Potential Biological Markers and Treatment Implications for Binge Eating Disorder and Behavioral Addictions
Abstract
1. Introduction
2. Materials and Methods
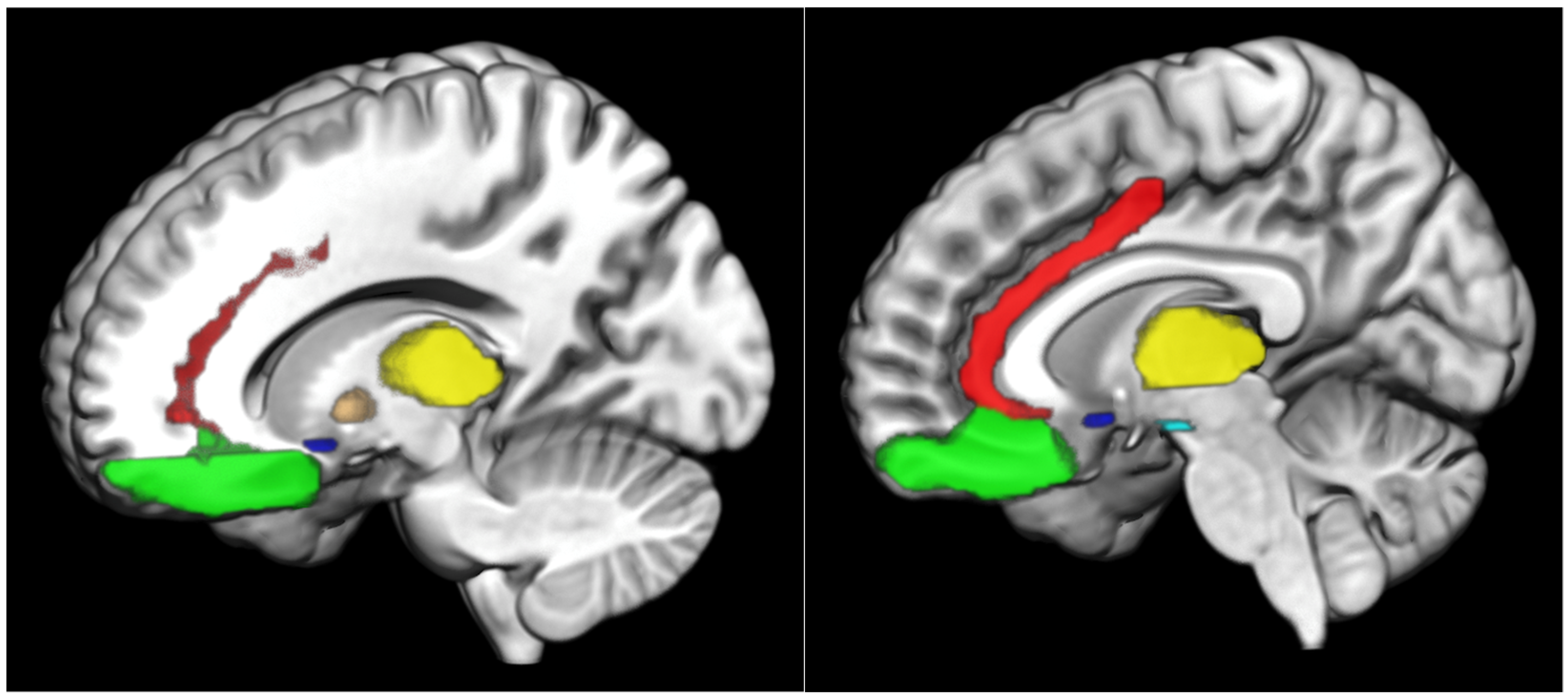
3. Results
3.1. Ventral Striatal Activation and GD
3.1.1. Neural Mechanisms of Recovery in GD
Pharmacological Interventions for GD
Psychobehavioral Interventions for GD
Neuromodulatory Interventions for GD
3.2. Ventral Striatal Activation and IGD
3.2.1. Neural Mechanisms of Recovery in IGD
Pharmacological Interventions for IGD
Psychobehavioral Interventions for IGD
Neuromodulatory Interventions for IGD
3.3. Ventral Striatal Activation and BED/FA
3.3.1. Neural Mechanisms of Recovery in BED/FA
Pharmacological Interventions for BED/FA
Psychobehavioral Interventions for BED/FA
Neuromodulatory Interventions for BED/FA
4. Limitations and Future Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Glossary
| ACC | anterior cingulate cortex |
| BED | binge eating disorder |
| CBT | cognitive behavioral therapy |
| CBI | craving behavioral intervention |
| DLPFC | dorsolateral prefrontal cortex |
| FA | food addiction |
| FC | functional connectivity |
| fMRI | functional magnetic resonance imaging |
| GD | gambling disorder |
| IGD | internet gaming disorder |
| NAc | nucleus accumbens |
| OFC | orbitofrontal cortex |
| PFC | prefrontal cortex |
| rTMS | repetitive transcranial magnetic stimulation |
| SUDs | substance use disorders |
| tDCS | transcranial direct current stimulation |
| VS | ventral striatum |
| VTA | ventral tegmental area |
| vmPFC | ventromedial prefrontal cortex |
References
- Warthen, K.G.; Boyse-Peacor, A.; Jones, K.G.; Sanford, B.; Love, T.M.; Mickey, B.J. Sex differences in the human reward system: Convergent behavioral, autonomic and neural evidence. Soc. Cogn. Affect. Neurosci. 2020, 15, 789–801. [Google Scholar] [CrossRef]
- Potenza, M.N.; Hong, K.A.; Lacadie, C.M.; Fulbright, R.K.; Tuit, K.L.; Sinha, R. Neural correlates of stress-induced and cue-induced drug craving: Influences of sex and cocaine dependence. Am. J. Psychiatry 2012, 169, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Nia, A.B.; Mann, C.; Kaur, H.; Ranganathan, M. Cannabis Use: Neurobiological, Behavioral, and Sex/Gender Considerations. Curr. Behav. Neurosci. Rep. 2018, 5, 271–280. [Google Scholar]
- Arias-Carrión, O.; Stamelou, M.; Murillo-Rodríguez, E.; Menéndez-González, M.; Pöppel, E. Dopaminergic reward system: A short integrative review. Int. Arch. Med. 2010, 3, 24. [Google Scholar] [CrossRef]
- Haber, S.N.; Knutson, B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology 2010, 35, 4–26. [Google Scholar] [CrossRef]
- Camara, E.; Rodriguez-Fornells, A.; Münte, T.F. Functional connectivity of reward processing in the brain. Front. Hum. Neurosci. 2008, 2, 19. [Google Scholar] [CrossRef]
