Mediation of 10-Year Cardiovascular Disease Risk between Inflammatory Diet and Handgrip Strength: Base on NHANES 2011–2014
Abstract
1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Measurements
2.2.1. Dietary Inflammatory Index (DII)
2.2.2. Cardiovascular Disease Risk
2.2.3. Assessment of Handgrip Strength
2.2.4. Covariate Assessment
2.3. Statistical Analysis
3. Results
3.1. Characteristics of Participants
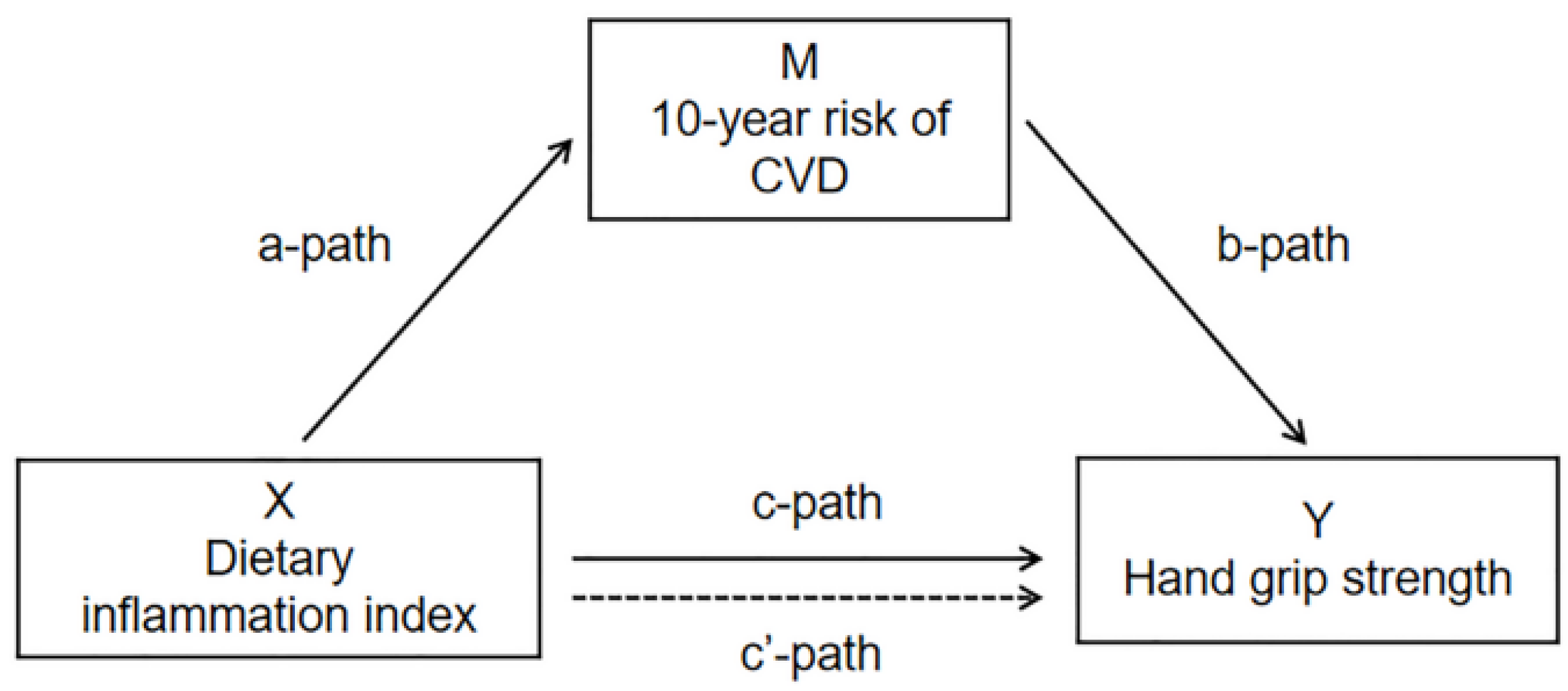
3.2. Mediation Analysis of 10-Year Risk of CVD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beenakker, K.G.; Ling, C.H.; Meskers, C.G.; de Craen, A.J.; Stijnen, T.; Westendorp, R.G.; Maier, A.B. Patterns of muscle strength loss with age in the general population and patients with a chronic inflammatory state. Ageing Res. Rev. 2010, 9, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Castro-Pinero, J.; Artero, E.G.; Espana-Romero, V.; Ortega, F.B.; Sjostrom, M.; Suni, J.; Ruiz, J.R. Criterion-related validity of field-based fitness tests in youth: A systematic review. Br. J. Sport. Med. 2010, 44, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef]
- Chainani, V.; Shaharyar, S.; Dave, K.; Choksi, V.; Ravindranathan, S.; Hanno, R.; Jamal, O.; Abdo, A.; Rafeh, N.A. Objective measures of the frailty syndrome (hand grip strength and gait speed) and cardiovascular mortality: A systematic review. Int. J. Cardiol. 2016, 215, 487–493. [Google Scholar] [CrossRef]
- Petermann-Rocha, F.; Balntzi, V.; Gray, S.R.; Lara, J.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. Global prevalence of sarcopenia and severe sarcopenia: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2022, 13, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Celis-Morales, C.A.; Welsh, P.; Lyall, D.M.; Steell, L.; Petermann, F.; Anderson, J.; Iliodromiti, S.; Sillars, A.; Graham, N.; Mackay, D.F.; et al. Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all cause mortality: Prospective cohort study of half a million UK Biobank participants. BMJ 2018, 361, k1651. [Google Scholar] [CrossRef] [PubMed]
- Georgousopoulou, E.N.; Kouli, G.M.; Panagiotakos, D.B.; Kalogeropoulou, A.; Zana, A.; Chrysohoou, C.; Tsigos, C.; Tousoulis, D.; Stefanadis, C.; Pitsavos, C. Anti-inflammatory diet and 10-year (2002–2012) cardiovascular disease incidence: The ATTICA study. Int. J. Cardiol. 2016, 222, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lee, D.H.; Hu, J.; Tabung, F.K.; Li, Y.P.; Bhupathiraju, S.N.; Rimm, E.B.; Rexrode, K.M.; Manson, J.E.; Willett, W.C.; et al. Dietary Inflammatory Potential and Risk of Cardiovascular Disease Among Men and Women in the US. J. Am. Coll. Cardiol. 2020, 76, 2181–2193. [Google Scholar] [CrossRef]
- Stewart, R. Cardiovascular Disease and Frailty: What Are the Mechanistic Links? Clin. Chem. 2019, 65, 80–86. [Google Scholar] [CrossRef]
- D’Agostino, R.B.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General cardiovascular risk profile for use in primary care: The Framingham heart study. Circulation 2008, 118, E86. [Google Scholar] [CrossRef]
