Caloric Restriction (CR) Plus High-Nitrate Beetroot Juice Does Not Amplify CR-Induced Metabolic Adaptation and Improves Vascular and Cognitive Functions in Overweight Adults: A 14-Day Pilot Randomised Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Nutritional Interventions
2.4. Study Protocol
2.5. Body Composition
2.6. Indirect Calorimetry
2.7. Resting Blood Pressure
2.8. Endothelial Function
2.9. Spectral Analysis
2.10. Hand-Grip Strength
2.11. Dietary Intake, Physical Activity, and Cognition Assessment
2.12. Urine and Saliva Collection
2.13. Compliance
2.14. Laboratory Analysis
2.15. Sample Size and Randomisation
2.16. Statistical Analysis
3. Results
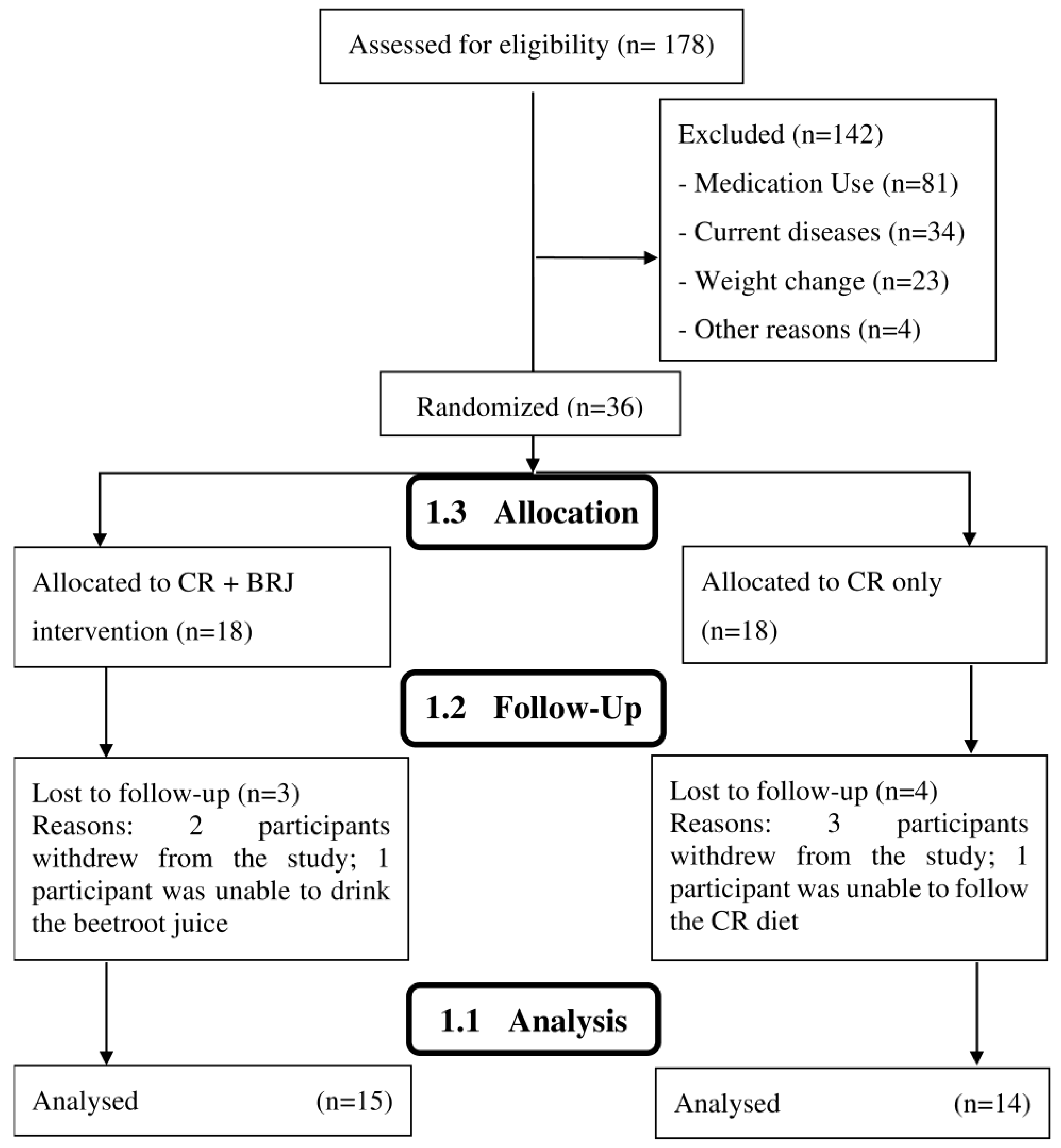
3.1. Participants
3.2. Body Composition, Hand-Grip Strength, and Resting Energy Expenditure
3.3. Vascular Function
3.4. Cognitive Function
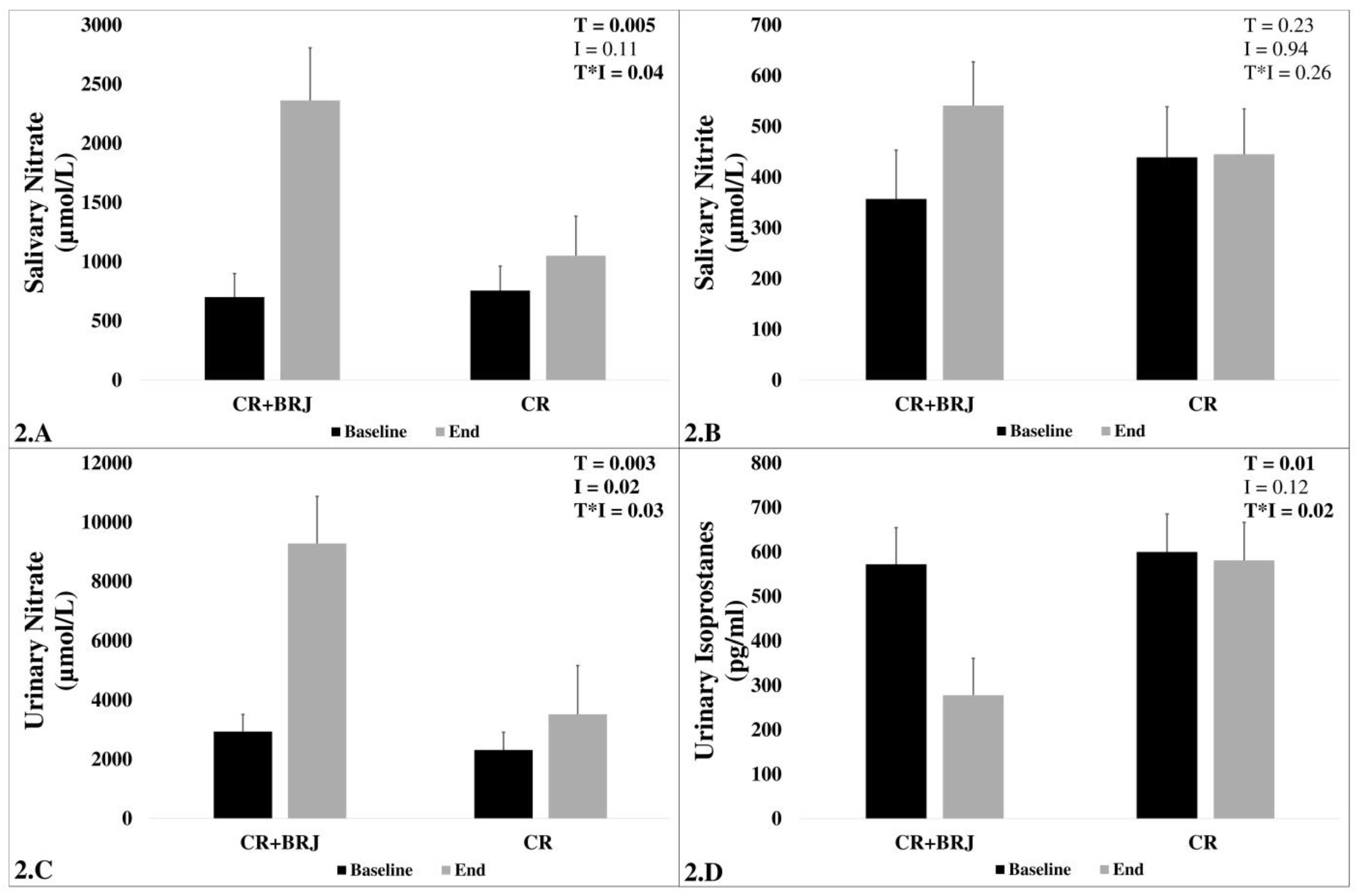
3.5. Biomarkers
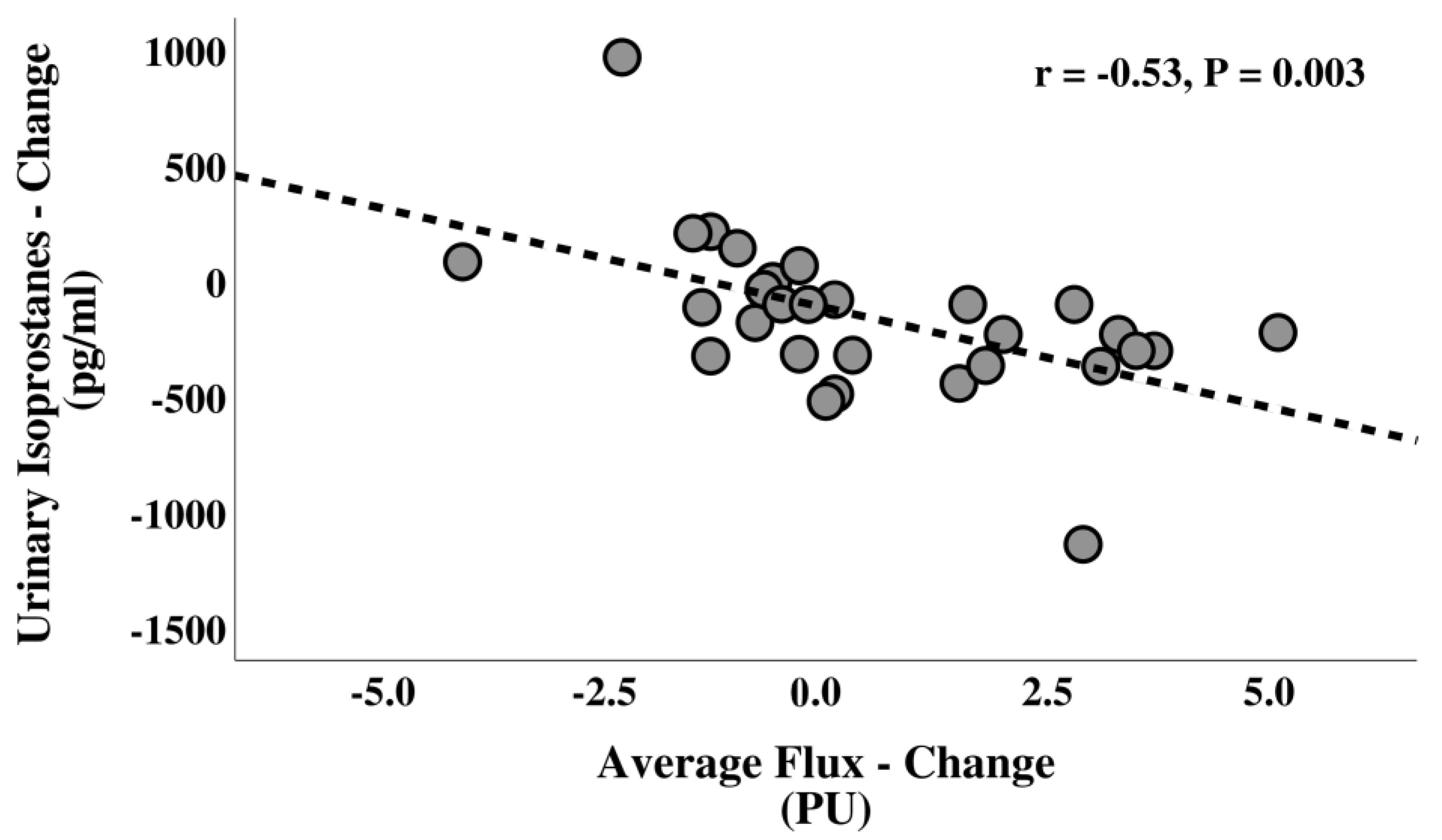
3.6. Correlations
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tiwari, A.; Balasundaram, P. Public Health Considerations Regarding Obesity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Wang, C.; Chan, J.S.Y.; Ren, L.; Yan, J.H. Obesity Reduces Cognitive and Motor Functions across the Lifespan. Neural Plast. 2016, 2016, 2473081. [Google Scholar] [CrossRef] [PubMed]
- Dye, L.; Boyle, N.B.; Champ, C.; Lawton, C. The relationship between obesity and cognitive health and decline. Proc. Nutr. Soc. 2017, 76, 443–454. [Google Scholar] [CrossRef]
- Khanna, D.; Rehman, A. Pathophysiology of Obesity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Parmar, R.M.; Can, A.S. Dietary Approaches to Obesity Treatment. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Donini, L.M.; Busetto, L.; Bauer, J.M.; Bischoff, S.; Boirie, Y.; Cederholm, T.; Cruz-Jentoft, A.J.; Dicker, D.; Frühbeck, G.; Giustina, A.; et al. Critical appraisal of definitions and diagnostic criteria for sarcopenic obesity based on a systematic review. Clin. Nutr. 2020, 39, 2368–2388. [Google Scholar] [CrossRef] [PubMed]
- Buie, J.J.; Watson, L.S.; Smith, C.J.; Sims-Robinson, C. Obesity-related cognitive impairment: The role of endothelial dysfunction. Neurobiol. Dis. 2019, 132, 104580. [Google Scholar] [CrossRef] [PubMed]
- Virdis, A. Endothelial Dysfunction in Obesity: Role of Inflammation. High Blood Press. Cardiovasc. Prev. 2016, 23, 83–85. [Google Scholar] [CrossRef]
- Raz, L.; Knoefel, J.E.; Bhaskar, K. The neuropathology and cerebrovascular mechanisms of dementia. J. Cereb. Blood Flow Metab. 2016, 36, 172–186. [Google Scholar] [CrossRef]
- Duong, S.; Patel, T.; Chang, F. Dementia: What pharmacists need to know. Can. Pharm. J. CPJ = Rev. Des Pharm. Du Can. RPC 2017, 150, 118–129. [Google Scholar] [CrossRef]
- Mudau, M.; Genis, A.; Lochner, A.; Strijdom, H. Endothelial dysfunction: The early predictor of atherosclerosis. Cardiovasc. J. Afr. 2012, 23, 222–231. [Google Scholar] [CrossRef]
- Hadi, H.A.R.; Carr, C.S.; Al Suwaidi, J. Endothelial Dysfunction: Cardiovascular Risk Factors, Therapy, and Outcome. Vasc. Health Risk Manag. 2005, 1, 183–198. [Google Scholar]
