Possible Combined Effects of Plasma Folate Levels, Global DNA Methylation, and Blood Cadmium Concentrations on Renal Cell Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Questionnaire Interview and Bio-Specimen Collection
2.3. Measurements of Plasma Folate and Vitamin B12 Levels and Blood Cadmium Concentrations
2.4. Global DNA Methylation Marker, 5mdC, Measurement
2.5. Statistical Analysis
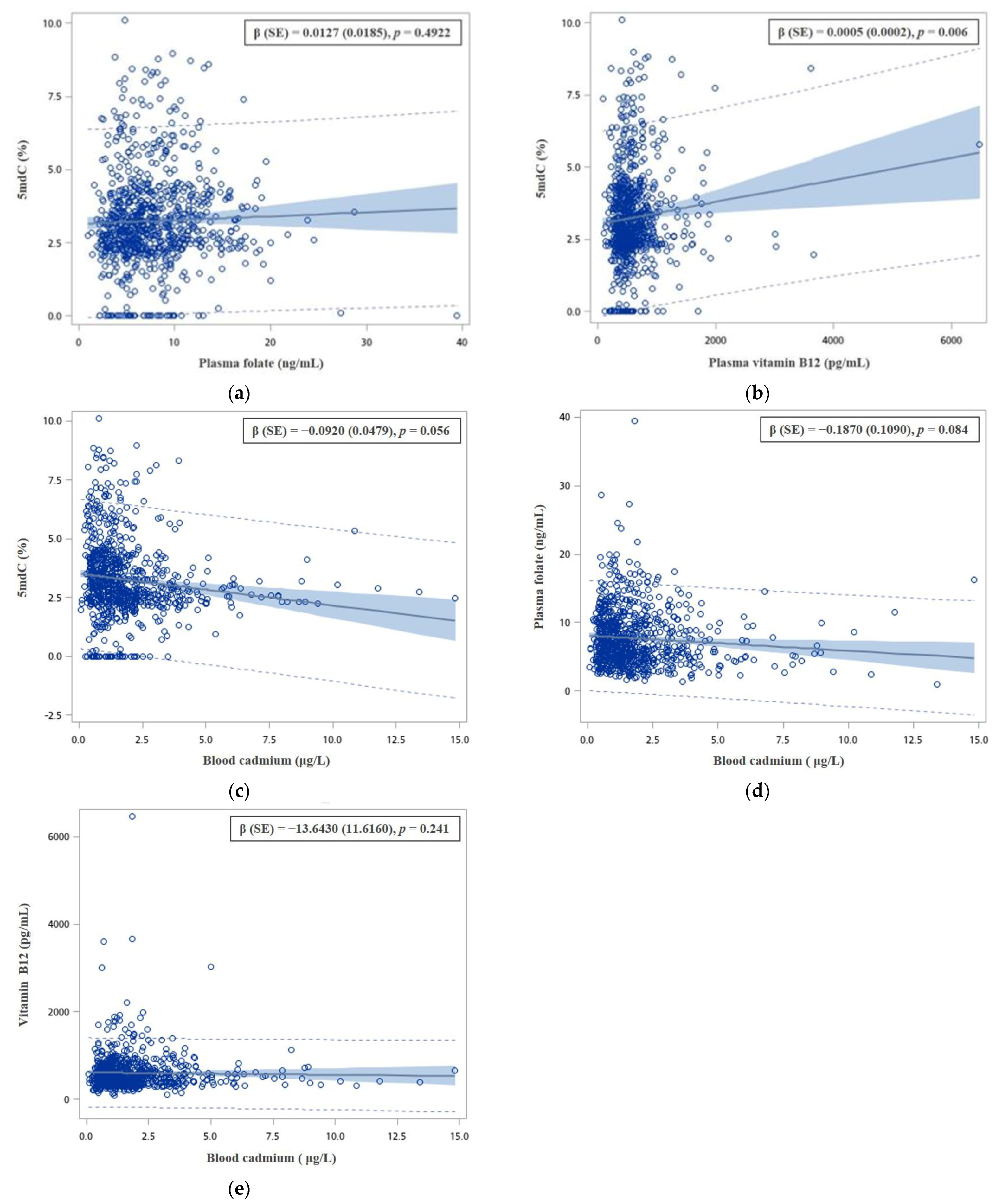
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Znaor, A.; Lortet-Tieulent, J.; Laversanne, M.; Jemal, A.; Bray, F. International variations and trends in renal cell carcinoma incidence and mortality. Eur. Urol. 2015, 67, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.J.; Lo, W.C.; Yang, Y.W.; You, S.L.; Chen, C.J.; Lai, M.S. Incidence and survival of adult cancer patients in Taiwan, 2002–2012. J. Formos. Med. Assoc. 2016, 115, 1076–1088. [Google Scholar] [CrossRef]
- Klaassen, Z.; Sayyid, R.K.; Wallis, C.J.D. Lessons Learned from the Global Epidemiology of Kidney Cancer: A Refresher in Epidemiology 101. Eur. Urol. 2019, 75, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, Y.M.; Lin, Y.C.; Huang, Y.L.; Shiue, H.S.; Pu, Y.S.; Huang, C.Y.; Chung, C.J. Effect of plasma selenium, red blood cell cadmium, total urinary arsenic levels, and eGFR on renal cell carcinoma. Sci. Total Environ. 