- Afilalo, J.; Karunananthan, S.; Eisenberg, M.J.; Alexander, K.P.; Bergman, H. Role of frailty in patients with cardiovascular disease. Am. J. Cardiol. 2009, 103, 1616–1621. [Google Scholar] [CrossRef]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Cervo, M.M.; Shivappa, N.; Hebert, J.; Oddy, W.; Winzenberg, T.; Balogun, S.; Wu, F.; Ebeling, P.R.; Aitken, D.; Jones, G.; et al. Longitudinal Associations Between Dietary Inflammatory Index and Musculoskeletal Health in Community-Dwelling Older Adults. Osteoporos. Int. 2019, 30, S181. [Google Scholar] [CrossRef] [PubMed]
- Johansson-Persson, A.; Ulmius, M.; Cloetens, L.; Karhu, T.; Herzig, K.H.; Onning, G. A high intake of dietary fiber influences C-reactive protein and fibrinogen, but not glucose and lipid metabolism, in mildly hypercholesterolemic subjects. Eur. J. Nutr. 2014, 53, 39–48. [Google Scholar] [CrossRef]
- Bano, G.; Trevisan, C.; Carraro, S.; Solmi, M.; Luchini, C.; Stubbs, B.; Manzato, E.; Sergi, G.; Veronese, N. Inflammation and sarcopenia: A systematic review and meta-analysis. Maturitas 2017, 96, 10–15. [Google Scholar] [CrossRef]
- Shahinfar, H.; Shahavandi, M.; Tijani, A.J.; Jafari, A.; Davarzani, S.; Djafarian, K.; Clark, C.C.T.; Shab-Bidar, S. The association between dietary inflammatory index, muscle strength, muscle endurance, and body composition in Iranian adults. Eat. Weight Disord. -Stud. Anorex. Bulim. Obes. 2022, 27, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Stubbs, B.; Hébert, J.R.; Cesari, M.; Schofield, P.; Soysal, P.; Maggi, S.; Veronese, N. The Relationship Between the Dietary Inflammatory Index and Incident Frailty: A Longitudinal Cohort Study. J. Am. Med. Dir. Assoc. 2018, 19, 77–82. [Google Scholar] [CrossRef]
- Alissa, E.M.; Ferns, G.A. Dietary fruits and vegetables and cardiovascular diseases risk. Crit. Rev. Food Sci. Nutr. 2017, 57, 1950–1962. [Google Scholar] [CrossRef]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hebert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef]
- Hariharan, R.; Odjidja, E.N.; Scott, D.; Shivappa, N.; Hebert, J.R.; Hodge, A.; de Courten, B. The dietary inflammatory index, obesity, type 2 diabetes, and cardiovascular risk factors and diseases. Obes. Rev. 2022, 23, e13349. [Google Scholar] [CrossRef]
- Phillips, C.M.; Chen, L.W.; Heude, B.; Bernard, J.Y.; Harvey, N.C.; Duijts, L.; Mensink-Bout, S.M.; Polanska, K.; Mancano, G.; Suderman, M.; et al. Dietary Inflammatory Index and Non-Communicable Disease Risk: A Narrative Review. Nutrients 2019, 11, 1873. [Google Scholar] [CrossRef]
- Mazidi, M.; Shivappa, N.; Wirth, M.D.; Hebert, J.R.; Mikhailidis, D.P.; Kengne, A.P.; Banach, M. Dietary inflammatory index and cardiometabolic risk in US adults. Atherosclerosis 2018, 276, 23–27. [Google Scholar] [CrossRef]