- Most, J.; Tosti, V.; Redman, L.M.; Fontana, L. Calorie restriction in humans: An update. Ageing Res. Rev. 2017, 39, 36–45. [Google Scholar] [CrossRef]
- Pellegrini, M.; Cioffi, I.; Evangelista, A.; Ponzo, V.; Goitre, I.; Ciccone, G.; Ghigo, E.; Bo, S. Effects of time-restricted feeding on body weight and metabolism. A systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2020, 21, 17–33. [Google Scholar] [CrossRef]
- Chaston, T.B.; Dixon, J.B.; O’Brien, P.E. Changes in fat-free mass during significant weight loss: A systematic review. Int. J. Obes. 2007, 31, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Wycherley, T.P.; Moran, L.J.; Clifton, P.M.; Noakes, M.; Brinkworth, G.D. Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2012, 96, 1281–1298. [Google Scholar] [CrossRef] [PubMed]
- Kraus, W.E.; Bhapkar, M.; Huffman, K.M.; Pieper, C.F.; Das, S.K.; Redman, L.M.; Villareal, D.T.; Rochon, J.; Roberts, S.B.; Ravussin, E.; et al. 2 years of calorie restriction and cardiometabolic risk (CALERIE): Exploratory outcomes of a multicentre, phase 2, randomised controlled trial. Lancet Diabetes Endocrinol. 2019, 7, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Anton, S.; Leeuwenburgh, C. Fasting or caloric restriction for Healthy Aging. Exp. Gerontol. 2013, 48, 1003–1005. [Google Scholar] [CrossRef]
- Mattson, M.P.; Duan, W.; Lee, J.; Guo, Z. Suppression of brain aging and neurodegenerative disorders by dietary restriction and environmental enrichment: Molecular mechanisms. Mech. Ageing Dev. 2001, 122, 757–778. [Google Scholar] [CrossRef]
- Mattson, M.P.; Wan, R. Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. J. Nutr. Biochem. 2005, 16, 129–137. [Google Scholar] [CrossRef]
- Prolla, T.A.; Mattson, M.P. Molecular mechanisms of brain aging and neurodegenerative disorders: Lessons from dietary restriction. Trends Neurosci. 2001, 24, 21–31. [Google Scholar] [CrossRef]
- Martin, B.; Mattson, M.P.; Maudsley, S. Caloric restriction and intermittent fasting: Two potential diets for successful brain aging. Ageing Res. Rev. 2006, 5, 332–353. [Google Scholar] [CrossRef]
- Contestabile, A. Benefits of caloric restriction on brain aging and related pathological States: Understanding mechanisms to devise novel therapies. Curr. Med. Chem. 2009, 16, 350–361. [Google Scholar] [CrossRef]
- Van Cauwenberghe, C.; Vandendriessche, C.; Libert, C.; Vandenbroucke, R. Caloric restriction: Beneficial effects on brain aging and Alzheimer’s disease. Mamm. Genome 2016, 27, 300–319. [Google Scholar] [CrossRef]
- Fontana, L.; Partridge, L. Promoting Health and Longevity through Diet: From Model Organisms to Humans. Cell 2015, 161, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Pifferi, F.; Terrien, J.; Marchal, J.; Dal-Pan, A.; Djelti, F.; Hardy, I.; Chahory, S.; Cordonnier, N.; Desquilbet, L.; Hurion, M.; et al. Caloric restriction increases lifespan but affects brain integrity in grey mouse lemur primates. Commun. Biol. 2018, 1, 30. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Ghezzi, L.; Cross, A.H.; Piccio, L. Effects of dietary restriction on neuroinflammation in neurodegenerative diseases. J. Exp. Med. 2021, 218, e20190086. [Google Scholar] [CrossRef] [PubMed]