2021, 750, 141547. [Google Scholar] [CrossRef]
- Panaiyadiyan, S.; Quadri, J.A.; Nayak, B.; Pandit, S.; Singh, P.; Seth, A.; Shariff, A. Association of heavy metals and trace elements in renal cell carcinoma: A case-controlled study. Urol.Oncol. 2022, 40, 111. [Google Scholar] [CrossRef] [PubMed]
- Lien, K.W.; Pan, M.H.; Ling, M.P. Levels of heavy metal cadmium in rice (Oryza sativa L.) produced in Taiwan and probabilistic risk assessment for the Taiwanese population. Environ. Sci. Pollut. Res. Int. 2021, 28, 28381–28390. [Google Scholar] [CrossRef]
- Chung, C.J.; Lee, H.L.; Chang, C.H.; Wu, C.D.; Liu, C.S.; Chung, M.C.; Hsu, H.T. Determination of potential sources of heavy metals in patients with urothelial carcinoma in central Taiwan: A biomonitoring case-control study. Environ. Geochem. Health 2023, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Faroon, O.; Ashizawa, A.; Wright, S.; Tucker, P.; Jenkins, K.; Ingerman, L.; Rudisill, C. Toxicological Profile for Cadmium; Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profiles: Atlanta, GA, USA, 2012.