- Delgado, M.R.; Nystrom, L.E.; Fissell, C.; Noll, D.C.; Fiez, J.A. Tracking the hemodynamic responses to reward and punishment in the striatum. J. Neurophysiol. 2000, 84, 3072–3077. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.R.; Locke, H.M.; Stenger, V.A.; Fiez, J.A. Dorsal striatum responses to reward and punishment: Effects of valence and magnitude manipulations. Cogn. Affect. Behav. Neurosci. 2003, 3, 27–38. [Google Scholar] [CrossRef]
- May, J.C.; Delgado, M.R.; Dahl, R.E.; Stenger, V.A.; Ryan, N.D.; Fiez, J.A.; Carter, C.S. Event-related functional magnetic resonance imaging of reward-related brain circuitry in children and adolescents. Biol. Psychiatry 2004, 55, 359–366. [Google Scholar] [CrossRef]
- Riba, J.; Krämer, U.M.; Heldmann, M.; Richter, S.; Münte, T.F. Dopamine agonist increases risk taking but blunts reward-related brain activity. PLoS ONE 2008, 3, e2479. [Google Scholar] [CrossRef]
- Tom, S.M.; Fox, C.R.; Trepel, C.; Poldrack, R.A. The neural basis of loss aversion in decision-making under risk. Science 2007, 315, 515–518. [Google Scholar] [CrossRef]
- Wei, D.; Lee, D.; Cox, C.D.; Karsten, C.A.; Peñagarikano, O.; Geschwind, D.H.; Gall, C.M.; Piomelli, D. Endocannabinoid signaling mediates oxytocin-driven social reward. Proc. Natl. Acad. Sci. USA 2015, 112, 14084–14089. [Google Scholar] [CrossRef]
- Filippi, S.; Luconi, M.; Granchi, S.; Vignozzi, L.; Bettuzzi, S.; Tozzi, P.; Ledda, F.; Forti, G.; Maggi, M. Estrogens, but not androgens, regulate expression and functional activity of oxytocin receptor in rabbit epididymis. Endocrinology 2002, 143, 4271–4280. [Google Scholar] [CrossRef]
- Vignozzi, L.; Filippi, S.; Luconi, M.; Morelli, A.; Mancina, R.; Marini, M.; Vannelli, G.B.; Granchi, S.; Orlando, C.; Gelmini, S.; et al. Oxytocin Receptor Is Expressed in the Penis and Mediates an Estrogen-Dependent Smooth Muscle Contractility. Endocrinology 2004, 145, 1823–1834. [Google Scholar] [CrossRef]
- Galbally, M.; Lewis, A.J.; van Ijzendoorn, M.; Permezel, M. The role of oxytocin in mother-infant relations: A systematic review of human studies. Harv. Rev. Psychiatry 2011, 19, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kor, A.; Djalovski, A.; Potenza, M.N.; Zagoory-Sharon, O.; Feldman, R. Alterations in oxytocin and vasopressin in men with problematic pornography use: The role of empathy. J. Behav. Addict. 2022, 11, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kwok, S.; Mayes, L.C.; Potenza, M.N.; Rutherford, H.J.V.; Strathearn, L. Early adverse experience and substance addiction: Dopamine, oxytocin, and glucocorticoid pathways. Ann. N. Y. Acad. Sci. 2017, 1394, 74–91. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Monjaras, M.; Mayes, L.C.; Potenza, M.N.; Rutherford, H.J. A developmental model of addictions: Integrating neurobiological and psychodynamic theories through the lens of attachment. Attach. Hum. Dev. 2019, 21, 616–637. [Google Scholar] [CrossRef]