- Gillette-Guyonnet, S.; Vellas, B. Caloric restriction and brain function. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 686–692. [Google Scholar] [CrossRef]
- Merry, B. Molecular mechanisms linking calorie restriction and longevity. Int. J. Biochem. Cell Biol. 2002, 34, 1340–1354. [Google Scholar] [CrossRef]
- Poljsak, B. Strategies for Reducing or Preventing the Generation of Oxidative Stress. Oxidative Med. Cell. Longev. 2011, 2011, 194586. [Google Scholar] [CrossRef]
- Witte, A.V.; Fobker, M.; Gellner, R.; Knecht, S.; Flöel, A. Caloric restriction improves memory in elderly humans. Proc. Natl. Acad. Sci. USA 2009, 106, 1255–1260. [Google Scholar] [CrossRef]
- Dolinsky, V.W.; Dyck, J.R. Calorie restriction and resveratrol in cardiovascular health and disease. Biochim. Et Biophys. Acta BBA Mol. Basis Dis. 2011, 1812, 1477–1489. [Google Scholar] [CrossRef]
- Bondonno, C.P.; Croft, K.D.; Hodgson, J.M. Dietary Nitrate, Nitric Oxide, and Cardiovascular Health. Crit. Rev. Food Sci. Nutr. 2016, 56, 2036–2052. [Google Scholar] [CrossRef]
- Omar, S.A.; Webb, A.J.; Lundberg, J.O.; Weitzberg, E. Therapeutic effects of inorganic nitrate and nitrite in cardiovascular and metabolic diseases. J. Intern. Med. 2016, 279, 315–336. [Google Scholar] [CrossRef]
- Ashor, A.W.; Lara, J.; Siervo, M. Medium-term effects of dietary nitrate supplementation on systolic and diastolic blood pressure in adults: A systematic review and meta-analysis. J. Hypertens. 2017, 35, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Siervo, M.; Lara, J.; Ogbonmwan, I.; Mathers, J.C. Inorganic Nitrate and Beetroot Juice Supplementation Reduces Blood Pressure in Adults: A Systematic Review and Meta-Analysis. J. Nutr. 2013, 143, 818–826. [Google Scholar] [CrossRef]
- Venturelli, M.; Pedrinolla, A.; Boscolo Galazzo, I.; Fonte, C.; Smania, N.; Tamburin, S.; Muti, E.; Crispoltoni, L.; Stabile, A.; Pistilli, A.; et al. Impact of Nitric Oxide Bioavailability on the Progressive Cerebral and Peripheral Circulatory Impairments During Aging and Alzheimer’s Disease. Front. Physiol. 2018, 9, 169. [Google Scholar] [CrossRef] [PubMed]
- Paul, V.; Ekambaram, P. Involvement of nitric oxide in learning & memory processes. Indian J. Med. Res. 2011, 133, 471–478. [Google Scholar]
- Clifford, T.; Babateen, A.; Shannon, O.; Capper, T.; Ashor, A.; Stephan, B.; Robinson, L.; O’Hara, J.P.; Mathers, J.C.; Stevenson, E.; et al. Effects of inorganic nitrate and nitrite consumption on cognitive function and cerebral blood flow: A systematic review and meta-analysis of randomized clinical trials. Crit. Rev. Food Sci. Nutr. 2019, 59, 2400–2410. [Google Scholar] [CrossRef] [PubMed]
- Aamand, R.; Dalsgaard, T.; Ho, Y.-C.L.; Møller, A.; Roepstorff, A.; Lund, T.E. A NO way to BOLD?: Dietary nitrate alters the hemodynamic response to visual stimulation. Neuroimage 2013, 83, 397–407. [Google Scholar] [CrossRef]
- Wightman, E.L.; Haskell-Ramsay, C.F.; Thompson, K.G.; Blackwell, J.R.; Winyard, P.G.; Forster, J.; Jones, A.M.; Kennedy, D.O. Dietary nitrate modulates cerebral blood flow parameters and cognitive performance in humans: A double-blind, placebo-controlled, crossover investigation. Physiol. Behav. 2015, 149, 149–158. [Google Scholar] [CrossRef]