- Peterson, M.D.; Zhang, P.; Choksi, P.; Markides, K.S.; Al Snih, S. Muscle Weakness Thresholds for Prediction of Diabetes in Adults. Sport. Med. 2016, 46, 619–628. [Google Scholar] [CrossRef]
- Cawthon, P.M.; Peters, K.W.; Shardell, M.D.; McLean, R.R.; Dam, T.T.; Kenny, A.M.; Fragala, M.S.; Harris, T.B.; Kiel, D.P.; Guralnik, J.M.; et al. Cutpoints for low appendicular lean mass that identify older adults with clinically significant weakness. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Moon, M.K.; Kim, W.; Koo, B.K. Association between muscle strength and advanced fibrosis in non-alcoholic fatty liver disease: A Korean nationwide survey. J. Cachexia Sarcopenia Muscle 2020, 11, 1232–1241. [Google Scholar] [CrossRef] [PubMed]
- Wong, N.D.; Dede, J.; Chow, V.H.; Wong, K.S.; Franklin, S.S. Global Cardiovascular Risk Associated With Hypertension and Extent of Treatment and Control According to Risk Group. Am. J. Hypertens. 2012, 25, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Laclaustra, M.; Moreno-Franco, B.; Lou-Bonafonte, J.M.; Mateo-Gallego, R.; Casasnovas, J.A.; Guallar-Castillon, P.; Cenarro, A.; Civeira, F. Impaired Sensitivity to Thyroid Hormones Is Associated With Diabetes and Metabolic Syndrome. Diabetes Care 2019, 42, 303–310. [Google Scholar] [CrossRef]
- Westerman, M.E.; Charchenko, C.M.; Ziegelmann, M.J.; Bailey, G.C.; Nippoldt, T.B.; Trost, L. Heavy Testosterone Use Among Bodybuilders: An Uncommon Cohort of Illicit Substance Users. Mayo Clin. Proc. 2016, 91, 175–182. [Google Scholar] [CrossRef]
- Brooks, J.M.; Titus, A.J.; Bruce, M.L.; Orzechowski, N.M.; Mackenzie, T.A.; Bartels, S.J.; Batsis, J.A. Depression and Handgrip Strength Among U.S. Adults Aged 60 Years and Older from NHANES 2011-2014. J. Nutr. Health Aging 2018, 22, 938–943. [Google Scholar] [CrossRef]
- Feng, Q.; Yang, Z.; May, M.; Tsoi, K.K.; Ingle, S.; Lee, E.K.; Wong, S.Y.; Kim, J.H. The role of body mass index in the association between dietary sodium intake and blood pressure: A mediation analysis with NHANES. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 3335–3344. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.J.; Lin, K.R.; Lin, M.T.; Chang, J.L. Associations Between Lifestyle Factors and Reduced Kidney Function in US Older Adults: NHANES 1999-2016. Int. J. Public Health 2021, 66, 1603966. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S.; Leiter, L.A.; Wiviott, S.D.; Giugliano, R.P.; Deedwania, P.; De Ferrari, G.M.; Murphy, S.A.; Kuder, J.F.; Gouni-Berthold, I.; Lewis, B.S.; et al. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new-onset diabetes: A prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol. 2017, 5, 941–950. [Google Scholar] [CrossRef]
- Alie, N.; Eldib, M.; Fayad, Z.A.; Mani, V. Inflammation, Atherosclerosis, and Coronary Artery Disease: PET/CT for the Evaluation of Atherosclerosis and Inflammation. Clin. Med. Insights Cardiol. 2014, 8, 13–21. [Google Scholar] [CrossRef]
