2 Diabetes Mellitus and Obesity and on Mortality in Patients With Preexisting Heart Failure: A Nationwide, Matched, Observational Cohort Study. J. Am. Heart Assoc. 2021, 10, e019323. [Google Scholar] [PubMed]
- Rassen, J.A.; Murk, W.; Schneeweiss, S. Real-world evidence of bariatric surgery and cardiovascular benefits using electronic health records data: A lesson in bias. Diabetes Obes. Metab. 2021, 23, 1453–1462. [Google Scholar] [CrossRef]
- Näslund, E.; Stenberg, E.; Hofmann, R.; Ottosson, J.; Sundbom, M.; Marsk, R.; Svensson, P.; Szummer, K.; Jernberg, T. Association of Metabolic Surgery With Major Adverse Cardiovascular Outcomes in Patients With Previous Myocardial Infarction and Severe Obesity: A Nationwide Cohort Study. Circulation 2021, 143, 1458–1467. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; Higgins, J.P.; Sterne, J.; Tugwell, P.; Reeves, B.C. Checklists of methodological issues for review authors to consider when including non-randomized studies in systematic reviews. Res. Synth. Methods 2013, 4, 63–77. [Google Scholar] [CrossRef]
- Hartung, J.; Knapp, G. A refined method for the meta-analysis of controlled clinical trials with binary outcome. Stat. Med. 2001, 20, 3875–3889. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Spiegelhalter, D.J. A re-evaluation of random-effects meta-analysis. J. R. Stat. Soc. Ser. A Stat. Soc. 2009, 172, 137–159. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Park, J.E.; Lee, Y.J.; Seo, H.-J.; Sheen, S.-S.; Hahn, S.; Jang, B.-H.; Son, H.-J. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J. Clin. Epidemiol. 2013, 66, 408–414. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Baujat, B.; Mahé, C.; Pignon, J.P.; Hill, C. A graphical method for exploring heterogeneity in meta-analyses: Application to a meta-analysis of 65 trials. Stat. Med. 2002, 21, 2641–2652. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Ambrosio, G.; Camm, A.J.; Bassand, J.P.; Corbalan, R.; Kayani, G.; Carluccio, E.; Mantovani, L.G.; Virdone, S.; Kakkar, A.K.; GARFIELD-AF Investigators. Characteristics, treatment, and outcomes of newly diagnosed atrial fibrillation patients with heart failure: GARFIELD-AF. ESC Heart Fail. 2021, 8, 1139–1149. [Google Scholar] [CrossRef]
- Neefs, J.; Boekholdt, S.M.; Khaw, K.T.; Luben, R.; Pfister, R.; Wareham, N.J.; Meulendijks, E.R.; Sanders, P.; de Groot, J.R. Body mass index and body fat distribution and new-onset atrial fibrillation: Substudy of the European Prospective Investigation into Cancer and Nutrition in Norfolk (EPIC-Norfolk) study. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Nuijten, M.A.H.; Eijsvogels, T.M.H.; Monpellier, V.M.; Janssen, I.M.C.; Hazebroek, E.J.; Hopman, M.T.E. The magnitude and progress of lean body mass, fat-free mass, and skeletal muscle mass loss following bariatric surgery: A systematic review and meta-analysis. Obes. Rev. 2022, 23, e13370. [Google Scholar] [CrossRef] [PubMed]
- Opacic, D.; van Bragt, K.A.; Nasrallah, H.M.; Schotten, U.; Verheule, S. Atrial metabolism and tissue perfusion as determinants of electrical and structural remodelling in atrial fibrillation. Cardiovasc. Res. 2016, 109, 527–541. [Google Scholar] [CrossRef] [PubMed]