- Walker, R. Naturally occuring nitrate/nitrite in foods. J. Sci. Food Agric. 1975, 26, 1735–1742. [Google Scholar] [CrossRef]
- Hord, N.G.; Tang, Y.; Bryan, N.S. Food sources of nitrates and nitrites: The physiologic context for potential health benefits. Am. J. Clin. Nutr. 2009, 90, 1–10. [Google Scholar] [CrossRef]
- Fredrix, E.W.; Soeters, P.B.; Deerenberg, I.M.; Kester, A.D.; Von Meyenfeldt, M.F.; Saris, W.H. Resting and sleeping energy expenditure in the elderly. Eur. J. Clin. Nutr. 1990, 44, 741–747. [Google Scholar] [PubMed]
- Mastantuono, T.; Di Maro, M.; Chiurazzi, M.; Battiloro, L.; Starita, N.; Nasti, G.; Lapi, D.; Iuppariello, L.; Cesarelli, M.; D’Addio, G.; et al. Microvascular Blood Flow Improvement in Hyperglycemic Obese Adult Patients by Hypocaloric Diet. Transl. Med. UniSa 2016, 15, 1–7. [Google Scholar] [PubMed]
- Kvandal, P.; Landsverk, S.A.; Bernjak, A.; Stefanovska, A.; Kvernmo, H.D.; Kirkebøen, K.A. Low-frequency oscillations of the laser Doppler perfusion signal in human skin. Microvasc. Res. 2006, 72, 120–127. [Google Scholar] [CrossRef]
- Pala, V.; Sieri, S.; Palli, D.; Salvini, S.; Berrino, F.; Bellegotti, M.; Frasca, G.; Tumino, R.; Sacerdote, C.; Fiorini, L.; et al. Diet in the Italian Epic Cohorts: Presentation of Data and Methodological Issues. Tumori J. 2003, 89, 594–607. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.H.; Macfarlane, D.J.; Lam, T.H.; Stewart, S.M. Validity of the international physical activity questionnaire short form (IPAQ-SF): A systematic review. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Llinàs-Reglà, J.; Vilalta-Franch, J.; López-Pousa, S.; Calvó-Perxas, L.; Rodas, D.T.; Garre-Olmo, J. The Trail Making Test. Assessment 2017, 24, 183–196. [Google Scholar] [CrossRef]
- Shannon, O.M.; Duckworth, L.; Barlow, M.J.; Woods, D.; Lara, J.; Siervo, M.; O’Hara, J.P. Dietary nitrate supplementation enhances high-intensity running performance in moderate normobaric hypoxia, independent of aerobic fitness. Nitric Oxide 2016, 59, 63–70. [Google Scholar] [CrossRef]
- Larsen, F.J.; Schiffer, T.A.; Borniquel, S.; Sahlin, K.; Ekblom, B.; Lundberg, J.O.; Weitzberg, E. Dietary Inorganic Nitrate Improves Mitochondrial Efficiency in Humans. Cell Metab. 2011, 13, 149–159. [Google Scholar] [CrossRef]
- Larsen, F.J.; Schiffer, T.A.; Ekblom, B.; Mattsson, M.P.; Checa, A.; Wheelock, C.E.; Nyström, T.; Lundberg, J.O.; Weitzberg, E. Dietary nitrate reduces resting metabolic rate: A randomized, crossover study in humans. Am. J. Clin. Nutr. 2014, 99, 843–850. [Google Scholar] [CrossRef]
- Carriker, C.R.; Harrison, C.D.; Bockover, E.J.; Ratcliffe, B.J.; Crowe, S.; Morales-Acuna, F.; Gurovich, A.N. Acute dietary nitrate does not reduce resting metabolic rate or oxidative stress marker 8-isoprostane in healthy males and females. Int. J. Food Sci. Nutr. 2019, 70, 887–893. [Google Scholar] [CrossRef]
- Beijers, R.J.; Huysmans, S.M.; van de Bool, C.; Kingma, B.R.; Verdijk, L.; van Loon, L.J.; Meex, S.J.; Gosker, H.R.; Schols, A.M. The effect of acute and 7-days dietary nitrate on mechanical efficiency, exercise performance and cardiac biomarkers in patients with chronic obstructive pulmonary disease. Clin. Nutr. 2018, 37, 1852–1861. [Google Scholar] [CrossRef] [PubMed]