- Amenyah, S.D.; Hughes, C.F.; Ward, M.; Rosborough, S.; Deane, J.; Thursby, S.J.; Walsh, C.P.; Kok, D.E.; Strain, J.J.; McNulty, H.; et al. Influence of nutrients involved in one-carbon metabolism on DNA methylation in adults-a systematic review and meta-analysis. Nutr.Rev. 2020, 78, 647–666. [Google Scholar] [CrossRef]
- Bock, C.H.; Ruterbusch, J.J.; Holowatyj, A.N.; Steck, S.E.; Van Dyke, A.L.; Ho, W.J.; Cote, M.L.; Hofmann, J.N.; Davis, F.; Graubard, B.I.; et al. Renal cell carcinoma risk associated with lower intake of micronutrients. Cancer Med. 2018, 7, 4087–4097. [Google Scholar] [CrossRef]
- Clasen, J.L.; Heath, A.K.; Scelo, G.; Muller, D.C. Components of one-carbon metabolism and renal cell carcinoma: A systematic review and meta-analysis. Eur. J. Nutr. 2020, 59, 3801–3813. [Google Scholar] [CrossRef] [PubMed]
- Mao, B.; Li, Y.; Zhang, Z.; Chen, C.; Chen, Y.; Ding, C.; Lei, L.; Li, J.; Jiang, M.; Wang, D.; et al. One-Carbon Metabolic Factors and Risk of Renal Cell Cancer: A Meta-Analysis. PLoS ONE 2015, 10, e0141762. [Google Scholar] [CrossRef] [PubMed]
- Srut, M. Ecotoxicological epigenetics in invertebrates: Emerging tool for the evaluation of present and past pollution burden. Chemosphere 2021, 282, 131026. [Google Scholar] [CrossRef] [PubMed]
- Law, P.P.; Holland, M.L. DNA methylation at the crossroads of gene and environment interactions. Essays Biochem. 2019, 63, 717–726. [Google Scholar]
- Esteller, M. Epigenetics in cancer. N. Engl. J. Med. 2008, 358, 1148–1159. [Google Scholar] [CrossRef]
- Kulis, M.; Esteller, M. DNA methylation and cancer. Adv. Genet. 2010, 70, 27–56. [Google Scholar]
- Chung, C.J.; Chang, C.H.; Liou, S.H.; Liu, C.S.; Liu, H.J.; Hsu, L.C.; Chen, J.S.; Lee, H.L. Relationships among DNA hypomethylation, Cd, and Pb exposure and risk of cigarette smoking-related urothelial carcinoma. Toxicol. Appl. Pharmacol. 2017, 316, 107–113. [Google Scholar] [CrossRef]
- Liou, S.H.; Wu, W.T.; Liao, H.Y.; Chen, C.Y.; Tsai, C.Y.; Jung, W.T.; Lee, H.L. Global DNA methylation and oxidative stress biomarkers in workers exposed to metal oxide nanoparticles. J. Hazard. Mater. 2017, 331, 329–335. [Google Scholar] [CrossRef]
- Mendoza-Perez, J.; Gu, J.; Herrera, L.A.; Tannir, N.M.; Matin, S.F.; Karam, J.A.; Huang, M.; Chang, D.W.; Wood, C.G.; Wu, X. Genomic DNA Hypomethylation and Risk of Renal Cell Carcinoma: A Case-Control Study. Clin. Cancer Res. 2016, 22, 2074–2082. [Google Scholar] [CrossRef]
- Wang, B.; Luo, Q.; Shao, C.; Li, X.; Li, F.; Liu, Y.; Sun, L.; Li, Y.; Cai, L. The late and persistent pathogenic effects of cadmium at very low levels on the kidney of rats. Dose. Response 2013, 11, 60–81. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chung, C.J.; Huang, Y.L.; Hsieh, R.L.; Huang, P.T.; Wu, M.Y.; Ao, P.L.; Shiue, H.S.; Huang, S.R.; Su, C.T.; et al. Association of plasma folate, vitamin B12 levels, and arsenic methylation capacity with developmental delay in preschool children in Taiwan. Arch.Toxicol. 2019, 93, 2535–2544. [Google Scholar] [CrossRef]