- Dall’Asta, C.; Paganelli, M.; Morabito, A.; Vedani, P.; Barbieri, M.; Paolisso, G.; Folli, F.; Pontiroli, A.E. Weight loss through gastric banding: Effects on TSH and thyroid hormones in obese subjects with normal thyroid function. Obesity 2010, 18, 854–857. [Google Scholar] [CrossRef]
- Abed, H.S.; Nelson, A.J.; Richardson, J.D.; Worthley, S.G.; Vincent, A.; Wittert, G.A.; Leong, D.P. Impact of weight reduction on pericardial adipose tissue and cardiac structure in patients with atrial fibrillation. Am. Heart J. 2015, 169, 655–662.e2. [Google Scholar] [CrossRef]
- IntHout, J.; Ioannidis, J.P.; Borm, G.F. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med. Res. Methodol. 2014, 14, 25. [Google Scholar] [CrossRef]
- Felsch, M.; Beckmann, L.; Bender, R.; Kuss, O.; Skipka, G.; Mathes, T. Performance of several types of beta-binomial models in comparison to standard approaches for meta-analyses with very few studies. BMC Med. Res. Methodol. 2022, 22, 319. [Google Scholar] [CrossRef]
- Jones, N.R.; Taylor, K.S.; Taylor, C.J.; Aveyard, P. Weight change and the risk of incident atrial fibrillation: A systematic review and meta-analysis. Heart 2019, 105, 1799–1805. [Google Scholar] [CrossRef]

| Authors and Year | Ref. | Study | Bariatric (BS) Surgical Procedure | BS Patients (% Women) | Follow-Up (y) | Age (y) | BMI (kg/m2) | Control Patients (% Women) | Follow-Up (y) | Age (y) | BMI (kg/m2) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Jamaly 2016 | [25] | cohort prospective | VGB (68%) AGB (19%), RYGB (13%) | 2000 (70.7) | 19 | 47.2 | 42.4 | 2021 (71.2) | 19 | 48.6 | 40.1 | ||||||
| Lynch 2019 | [26] | cohort retrospective | RYGB or LSG | 2522 (83.1) | 6.2 | 42 | 47.1 | 2522 (83.4) | 8 | 42 | 47.7 | ||||||
| Aminian 2019 | [27] | cohort retrospective | RYGB or LSG or AGB or BPD | 2135 (70.2) | 3.3 | 52.5 | 45.1 | 10734 (68.4) | 4 | 54.8 | 42.6 | ||||||
| Singh 2020 | [28] | cohort retrospective | RYGB or LSG or AGB-or BPD | 5087 (80.4) | 3.9 | 45.3 | -- | 9858 (81.1) | 3.9 | 45.3 | -- | ||||||
| Srivatsa 2020 | [29] | cohort retrospective | RYGB or LSG | 1581 (78.7) | 5.5 | 49 | -- | 3162 (78.7) | 5.5 | 49 | -- | ||||||
| Moussa 2021 | [30] | cohort retrospective | Various BS procedures | 3077 (77.3) | 12.3 | 50 | 43.5 | 3077 (77.3) | 12.3 | 50 | 43.1 | ||||||
| Höskuldsdóttir 2021 | [31] | cohort retrospective | RYGB | 5321 (66.6) | 4.5 | 49 | 42 | 5321 (68.1) | 4.5 | 47 | 41 | ||||||
| Rassen 2021 | [32] | cohort retrospective | 44% GA, 8% GR, 50% GIB | 291 (69.2) | 2.5 | 57.9 | 42.6 | 461 (61.6) | 2.5 | 59 | 42.1 | ||||||
| Näslund 2021 | [33] | cohort prospective | RYGB or LSG | 509 (42.8) | 4.6 | 53 | 40.6 | 509 (42.8) | 4.6 | 53.2 | 39.7 | ||||||
| Yuan 2021 | [22] | cohort retrospective | RYGB | 308 (82.5) | 15 | 44.2 | 46.4 | 701 (76.6) | 15 | 43.6 | 44.8 | ||||||
| Authors and year | Ref. | Total patients | Main BS type | Mean age(y) | Mean Follow-up (y) | Mean BMI (kg/m2) | WL (%) BS patients | WL (%) control patients | % DM BS patients | % DM control patients | % HTN BS | % HTN con | % CAD BS | % CAD con | % HF BS | % HF con | NOS score |
| Jamaly 2016 | [25] | 4021 | 1 | 47.9 | 19 | 41.2 | −20 | 0 | 17 | 13 | 78.3 | 63.6 | 3.0 | 2.9 | 3.0 | 2.9 | 8 |