- Doménech, M.; Roman, P.; Lapetra, J.; García de la Corte, F.J.; Sala-Vila, A.; de la Torre, R.; Corella, D.; Salas-Salvadó, J.; Ruiz-Gutiérrez, V.; Lamuela-Raventós, R.M.; et al. Mediterranean diet reduces 24-h ambulatory blood pressure, blood glucose, and lipids: One-year randomized, clinical trial. Hypertension 2014, 64, 69–76. [Google Scholar] [CrossRef]
- Johnson, C.B.; Davis, M.K.; Law, A.; Sulpher, J. Shared Risk Factors for Cardiovascular Disease and Cancer: Implications for Preventive Health and Clinical Care in Oncology Patients. Can. J. Cardiol. 2016, 32, 900–907. [Google Scholar] [CrossRef]
- Estruch, R. Anti-inflammatory effects of the Mediterranean diet: The experience of the PREDIMED study. Proc. Nutr. Soc. 2010, 69, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.; Kuh, D.; Hardy, R.; Mortality Review Group. Objectively measured physical capability levels and mortality: Systematic review and meta-analysis. BMJ 2010, 341, c4467. [Google Scholar] [CrossRef] [PubMed]
- Walrand, S.; Guillet, C.; Salles, J.; Cano, N.; Boirie, Y. Physiopathological mechanism of sarcopenia. Clin. Geriatr. Med. 2011, 27, 365–385. [Google Scholar] [CrossRef]
- Meng, S.J.; Yu, L.J. Oxidative stress, molecular inflammation and sarcopenia. Int. J. Mol. Sci. 2010, 11, 1509–1526. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.; Jones, G. Impact of nutrition on muscle mass, strength, and performance in older adults. Osteoporos. Int. 2014, 25, 791–792. [Google Scholar] [CrossRef] [PubMed]
- Aleman-Mateo, H.; Carreon, V.R.; Macias, L.; Astiazaran-Garcia, H.; Gallegos-Aguilar, A.C.; Enriquez, J.R. Nutrient-rich dairy proteins improve appendicular skeletal muscle mass and physical performance, and attenuate the loss of muscle strength in older men and women subjects: A single-blind randomized clinical trial. Clin. Interv. Aging 2014, 9, 1517–1525. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, S.; Wang, J.; Chi, V.T.Q.; Zhang, Q.; Liu, L.; Meng, G.; Yao, Z.; Wu, H.; Bao, X.; et al. Relationship between consumption of raw garlic and handgrip strength in a large-scale adult population. Clin. Nutr. 2020, 39, 1234–1241. [Google Scholar] [CrossRef]
- Son, E.W.; Mo, S.J.; Rhee, D.K.; Pyo, S. Inhibition of ICAM-1 expression by garlic component, allicin, in gamma-irradiated human vascular endothelial cells via downregulation of the JNK signaling pathway. Int. Immunopharmacol. 2006, 6, 1788–1795. [Google Scholar] [CrossRef]
- Beyer, I.; Mets, T.; Bautmans, I. Chronic low-grade inflammation and age-related sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 12–22. [Google Scholar] [CrossRef]
- McNallan, S.M.; Singh, M.; Chamberlain, A.M.; Kane, R.L.; Dunlay, S.M.; Redfield, M.M.; Weston, S.A.; Roger, V.L. Frailty and healthcare utilization among patients with heart failure in the community. JACC Heart Fail. 2013, 1, 135–141. [Google Scholar] [CrossRef]