- Hsueh, Y.M.; Lee, C.Y.; Chien, S.N.; Chen, W.J.; Shiue, H.S.; Huang, S.R.; Lin, M.I.; Mu, S.C.; Hsieh, R.L. Association of blood heavy metals with developmental delays and health status in children. Sci. Rep. 2017, 7, 43608. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S. Confidence interval estimation of interaction. Epidemiology 1992, 3, 452–456. [Google Scholar] [CrossRef]
- Terry, M.B.; Delgado-Cruzata, L.; Vin-Raviv, N.; Wu, H.C.; Santella, R.M. DNA methylation in white blood cells: Association with risk factors in epidemiologic studies. Epigenetics 2011, 6, 828–837. [Google Scholar] [CrossRef]
- Cappetta, M.; Berdasco, M.; Hochmann, J.; Bonilla, C.; Sans, M.; Hidalgo, P.C.; Artagaveytia, N.; Kittles, R.; Martinez, M.; Esteller, M.; et al. Effect of genetic ancestry on leukocyte global DNA methylation in cancer patients. BMC Cancer 2015, 15, 434. [Google Scholar] [CrossRef]
- Sturgeon, S.R.; Pilsner, J.R.; Arcaro, K.F.; Ikuma, K.; Wu, H.; Kim, S.M.; Chopra-Tandon, N.; Karpf, A.R.; Ziegler, R.G.; Schairer, C.; et al. White blood cell DNA methylation and risk of breast cancer in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO). Breast Cancer Res. 2017, 19, 94. [Google Scholar] [CrossRef]
- Boeke, C.E.; Baccarelli, A.; Kleinman, K.P.; Burris, H.H.; Litonjua, A.A.; Rifas-Shiman, S.L.; Tarantini, L.; Gillman, M. Gestational intake of methyl donors and global LINE-1 DNA methylation in maternal and cord blood: Prospective results from a folate-replete population. Epigenetics 2012, 7, 253–260. [Google Scholar] [CrossRef]
- Hu, J.; Liu, J.; Li, J.; Lv, X.; Yu, L.; Wu, K.; Yang, Y. Metal contamination, bioaccumulation, ROS generation, and epigenotoxicity influences on zebrafish exposed to river water polluted by mining activities. J. Hazard. Mater. 2021, 405, 124150. [Google Scholar] [CrossRef]
- Hu, F.; Yin, L.; Dong, F.; Zheng, M.; Zhao, Y.; Fu, S.; Zhang, W.; Chen, X. Effects of long-term cadmium exposure on growth, antioxidant defense and DNA methylation in juvenile Nile tilapia (Oreochromis niloticus). Aquat. Toxicol. 2021, 241, 106014. [Google Scholar] [CrossRef]
- Gibson, T.M.; Weinstein, S.J.; Mayne, S.T.; Pfeiffer, R.M.; Selhub, J.; Taylor, P.R.; Virtamo, J.; Albanes, D.; Stolzenberg-Solomon, R. A prospective study of one-carbon metabolism biomarkers and risk of renal cell carcinoma. Cancer Causes Control. 2010, 21, 1061–1069. [Google Scholar] [CrossRef]