- Lara, J.; Ashor, A.; Oggioni, C.; Ahluwalia, A.; Mathers, J.C.; Siervo, M. Effects of inorganic nitrate and beetroot supplementation on endothelial function: A systematic review and meta-analysis. Eur. J. Nutr. 2016, 55, 451–459. [Google Scholar] [CrossRef] [PubMed]
- El Assar, M.; Angulo, J.; Rodríguez-Mañas, L. Oxidative stress and vascular inflammation in aging. Free. Radic. Biol. Med. 2013, 65, 380–401. [Google Scholar] [CrossRef] [PubMed]
- Shinmura, K. Cardiovascular protection afforded by caloric restriction: Essential role of nitric oxide synthase. Geriatr. Gerontol. Int. 2011, 11, 143–156. [Google Scholar] [CrossRef] [PubMed]
- García-Prieto, C.F.; Gil-Ortega, M.; Plaza, A.; Manzano-Lista, F.J.; Gonzalez-Blazquez, R.; Alcala, M.; Rodríguez-Rodríguez, P.; Viana, M.; Aranguez, I.; Gollasch, M.; et al. Caloric restriction induces H(2)O(2) formation as a trigger of AMPK-eNOS-NO pathway in obese rats: Role for CAMKII. Free Radic. Biol. Med. 2019, 139, 35–45. [Google Scholar] [CrossRef]
- Nicoll, R.; Henein, M.Y. Caloric Restriction and Its Effect on Blood Pressure, Heart Rate Variability and Arterial Stiffness and Dilatation: A Review of the Evidence. Int. J. Mol. Sci. 2018, 19, 751. [Google Scholar] [CrossRef]
- Guarente, L. Sirtuins in aging and disease. Cold Spring Harb. Symp. Quant Biol. 2007, 72, 483–488. [Google Scholar] [CrossRef]
- Villar, M.L.; Godwin, I.R.; Hegarty, R.S.; Dobos, R.C.; Smith, K.A.; Clay, J.W.; Nolan, J.V. The effects of dietary nitrate on plasma glucose and insulin sensitivity in sheep. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1657–1662. [Google Scholar] [CrossRef]
- Ashor, A.W.; Chowdhury, S.; Oggioni, C.; Qadir, O.; Brandt, K.; Ishaq, A.; Mathers, J.C.; Saretzki, G.; Siervo, M. Inorganic Nitrate Supplementation in Young and Old Obese Adults Does Not Affect Acute Glucose and Insulin Responses but Lowers Oxidative Stress. J. Nutr. 2016, 146, 2224–2232. [Google Scholar] [CrossRef]
- McNally, B.D.; Moran, A.; Watt, N.T.; Ashmore, T.; Whitehead, A.; Murfitt, S.A.; Kearney, M.T.; Cubbon, R.M.; Murray, A.J.; Griffin, J.L.; et al. Inorganic Nitrate Promotes Glucose Uptake and Oxidative Catabolism in White Adipose Tissue Through the XOR-Catalyzed Nitric Oxide Pathway. Diabetes 2020, 69, 893–901. [Google Scholar] [CrossRef]
- Norouzirad, R.; González-Muniesa, P.; Ghasemi, A. Hypoxia in Obesity and Diabetes: Potential Therapeutic Effects of Hyperoxia and Nitrate. Oxid. Med. Cell. Longev. 2017, 2017, 5350267. [Google Scholar] [CrossRef]
- Baião, D.D.S.; da Silva, V.; Paschoalin, M. Beetroot, a Remarkable Vegetable: Its Nitrate and Phytochemical Contents Can be Adjusted in Novel Formulations to Benefit Health and Support Cardiovascular Disease Therapies. Antioxidants 2020, 9, 960. [Google Scholar] [CrossRef]