- Baik, J.-H. Dopamine signaling in reward-related behaviors. Front. Neural. Circuits 2013, 7, 152. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.-J.; Baler, R.D. Reward, dopamine and the control of food intake: Implications for obesity. Trends Cogn. Sci. 2011, 15, 37–46. [Google Scholar] [CrossRef]
- Gola, M.; Draps, M. Ventral Striatal Reactivity in Compulsive Sexual Behaviors. Front. Psychiatry 2018, 9, 546. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.E.; Berridge, K.C. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res. Brain Res. Rev. 1993, 18, 247–291. [Google Scholar] [CrossRef] [PubMed]
- Blum, K.; Gardner, E.; Oscar-Berman, M.; Gold, M. “Liking” and “wanting” linked to Reward Deficiency Syndrome (RDS): Hypothesizing differential responsivity in brain reward circuitry. Curr. Pharm. Des. 2012, 18, 113–118. [Google Scholar] [CrossRef]
- Comings, D.E.; Blum, K. Reward deficiency syndrome: Genetic aspects of behavioral disorders. Prog. Brain Res. 2000, 126, 325–341. [Google Scholar] [CrossRef]
- Volkow, N.D.; Fowler, J.S.; Wang, G.-J.; Swanson, J.M. Dopamine in drug abuse and addiction: Results from imaging studies and treatment implications. Mol. Psychiatry 2004, 9, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Denier, N.; Magon, S.; Radue, E.-W.; Huber, C.G.; Riecher-Rossler, A.; Wiesbeck, G.A.; Lang, U.E.; Borgwardt, S.; Walter, M. Increased functional connectivity in the resting-state basal ganglia network after acute heroin substitution. Transl. Psychiatry 2015, 5, e533. [Google Scholar] [CrossRef] [PubMed]
- Forbes, E.E.; Rodriguez, E.E.; Musselman, S.; Narendran, R. Prefrontal response and frontostriatal functional connectivity to monetary reward in abstinent alcohol-dependent young adults. PLoS ONE 2014, 9, e94640. [Google Scholar] [CrossRef]
- Gelskov, S.V.; Madsen, K.H.; Ramsøy, T.Z.; Siebner, H.R. Aberrant neural signatures of decision-making: Pathological gamblers display cortico-striatal hypersensitivity to extreme gambles. Neuroimage 2016, 128, 342–352. [Google Scholar] [CrossRef]
- Hong, S.-B.; Harrison, B.J.; Dandash, O.; Choi, E.-J.; Kim, S.-C.; Kim, H.-H.; Shim, D.-H.; Kim, C.-D.; Kim, J.-W.; Yi, S.-H. A selective involvement of putamen functional connectivity in youth with internet gaming disorder. Brain Res. 2015, 1602, 85–95. [Google Scholar] [CrossRef]
- APA. Diagnostic and Statistical Manual of Mental Disorders 5 Edition (DSM-5) 5; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- World Health Organization. International Classification of Diseases 11th Revision; ICD-11; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Schulte, E.M.; Joyner, M.A.; Potenza, M.N.; Grilo, C.M.; Gearhardt, A.N. Current considerations regarding food addiction. Curr. Psychiatry Rep. 2015, 17, 563. [Google Scholar] [CrossRef]
- Kessler, R.M.; Hutson, P.H.; Herman, B.K.; Potenza, M.N. The neurobiological basis of binge-eating disorder. Neurosci. Biobehav. Rev. 2016, 63, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Novelle, M.G.; Diéguez, C. Food Addiction and Binge Eating: Lessons Learned from Animal Models. Nutrients 2018, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Gearhardt, A.N.; White, M.A.; Potenza, M.N. Binge eating disorder and food addiction. Curr. Drug Abus. Rev. 2011, 4, 201–207. [Google Scholar] [CrossRef]