- Lonn, E.; Yusuf, S.; Arnold, M.J.; Sheridan, P.; Pogue, J.; Micks, M.; McQueen, M.J.; Probstfield, J.; Fodor, G.; Held, C.; et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. N. Engl. J. Med. 2006, 354, 1567–1577. [Google Scholar] [CrossRef] [PubMed]
- Barnabe, A.; Alessio, A.C.; Bittar, L.F.; de Moraes Mazetto, B.; Bicudo, A.M.; de Paula, E.V.; Hoehr, N.F.; Annichino-Bizzacchi, J.M. Folate, vitamin B12 and Homocysteine status in the post-folic acid fortification era in different subgroups of the Brazilian population attended to at a public health care center. Nutr. J. 2015, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- Szigeti, K.A.; Kalmar, A.; Galamb, O.; Valcz, G.; Bartak, B.K.; Nagy, Z.B.; Zsigrai, S.; Felletar, I.; Patai, Á.V.; Micsik, T.; et al. Global DNA hypomethylation of colorectal tumours detected in tissue and liquid biopsies may be related to decreased methyl-donor content. BMC Cancer 2022, 22, 605. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Ali, M.M. Methyl Donor Micronutrients that Modify DNA Methylation and Cancer Outcome. Nutrients. 2019, 11, 608. [Google Scholar] [CrossRef] [PubMed]
- Poirier, L.A.; Vlasova, T.I. The prospective role of abnormal methyl metabolism in cadmium toxicity. Environ. Health Perspect. 2002, 110 (Suppl. S5), 793–795. [Google Scholar] [CrossRef]
- Jacques, P.F.; Selhub, J.; Bostom, A.G.; Wilson, P.W.; Rosenberg, I.H. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N. Engl. J. Med. 1999, 340, 1449–1454. [Google Scholar] [CrossRef]
| Characteristic | RCC Cases n (%) | Controls n (%) | p-Value a | Age-Sex Adjusted OR (95% CI) |
|---|---|---|---|---|
| Age (years) | 58 (49, 69) b | 59 (51, 71) b | 0.22 | 0.99 (0.98–1.01) c |
| Sex | 0.15 | |||
| Male | 121 (69.54) | 429 (63.74) | 1.00 d | |
| Female | 53 (30.46) | 244 (36.26) | 0.77 (0.54–1.11) | |
| Education | 0.10 | |||
| Elementary school or below | 37 (21.26) | 129 (19.23) | 1.00 | |
| Junior/Senior high school | 71 (40.80) | 228 (33.98) | 0.92 (0.57–1.47) | |
| College or above | 66 (37.94) | 314 (46.80) | 0.56 (0.34–0.93) ** | |
| BMI (kg/m2) | <0.01 | |||
| Normal (≤23.99) | 70 (40.23) | 456 (67.76) | 1.00 | |
| Overweight (24.0–26.99) | 53 (30.46) | 125 (18.57) | 2.71 (1.79–4.10) ** | |
| Obese (≥27.0) | 51 (26.31) | 92 (13.67) | 3.55 (2.32–5.45) ** | |
| Cigarette smoking | 0.03 | |||
| Non-smoker | 104 (59.77) | 462 (68.65) | 1.00 | |
| Former or current smoker | 70 (40.23) | 211 (31.35) | 1.42 (0.96–2.10) + | |
| Cumulative cigarette smoking (pack years) | 0.02 | |||
| 0 | 104 (61.18) | 456 (67.76) | 1.00 | |
| 0 < pack year ≤ 21 | 30 (17.65) | 125 (18.57) | 1.31 (0.80–2.14) | |
| Pack year > 21 | 36 (21.18) | 92 (13.67) | 1.64 (1.01–2.66) * | |
| Alcohol consumption | <0.01 | |||
| Never | 132 (75.86) | 400 (59.44) | 1.00 | |
| Occasional or frequent | 42 (24.14) | 273 (40.56) | 0.39 (0.26–0.58) ** | |