- Georgiev, V.G.; Weber, J.; Kneschke, E.-M.; Denev, P.N.; Bley, T.; Pavlov, A.I. Antioxidant Activity and Phenolic Content of Betalain Extracts from Intact Plants and Hairy Root Cultures of the Red Beetroot Beta vulgaris cv. Detroit Dark Red. Plant Foods Hum. Nutr. 2010, 65, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Forte, M.; Conti, V.; Damato, A.; Ambrosio, M.; Puca, A.A.; Sciarretta, S.; Frati, G.; Vecchione, C.; Carrizzo, A. Targeting Nitric Oxide with Natural Derived Compounds as a Therapeutic Strategy in Vascular Diseases. Oxidative Med. Cell. Longev. 2016, 2016, 7364138. [Google Scholar] [CrossRef] [PubMed]
- Ho, E.; Karimi Galougahi, K.; Liu, C.-C.; Bhindi, R.; Figtree, G.A. Biological markers of oxidative stress: Applications to cardiovascular research and practice. Redox Biol. 2013, 1, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Erve, T.J.V.; Lih, F.B.; Jelsema, C.; Deterding, L.J.; Eling, T.E.; Mason, R.P.; Kadiiska, M.B. Reinterpreting the best biomarker of oxidative stress: The 8-iso-prostaglandin F2α/prostaglandin F2α ratio shows complex origins of lipid peroxidation biomarkers in animal models. Free. Radic. Biol. Med. 2016, 95, 65–73. [Google Scholar] [CrossRef]
- Qiu, R.; Chen, S.; Hua, F.; Bian, S.; Chen, J.; Li, G.; Wu, X. Betanin Prevents Experimental Abdominal Aortic Aneurysm Progression by Modulating the TLR4/NF-κB and Nrf2/HO-1 Pathways. Biol. Pharm. Bull. 2021, 44, 1254–1262. [Google Scholar] [CrossRef]
- Tural, K.; Ozden, O.; Bilgi, Z.; Merhan, O.; Ermutlu, C.S.; Aksoyek, A. Protective Effects of Betanin against Oxidative Stress in a Peripheral Artery Vasospasm Model in Rat. J. Investig. Surg. 2021, 34, 208–213. [Google Scholar] [CrossRef]
- Justice, J.N.; Johnson, L.C.; DeVan, A.E.; Cruickshank-Quinn, C.; Reisdorph, N.; Bassett, C.J.; Evans, T.D.; Brooks, F.A.; Bryan, N.S.; Chonchol, M.B.; et al. Improved motor and cognitive performance with sodium nitrite supplementation is related to small metabolite signatures: A pilot trial in middle-aged and older adults. Aging 2015, 7, 1004–1021. [Google Scholar] [CrossRef]
- Gabbouj, S.; Ryhänen, S.; Marttinen, M.; Wittrahm, R.; Takalo, M.; Kemppainen, S.; Martiskainen, H.; Tanila, H.; Haapasalo, A.; Hiltunen, M.; et al. Altered Insulin Signaling in Alzheimer’s Disease Brain—Special Emphasis on PI3K-Akt Pathway. Front. Neurosci. 2019, 13, 629. [Google Scholar] [CrossRef]
- Ma, L.; Zhao, Z.; Wang, R.; Zhang, X.; Zhang, J.; Dong, W.; Xu, B.; Zhang, J. Caloric restriction can improve learning ability in C57/BL mice via regulation of the insulin-PI3K/Akt signaling pathway. Neurol. Sci. 2014, 35, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Picón-Pagès, P.; Garcia-Buendia, J.; Muñoz, F.J. Functions and dysfunctions of nitric oxide in brain. Biochim. Et Biophys. Acta BBA Mol. Basis Dis. 2019, 1865, 1949–1967. [Google Scholar] [CrossRef] [PubMed]
- Reis, P.A.; de Albuquerque, T.M.C.F.G.; Maron-Gutierrez, T.; Silva, A.R.; Neto, H.C.D.C.F. Role of Nitric Oxide Synthase in the Function of the Central Nervous System under Normal and Infectious Conditions; Harvard Medical School: Boston, MA, USA; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar]
- Lee, J.; Yanckello, L.M.; Ma, D.; Hoffman, J.D.; Parikh, I.; Thalman, S.; Bauer, B.; Hartz, A.M.S.; Hyder, F.; Lin, A.-L. Neuroimaging Biomarkers of mTOR Inhibition on Vascular and Metabolic Functions in Aging Brain and Alzheimer’s Disease. Front. Aging Neurosci. 2018, 10, 225. [Google Scholar] [CrossRef] [PubMed]