| Lynch 2019 | [26] | 5044 | 2 | 42 | 7.1 | 47.4 | −58 | −3.8 | 29 | 10 | 42.5 | 43.5 | -- | -- | 4.9 | 4.6 | 7 |
| Aminian 2019 | [27] | 12869 | 2 | 53.6 | 3.65 | 43.8 | −18 | -- | 100 | 100 | 91.5 | 79.8 | 9.6 | 5.5 | 11.1 | 12.5 | 6 |
| Singh 2020 | [28] | 14945 | 3 | 45.3 | 3.9 | -- | −20 | −0.8 | 22 | 20 | 31.5 | 29.9 | .04 | 3.3 | .8 | .8 | 7 |
| Srivatsa 2020 | [29] | 4743 | 2 | -- | 5.5 | -- | −60 | -- | 23 | 16 | 37.4 | 33.7 | 2.7 | 2.9 | 6 | ||
| Moussa 2021 | [30] | 6154 | 3 | 50 | 12.3 | 43.3 | −9 | +4.1 | 22 | 23 | 41.4 | 30.3 | 11.9 | 16.9 | 15.7 | 14.5 | 6 |
| Höskuldsdóttir 2021 | [31] | 10642 | 2 | 48 | 4.5 | 41.5 | −22 | −4.8 | 100 | 100 | 47.1 | 59.8 | 7.4 | 5.9 | 2.7 | 3.7 | 6 |
| Rassen 2021 | [32] | 752 | 3 | 58.4 | 2.5 | 42.3 | −20 | -- | 100 | 100 | 65.2 | 71.7 | 24.6 | 24.9 | 15.9 | 26.6 | 4 |
| Näslund 2021 | [33] | 1018 | 2 | 53.1 | 4.6 | 40.1 | −28 | -- | 41 | 45 | 88.9 | 88.4 | 12.5 | 12.9 | 9.8 | 10 | 5 |
| Yuan 2021 | [22] | 1009 | 2 | 43.9 | 15 | 45.6 | −26.5 | −8.25 | 21 | 39 | 44.15 | 56.0 | 4.9 | 5.2 | .3 | 3.7 | 8 |
| Studies Author/Pub Year | Selection | Comparability | Outcome | Total Score | Conclusion | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 2 | 1 | 2 | 3 | |||
| Jamaly 2016 [25] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 8 | Good |
| Lynch 2019 [26] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 7 | Good |
| Aminian 2019 [27] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 6 | Intermediate |
| Singh 2020 [28] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 7 | Good |
| Srivatsa 2020 [29] | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 6 | Intermediate |
| Moussa 2021 [30] | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 6 | Intermediate |
| Höskuldsdóttir 2021 [31] | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 6 | Intermediate |
| Rassen 2021 [32] | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 4 | Poor |
| Näslund 2021 [33] | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 5 | Poor |
| Yuan 2021 [22] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 8 | Good |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pontiroli, A.E.; Centofanti, L.; Le Roux, C.W.; Magnani, S.; Tagliabue, E.; Folli, F. Effect of Prolonged and Substantial Weight Loss on Incident Atrial Fibrillation: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 940. https://doi.org/10.3390/nu15040940
Pontiroli AE, Centofanti L, Le Roux CW, Magnani S, Tagliabue E, Folli F. Effect of Prolonged and Substantial Weight Loss on Incident Atrial Fibrillation: A Systematic Review and Meta-Analysis. Nutrients. 2023; 15(4):940. https://doi.org/10.3390/nu15040940
Chicago/Turabian StylePontiroli, Antonio E., Lucia Centofanti, Carel W. Le Roux, Silvia Magnani, Elena Tagliabue, and Franco Folli. 2023. "Effect of Prolonged and Substantial Weight Loss on Incident Atrial Fibrillation: A Systematic Review and Meta-Analysis" Nutrients 15, no. 4: 940. https://doi.org/10.3390/nu15040940
APA StylePontiroli, A. E., Centofanti, L., Le Roux, C. W., Magnani, S., Tagliabue, E., & Folli, F. (2023). Effect of Prolonged and Substantial Weight Loss on Incident Atrial Fibrillation: A Systematic Review and Meta-Analysis. Nutrients, 15(4), 940. https://doi.org/10.3390/nu15040940