| Tea consumption | <0.01 | |||
| Never | 84 (48.28) | 222 (32.99) | 1.00 | |
| Occasional or frequent | 90 (51.72) | 451 (67.01) | 0.50 (0.35–0.70) ** | |
| Coffee consumption | 0.01 | |||
| Never | 107 (61.49) | 316 (46.95) | 1.00 | |
| Occasional or frequent | 67 (38.51) | 357 (53.05) | 0.54 (0.39–0.77) ** | |
| Diabetes mellitus | <0.01 | |||
| No | 139 (79.89) | 617 (92.09) | 1.00 | |
| Yes | 35 (20.11) | 53 (7.91) | 3.16 (1.96–5.09) ** | |
| Hypertension | <0.01 | |||
| No | 94 (54.02) | 502 (74.93) | 1.00 | |
| Yes | 80 (45.98) | 168 (25.07) | 2.84 (1.97–4.09) ** | |
| Chronic kidney disease | <0.01 | |||
| No | 135 (77.59) | 591 (87.82) | 1.00 | |
| Yes | 39 (22.41) | 82 (12.18) | 2.32 (1.49–3.63) ** |
| Variables | RCC Cases n (%) | Controls n (%) | Age-Sex Adjusted OR (95% CI) | Multivariate Adjusted OR (95% CI) b |
|---|---|---|---|---|
| 5mdC (%) | 2.47 (2.07, 3.17) a | 3.83 (4.90, 7.26) a,** | ||
| ≤2.70 | 109 (62.64) | 225 (33.43) | 1.00 # | 1.00 # |
| 2.71–3.79 | 47 (27.01) | 244 (33.28) | 0.43 (0.29–0.64) ** | 0.53 (0.33–0.84) ** |
| ≥3.80 | 18 (10.74) | 224 (33.28) | 0.17 (0.10–0.28) ** | 0.16 (0.09–0.30) ** |
| Plasma folate levels (ng/mL) | 4.95 (3.29, 7.34) a | 7.39 (5.18, 10.20) a,** | ||
| ≤5.85 | 113 (64.94) | 226 (33.58) | 1.00 # | 1.00 # |
| 5.86–9.20 | 33 (18.97) | 223 (33.14) | 0.30 (0.19–0.46) ** | 0.27 (0.16–0.45) ** |
| ≥9.21 | 28 (16.09) | 224 (33.28) | 0.25 (0.16–0.40) ** | 0.26 (0.15–0.44) ** |
| Plasma vitamin B12 levels (pg/mL) | 468 (360, 655) a | 532 (410, 715) a,** | ||
| ≤445 | 79 (45.40) | 225 (33.43) | 1.00 # | 1.00 # |
| 446–621 | 48 (27.58) | 224 (33.28) | 0.61 (0.41–0.92) * | 0.59 (0.37–0.95) * |
| ≥622 | 47 (27.01) | 224 (33.28) | 0.63 (0.42–0.95) * | 0.57 (0.35–0.94) * |
| Blood cadmium concentrations (μg/L) | 1.86 (1.16, 2.86) a | 1.27 (0.78, 2.08) a,** | ||
| ≤0.92 | 30 (17.24) | 236 (35.07) | 1.00 # | 1.00 # |
| 0.93–1.66 | 44 (25.29) | 218 (32.39) | 1.74 (1.05–2.88) * | 2.17 (1.21–3.88) ** |
| ≥1.67 | 100 (57.49) | 219 (32.54) | 3.97 (2.52–6.26) ** | 5.13 (2.92–9.02) ** |
| Variables 1 | Variables 2 | Cases/Controls | Age-sex Adjusted OR (95% CI) | Multivariate Adjusted OR (95% CI) a |
|---|---|---|---|---|
| Plasma folate levels (ng/mL) | 5mdC (%) | |||
| >7.39 | >3.16 | 9/176 | 1.00 # | 1.00 # |
| >7.39 | ≤3.16 | 32/159 | 4.01 (1.85–8.68) ** | 3.79 (1.60–8.97) ** |
| ≤7.39 | >3.16 | 36/159 | 4.49 (2.09–9.63) ** | 4.61 (1.99–10.70) ** |
| ≤7.39 | ≤3.16 | 97/179 | 10.78 (5.24–22.19) ** | 11.86 (5.27–26.65) ** |
| S = 1.50 (0.95–2.39) | S = 1.70 (0.97–2.96) | |||
| pInteraction < 0.01 | pInteraction < 0.01 | |||
| Plasma vitamin B12 levels (pg/mL) | 5mdC (%) | |||
| >532 | >3.16 | 20/173 | 1.00 # | 1.00 # |
| >532 | ≤3.16 | 45/161 | 2.45 (1.38–4.34) ** | 2.32 (1.09–4.52) * |