- Babateen, A.M.; Shannon, O.M.; O’Brien, G.M.; Okello, E.; Smith, E.; Olgacer, D.; Koehl, C.; Fostier, W.; Wightman, E.; Kennedy, D.; et al. Incremental Doses of Nitrate-Rich Beetroot Juice Do Not Modify Cognitive Function and Cerebral Blood Flow in Overweight and Obese Older Adults: A 13-Week Pilot Randomised Clinical Trial. Nutrients 2022, 14, 1052. [Google Scholar] [CrossRef]
- Kirkham, A.A.; Beka, V.; Prado, C.M. The effect of caloric restriction on blood pressure and cardiovascular function: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2021, 40, 728–739. [Google Scholar] [CrossRef]
- Eichholzer, M.; Gutzwiller, F. Dietary Nitrates, Nitrites, and N-Nitroso Compounds and Cancer Risk: A Review of the Epidemiologic Evidence. Nutr. Rev. 1998, 56 Pt 1, 95–105. [Google Scholar] [CrossRef]
- DellaValle, C.T.; Xiao, Q.; Yang, G.; Shu, X.O.; Aschebrook-Kilfoy, B.; Zheng, W.; Lan Li, H.; Ji, B.T.; Rothman, N.; Chow, W.H.; et al. Dietary nitrate and nitrite intake and risk of colorectal cancer in the Shanghai Women’s Health Study. Int. J. Cancer 2014, 134, 2917–2926. [Google Scholar] [CrossRef]
- Abasse, K.S.; Essien, E.E.; Abbas, M.; Yu, X.; Xie, W.; Sun, J.; Akter, L.; Cote, A. Association between Dietary Nitrate, Nitrite Intake, and Site-Specific Cancer Risk: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 666. [Google Scholar] [CrossRef]
| Total (n = 29) | CR + BRJ (n = 15) | CR (n = 14) | p | |
|---|---|---|---|---|
| Age, y | 61.3 ± 5.9 | 59.5 ± 5.9 | 63.2 ± 5.3 | 0.08 |
| Female, n (%) | 22 (75.9) | 11 (73.3) | 11 (78.5) | 0.74 |
| Weight, kg | 89.0 ± 19.1 | 91.7 ± 19.6 | 86.1 ± 18.8 | 0.44 |
| Height, cm | 160.1 ± 10.3 | 161.5 ± 12.7 | 158.6 ± 7.0 | 0.46 |
| BMI, kg/m2 | 34.5 ± 5.8 | 34.8 ± 4.4 | 34.1 ± 7.2 | 0.74 |
| WC, cm | 104.9 ± 13.2 | 104.6 ± 12.6 | 105.3 ± 14.2 | 0.88 |
| FM, kg | 35.2 ± 10.6 | 35.8 ± 9.5 | 34.5 ± 12.0 | 0.75 |
| FFM, kg | 53.7 ± 13.1 | 55.8 ± 15.6 | 51.5 ± 9.9 | 0.38 |
| HGS, kg | 25.7 ± 10.2 | 26.7 ± 12.2 | 24.7 ± 7.8 | 0.61 |
| IPAQ, METs/w | 3401 ± 4470 * | 4030 ± 5319 | 2727 ± 3408 | 0.44 |
| EI, kcal/d | 1448 ± 165 | 1460 ± 183 | 1434 ± 149 | 0.68 |
| Variable | CR + BRJ | CR | p | ||||
|---|---|---|---|---|---|---|---|
| Baseline | End | ∆% | Baseline | End | ∆% | ||
| Weight, kg | 91.7 ± 19.6 | 88.9 ± 18.1 | −2.8 ± 1.2 | 86.1 ± 18.8 | 83.8 ± 18.0 | −2.5 ± 1.1 | T < 0.001 I = 0.44 T*I = 0.32 ∆% = 0.44 |
| WC, cm | 104.6 ± 12.6 | 101.0 ± 12.5 | −3.4 ± 2.2 | 105.3 ± 14.2 | 102.5 ± 13.3 | −2.6 ± 1.7 | T < 0.001 I = 0.82 T*I = 0.33 ∆% = 0.27 |
| FM, kg | 35.8 ± 9.5 | 34.5 ± 10.2 | −4.4 ± 7.9 | 34.5 ± 12.0 | 33.3 ± 12.3 | −3.7 ± 7.2 | T = 0.003 I = 0.76 T*I = 0.91 ∆% = 0.8 |
| FFM, kg | 55.8 ± 15.6 | 54.4 ± 15.7 | −2.7 ± 3.5 | 51.5 ± 9.9 | 50.5 ± 9.2 | −1.6 ± 4.3 | T = 0.006 I = 0.4 T*I = 0.56 ∆% = 0.47 |
| HGS, kg | 26.7 ± 12.2 | 29.7 ± 10.2 | 18.4 ± 25.0 | 24.7 ± 7.8 | 25.4 ± 7.1 | 4.0 ± 7.4 | T < 0.001 I = 0.38 T*I = 0.34 ∆% = 0.04 |
| REE, kcal/d | 1438 ± 222 | 1411 ± 263 | −1.8 ± 10.3 | 1355 ± 216 | 1313 ± 213 | −2.9 ± 6.7 | T = 0.12 I = 0.28 T*I = 0.71 ∆% = 0.74 |
| REE/FFM, kcal/d/kg | 26.6 ± 4.2 | 26.6 ± 3.4 | 0.8 ± 9.9 | 26.5 ± 2.4 | 26.1 ± 1.8 | −1.1 ± 8.3 | T = 0.63 I = 0.79 T*I = 0.66 ∆% = 0.56 |