- Wiedemann, A.A.; Ivezaj, V.; Gueorguieva, R.; Potenza, M.N.; Grilo, C.M. Examining Self-Weighing Behaviors and Associated Features and Treatment Outcomes in Patients with Binge-Eating Disorder and Obesity with and without Food Addiction. Nutrients 2020, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Georgel, P.T.; Georgel, P. Where Epigenetics Meets Food Intake: Their Interaction in the Development/Severity of Gout and Therapeutic Perspectives. Front. Immunol. 2021, 12, 752359. [Google Scholar] [CrossRef]
- Nestler, E.J.; Lüscher, C. The Molecular Basis of Drug Addiction: Linking Epigenetic to Synaptic and Circuit Mechanisms. Neuron 2019, 102, 48–59. [Google Scholar] [CrossRef]
- Bullock, S.A.; Potenza, M.N. Pathological Gambling: Neuropsychopharmacology and Treatment. Curr. Psychopharmacol. 2012, 1, 67–85. [Google Scholar] [CrossRef]
- Koehler, S.; Hasselmann, E.; Wüstenberg, T.; Heinz, A.; Romanczuk-Seiferth, N. Higher volume of ventral striatum and right prefrontal cortex in pathological gambling. Brain Struct. Funct. 2015, 220, 469–477. [Google Scholar] [CrossRef]
- Van Holst, R.J.; van den Brink, W.; Veltman, D.J.; Goudriaan, A.E. Brain imaging studies in pathological gambling. Curr. Psychiatry Rep. 2010, 12, 418–425. [Google Scholar] [CrossRef]
- Limbrick-Oldfield, E.H.; van Holst, R.J.; Clark, L. Fronto-striatal dysregulation in drug addiction and pathological gambling: Consistent inconsistencies? NeuroImage. Clin. 2013, 2, 385–393. [Google Scholar] [CrossRef]
- Potenza, M.N. The neural bases of cognitive processes in gambling disorder. Trends Cogn. Sci. 2014, 18, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Studer, B.; Apergis-Schoute, A.M.; Robbins, T.W.; Clark, L. What are the Odds? The Neural Correlates of Active Choice during Gambling. Front. Neurosci. 2012, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Koehler, S.; Ovadia-Caro, S.; van der Meer, E.; Villringer, A.; Heinz, A.; Romanczuk-Seiferth, N.; Margulies, D. Increased functional connectivity between prefrontal cortex and reward system in pathological gambling. PLoS ONE 2013, 8, e84565. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.; Boileau, I.; Zack, M. Neuroimaging of reward mechanisms in Gambling disorder: An integrative review. Mol. Psychiatry 2019, 24, 674–693. [Google Scholar] [CrossRef] [PubMed]
- Brevers, D.; Noël, X.; He, Q.; Melrose, J.A.; Bechara, A. Increased ventral-striatal activity during monetary decision making is a marker of problem poker gambling severity. Addict. Biol. 2016, 21, 688–699. [Google Scholar] [CrossRef]
- Raimo, S.; Cropano, M.; Trojano, L.; Santangelo, G. The neural basis of gambling disorder: An activation likelihood estimation meta-analysis. Neurosci. Biobehav. Rev. 2021, 120, 279–302. [Google Scholar] [CrossRef] [PubMed]