| ≤532 | >3.16 | 25/162 | 1.32 (0.71–2.47) | 1.50 (0.74–3.05) |
| ≤532 | ≤3.16 | 84/177 | 4.01 (2.35–6.86) ** | 4.62 (2.46–8.66) ** |
| S = 1.70 (0.80–3.62) | S = 1.99 (0.83–4.76) | |||
| pInteraction = 0.44 | pInteraction = 0.25 | |||
| 5mdC (%) | Blood cadmium concentrations (μg/L) | |||
| >3.16 | ≤1.27 | 14/200 | 1.00 # | 1.00 # |
| >3.16 | >1.27 | 31/135 | 3.46 (1.77–6.76) ** | 4.54 (2.10–9.78) ** |
| ≤3.16 | ≤1.27 | 39/144 | 3.79 (1.98–7.26) ** | 3.89 (1.88–8.03) ** |
| ≤3.16 | >1.27 | 90/194 | 6.84 (3.76–12.45) ** | 8.16 (4.10–16.24) ** |
| S = 1.11 (0.69–1.80) | S = 1.11 (0.64–1.93) | |||
| pInteraction = 0.31 | pInteraction = 0.84 | |||
| Plasma folate levels (ng/mL) | Blood cadmium concentrations (μg/L) | |||
| >7.39 | ≤1.27 | 15/174 | 1.00 # | 1.00 # |
| >7.39 | >1.27 | 26/161 | 1.93 (0.98–3.78) + | 1.98 (0.91–4.31) + |
| ≤7.39 | ≤1.27 | 38/170 | 2.49 (1.31–4.72) ** | 2.29 (1.10–4.75) * |
| ≤7.39 | >1.27 | 95/168 | 6.47 (3.60–11.61) ** | 8.15 (4.07–16.29) ** |
| S = 2.26 (1.16–4.42) | S = 3.15 (1.39–7.13) | |||
| pInteraction = 0.34 | pInteraction = 0.05 | |||
| Plasma vitamin B12 levels (pg/mL) | Blood cadmium concentrations (μg/L) | |||
| >532 | ≤1.27 | 15/174 | 1.00 # | 1.00 # |
| >532 | >1.27 | 50/160 | 3.71 (2.00–6.89) ** | 4.89 (2.31–10.32) ** |
| ≤532 | ≤1.27 | 38/170 | 2.40 (1.27–4.56) ** | 3.07 (1.44–6.54) ** |
| ≤532 | >1.27 | 71/163 | 4.83 (2.66–8.77) ** | 7.86 (3.76–16.42) ** |
| S = 0.93 (0.56–1.56) | S = 1.15 (0.66–2.01) | |||
| pInteraction = 0.63 | pInteraction = 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-Y.; Chen, W.-J.; Lee, H.-L.; Lin, Y.-C.; Huang, Y.-L.; Shiue, H.-S.; Pu, Y.-S.; Hsueh, Y.-M. Possible Combined Effects of Plasma Folate Levels, Global DNA Methylation, and Blood Cadmium Concentrations on Renal Cell Carcinoma. Nutrients 2023, 15, 937. https://doi.org/10.3390/nu15040937
Huang C-Y, Chen W-J, Lee H-L, Lin Y-C, Huang Y-L, Shiue H-S, Pu Y-S, Hsueh Y-M. Possible Combined Effects of Plasma Folate Levels, Global DNA Methylation, and Blood Cadmium Concentrations on Renal Cell Carcinoma. Nutrients. 2023; 15(4):937. https://doi.org/10.3390/nu15040937
Chicago/Turabian StyleHuang, Chao-Yuan, Wei-Jen Chen, Hui-Ling Lee, Ying-Chin Lin, Ya-Li Huang, Horng-Sheng Shiue, Yeong-Shiau Pu, and Yu-Mei Hsueh. 2023. "Possible Combined Effects of Plasma Folate Levels, Global DNA Methylation, and Blood Cadmium Concentrations on Renal Cell Carcinoma" Nutrients 15, no. 4: 937. https://doi.org/10.3390/nu15040937
APA StyleHuang, C.-Y., Chen, W.-J., Lee, H.-L., Lin, Y.-C., Huang, Y.-L., Shiue, H.-S., Pu, Y.-S., & Hsueh, Y.-M. (2023). Possible Combined Effects of Plasma Folate Levels, Global DNA Methylation, and Blood Cadmium Concentrations on Renal Cell Carcinoma. Nutrients, 15(4), 937. https://doi.org/10.3390/nu15040937