| Variable | CR + BRJ | CR | p | ||||
|---|---|---|---|---|---|---|---|
| Baseline | End | ∆% | Baseline | End | ∆% | ||
| SBP, mmHg | 131.2 ± 6.1 | 123.2 ± 6.0 | −5.9 ± 4.9 | 129.0 ± 9.0 | 125.5 ± 8.4 | −2.6 ± 4.4 | T < 0.001 I = 0.97 T*I = 0.06 ∆% = 0.06 |
| DBP, mmHg | 81.0 ± 8.0 | 78.1 ± 6.2 | −3.2 ± 4.8 | 79.1 ± 7.3 | 77.5 ± 7.7 | −1.8 ± 6.0 | T < 0.01 I = 0.64 T*I = 0.46 ∆% = 0.50 |
| Average flux, PU | 7.2 ± 3.0 | 8.5 ± 2.5 | 26.1 ± 35.8 | 7.6 ± 2.1 | 7.3 ± 1.8 | −2.2 ± 21.2 | T = 0.18 I = 0.57 T*I = 0.03 ∆% < 0.01 |
| Peak flux, PU | 54.4 ± 10.8 | 58.1 ± 11.2 | 7.6 ± 13.7 | 46.6 ± 15.6 | 50.4 ± 22.3 | 9.2 ± 43.6 | T = 0.13 I = 0.15 T*I = 0.99 ∆% = 0.89 |
| NO-dependent EA, % | 10.5 ± 4.7 | 17.4 ± 3.7 | 95.4 ± 96.3 | 10.4 ± 4.6 | 11.7 ± 5.0 | 24.9 ± 67.1 | T < 0.001 I = 0.02 T*I = 0.02 ∆% = 0.03 |
| NO-independent EA, % | 2.2 ± 1.1 | 1.9 ± 1.6 | 10.7 ± 145.3 | 3.0 ± 2.0 | 3.5 ± 2.4 | 63.2 ± 161.3 | T = 0.86 I = 0.03 T*I = 0.36 ∆% = 0.36 |
| Variable | CR + BRJ | CR | p | ||||
|---|---|---|---|---|---|---|---|
| Baseline | End | ∆% | Baseline | End | ∆% | ||
| TMT-A, s | 40.8 ± 19.2 | 34.4 ± 12.3 | −13.5 ± 12.6 | 49.2 ± 23.3 | 44.9 ± 17.5 | −3.6 ± 19.9 | T = 0.005 I = 0.16 T*I = 0.54 ∆% = 0.12 |
| TMT-B, s | 94.1 ± 19.5 | 81.5 ± 19.7 | −13.6 ± 8.7 | 95.8 ± 33.4 | 91.5 ± 30.7 | −3.2 ± 11.6 | T < 0.001 I = 0.54 T*I = 0.012 ∆% = 0.011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alharbi, M.; Chiurazzi, M.; Nasti, G.; Muscariello, E.; Mastantuono, T.; Koechl, C.; Stephan, B.C.; Shannon, O.M.; Colantuoni, A.; Siervo, M. Caloric Restriction (CR) Plus High-Nitrate Beetroot Juice Does Not Amplify CR-Induced Metabolic Adaptation and Improves Vascular and Cognitive Functions in Overweight Adults: A 14-Day Pilot Randomised Trial. Nutrients 2023, 15, 890. https://doi.org/10.3390/nu15040890
Alharbi M, Chiurazzi M, Nasti G, Muscariello E, Mastantuono T, Koechl C, Stephan BC, Shannon OM, Colantuoni A, Siervo M. Caloric Restriction (CR) Plus High-Nitrate Beetroot Juice Does Not Amplify CR-Induced Metabolic Adaptation and Improves Vascular and Cognitive Functions in Overweight Adults: A 14-Day Pilot Randomised Trial. Nutrients. 2023; 15(4):890. https://doi.org/10.3390/nu15040890
Chicago/Turabian StyleAlharbi, Mushari, Martina Chiurazzi, Gilda Nasti, Espedita Muscariello, Teresa Mastantuono, Christina Koechl, Blossom CM Stephan, Oliver M Shannon, Antonio Colantuoni, and Mario Siervo. 2023. "Caloric Restriction (CR) Plus High-Nitrate Beetroot Juice Does Not Amplify CR-Induced Metabolic Adaptation and Improves Vascular and Cognitive Functions in Overweight Adults: A 14-Day Pilot Randomised Trial" Nutrients 15, no. 4: 890. https://doi.org/10.3390/nu15040890
APA StyleAlharbi, M., Chiurazzi, M., Nasti, G., Muscariello, E., Mastantuono, T., Koechl, C., Stephan, B. C., Shannon, O. M., Colantuoni, A., & Siervo, M. (2023). Caloric Restriction (CR) Plus High-Nitrate Beetroot Juice Does Not Amplify CR-Induced Metabolic Adaptation and Improves Vascular and Cognitive Functions in Overweight Adults: A 14-Day Pilot Randomised Trial. Nutrients, 15(4), 890. https://doi.org/10.3390/nu15040890