- Kang, J.; Moser, D.K.; Biddle, M.J.; Lennie, T.A.; Smyth, S.S.; Vsevolozhskaya, O.A. Inflammatory properties of diet mediate the effect of depressive symptoms on Framingham risk score in men and women: Results from the National Health and Nutrition Examination Survey (2007–2014). Nutr. Res. 2020, 74, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Nakhaie, M.R.; Koor, B.E.; Salehi, S.O.; Karimpour, F. Prediction of cardiovascular disease risk using framingham risk score among office workers, Iran, 2017. Saudi J. Kidney Dis. Transpl. 2018, 29, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, M.; Shivappa, N.; de Souza Genaro, P.; Martini, L.A.; Schuch, N.J.; Hebert, J.R.; Pinheiro, M.M. Lack of association between dietary inflammatory index and low impact fractures in the Brazilian population: The Brazilian Osteoporosis Study (BRAZOS). Adv. Rheumatol. 2019, 59, 16. [Google Scholar] [CrossRef]
- Alosaimi, F.D.; Al-Sultan, O.A.; Alghamdi, Q.A.; Almohaimeed, I.K.; Alqannas, S.I. Gender-specific differences in depression and anxiety symptoms and help-seeking behavior among gastroenterology patients in Riyadh, Saudi Arabia. Neurosci. J. 2014, 19, 203–209. [Google Scholar]

| Characteristics | Anti-Inflammatory Diet (n = 2846) | Pro-Inflammatory Diet (n = 2845) | χ2/t | p | |||
|---|---|---|---|---|---|---|---|
| n | Mean (SE)/% (SE) | n | Mean (SE)/% (SE) | ||||
| 10-year risk of CVD (%) | — | 8.9 (7.4) | — | 8.8 (7.5) | −0.12 | 0.905 | |
| Relative handgrip strength (kg/BMI) | — | 1.3 (0.4) | — | 1.1 (0.4) | −14.29 | <0.001 | |
| Age (year) | — | 51.4 (14.9) | — | 51.9 (14.3) | 1.33 | 0.185 | |
| BMI (kg/m2) | — | 28.7 (6.3) | — | 29.6 (6.9) | 5.47 | <0.001 | |
| Energy Intake (kcal) | — | 2445.0 (809.3) | — | 1601.5 (557.5) | −45.78 | <0.001 | |
| Gender | 206.94 | <0.001 | |||||
| Males | 1667 | 59.7 (1.5) | 1124 | 40.3 (1.5) | |||
| Females | 1179 | 40.3 (1.5) | 1721 | 59.7 (1.5) | |||
| Race | 57.28 | <0.001 | |||||
| White | 1619 | 52.3 (1.4) | 1478 | 47.7 (1.4) | |||
| Black | 508 | 40.6 (2.0) | 743 | 59.4 (2.0) | |||
| Other | 719 | 53.5 (2.5) | 624 | 46.5 (2.5) | |||
| Education | 54.61 | <0.001 | |||||
| <12 years | 459 | 40.2 (2.4) | 682 | 59.8 (2.4) | |||
| >=12 years | 2387 | 52.5 (1.1) | 2163 | 47.5 (1.1) | |||
| Marital status | 62.04 | <0.001 | |||||
| Single | 736 | 44.3 (3.4) | 925 | 55.7 (3.4) | |||
| Married | 1796 | 54.4 (3.5) | 1503 | 45.6 (3.5) | |||
| Divorced | 314 | 43.0 (2.0) | 417 | 57.0 (2.0) | |||
| Physical activity | 59.79 | <0.001 | |||||
| Inactive | 965 | 47.1 (1.4) | 1249 | 52.9 (1.4) | |||
| Active | 1881 | 57.7 (1.4) | 1596 | 42.3 (1.4) | |||
| Smoking status | 86.72 | <0.001 | |||||
| No | 2517 | 57.1 (1.1) | 2258 | 42.9 (1.1) | |||
| Yes | 329 | 36.5 (2.7) | 587 | 63.5 (2.7) | |||
| Diabetes | 2.03 | 0.155 | |||||
| No | 2539 | 54.0 (1.0) | 2504 | 46.0 (1.0) | |||
| Yes | 307 | 47.3 (3.7) | 341 | 52.7 (3.7) | |||
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β | 95%CI | p | β | 95%CI | p | β | 95%CI | p | |
| Inflammatory diet (reference = Anti) | −0.160 | (−0.190,−0.130) | <0.001 | −0.053 | (−0.083,−0.023) | 0.001 | −0.054 | (−0.086,−0.022) | 0.002 |
| 10-year risk of CVD | 0.001 | (−0.001,0.004) | 0.383 | −0.009 | (−0.011,−0.007) | <0.001 | −0.014 | (−0.016,−0.012) | <0.001 |
| Mediator | Sample | Exposure to Mediator | Mediator to Outcome | Direct Effect | Mediated (Indirect Effect) | Total Effect (Exposure to Outcome) | Proportion Mediated (%) |
|---|---|---|---|---|---|---|---|
| 10-year risk of CVD | 5691 | 0.123 (0.037) p < 0.001 | −0.013 (0.001) p < 0.001 | −0.015 (0.003) p < 0.001 | −0.002(0.001) 95%CI (−0.003,−0.001) | −0.017 (0.003) p < 0.001 | 11.8 |
| Sample | Exposure to Mediator | Mediator to Outcome | Direct Effect | Mediated (Indirect Effect) | Total Effect (Exposure to Outcome) | Proportion Mediated (%) | |
|---|---|---|---|---|---|---|---|
| Stratification by Sex | |||||||
| Males | 2791 | 0.058 (0.053) p = 0.274 | −0.015 (0.002) p < 0.001 | −0.014 (0.004) p = 0.002 | −0.001(0.001) 95%CI (−0.003, 0.001) | −0.014 (0.004) p = 0.001 | - |
| Females | 2900 | 0.138 (0.035) p < 0.001 | −0.014 (0.002) p < 0.001 | −0.018 (0.003) p < 0.001 | −0.002(0.001) 95%CI (−0.003, −0.001) | −0.020 (0.003) p < 0.001 | 10.0 |
| Stratification by Age group | |||||||
| 30–44 | 2070 | 0.068 (0.033) p = 0.038 | −0.032 (0.003) p < 0.001 | −0.016 (0.005) p = 0.002 | −0.002 (0.001) 95%CI (−0.005, −0.001) | 0.018 (0.005) p < 0.001 | 11.1 |
| 45–64 | 2450 | −0.087 (0.064) p = 0.172 | −0.016 (0.001) p < 0.001 | −0.015 (0.004) p < 0.001 | 0.001(0.001) 95%CI (−0.001,0.003) | −0.014 (0.004) p < 0.001 | - |
| ≥65 | 1171 | 0.187 (0.102) p = 0.067 | −0.011 (0.002) p < 0.001 | −0.010 (0.006) p = 0.076 | −0.002(0.001) 95%CI (−0.005,0.000) | −0.012 (0.006) p = 0.035 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Z.; Wang, L.; Sun, M.; Wang, R.; Li, J.; Wang, X.; Guo, R.; Dong, Y.; Wang, Y.; Li, B. Mediation of 10-Year Cardiovascular Disease Risk between Inflammatory Diet and Handgrip Strength: Base on NHANES 2011–2014. Nutrients 2023, 15, 918. https://doi.org/10.3390/nu15040918
Xie Z, Wang L, Sun M, Wang R, Li J, Wang X, Guo R, Dong Y, Wang Y, Li B. Mediation of 10-Year Cardiovascular Disease Risk between Inflammatory Diet and Handgrip Strength: Base on NHANES 2011–2014. Nutrients. 2023; 15(4):918. https://doi.org/10.3390/nu15040918
Chicago/Turabian StyleXie, Zechun, Ling Wang, Mengzi Sun, Rui Wang, Jing Li, Xuhan Wang, Ruirui Guo, Yibo Dong, Yuxiang Wang, and Bo Li. 2023. "Mediation of 10-Year Cardiovascular Disease Risk between Inflammatory Diet and Handgrip Strength: Base on NHANES 2011–2014" Nutrients 15, no. 4: 918. https://doi.org/10.3390/nu15040918
APA StyleXie, Z., Wang, L., Sun, M., Wang, R., Li, J., Wang, X., Guo, R., Dong, Y., Wang, Y., & Li, B. (2023). Mediation of 10-Year Cardiovascular Disease Risk between Inflammatory Diet and Handgrip Strength: Base on NHANES 2011–2014. Nutrients, 15(4), 918. https://doi.org/10.3390/nu15040918