- Reuter, J.; Raedler, T.; Rose, M.; Hand, I.; Gläscher, J.; Büchel, C. Pathological gambling is linked to reduced activation of the mesolimbic reward system. Nat. Neurosci. 2005, 8, 147–148. [Google Scholar] [CrossRef] [PubMed]
- de Ruiter, M.B.; Veltman, D.J.; Goudriaan, A.E.; Oosterlaan, J.; Sjoerds, Z.; van den Brink, W. Response perseveration and ventral prefrontal sensitivity to reward and punishment in male problem gamblers and smokers. Neuropsychopharmacology 2009, 34, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Balodis, I.M.; Kober, H.; Worhunsky, P.D.; Stevens, M.C.; Pearlson, G.D.; Potenza, M.N. Diminished frontostriatal activity during processing of monetary rewards and losses in pathological gambling. Biol. Psychiatry 2012, 71, 749–757. [Google Scholar] [CrossRef]
- Sescousse, G.; Barbalat, G.; Domenech, P.; Dreher, J.-C. Imbalance in the sensitivity to different types of rewards in pathological gambling. Brain 2013, 136, 2527–2538. [Google Scholar] [CrossRef] [PubMed]
- van Holst, R.J.; van Holstein, M.; van den Brink, W.; Veltman, D.J.; Goudriaan, A.E. Response inhibition during cue reactivity in problem gamblers: An fMRI study. PLoS ONE 2012, 7, e30909. [Google Scholar] [CrossRef]
- EH, L.-O.; Mick, I.; RE, C.; McGonigle, J.; SP, S.; AP, G.; PR, S.; Waldman, A.; Erritzoe, D.; Bowden-Jones, H.; et al. Neural substrates of cue reactivity and craving in gambling disorder. Transl. Psychiatry 2017, 7, e992. [Google Scholar] [CrossRef]
- Chase, H.W.; Clark, L. Gambling severity predicts midbrain response to near-miss outcomes. J. Neurosci. 2010, 30, 6180–6187. [Google Scholar] [CrossRef]
- Clark, L.; Lawrence, A.J.; Astley-Jones, F.; Gray, N. Gambling near-misses enhance motivation to gamble and recruit win-related brain circuitry. Neuron 2009, 61, 481–490. [Google Scholar] [CrossRef]
- Worhunsky, P.D.; Malison, R.T.; Rogers, R.D.; Potenza, M.N. Altered neural correlates of reward and loss processing during simulated slot-machine fMRI in pathological gambling and cocaine dependence. Drug Alcohol. Depend. 2014, 145, 77–86. [Google Scholar] [CrossRef]
- Sescousse, G.; Janssen, L.K.; Hashemi, M.M.; Timmer, M.H.M.; Geurts, D.E.M.; Ter Huurne, N.P.; Clark, L.; Cools, R. Amplified Striatal Responses to Near-Miss Outcomes in Pathological Gamblers. Neuropsychopharmacology 2016, 41, 2614–2623. [Google Scholar] [CrossRef]
- Potenza, M.N.; Balodis, I.M.; Derevensky, J.; Grant, J.E.; Petry, N.M.; Verdejo-Garcia, A.; Yip, S.W. Gambling disorder. Nat. Rev. Dis. Prim. 2019, 5, 51. [Google Scholar] [CrossRef]
- Bae, S.; Hong, J.S.; Kim, S.M.; Han, D.H. Bupropion shows different effects on brain functional connectivity in patients with Internet-based gambling disorder and internet gaming disorder. Front. Psychiatry 2018, 9, 130. [Google Scholar] [CrossRef]
- Grant, J.E.; Odlaug, B.L.; Chamberlain, S.R.; Hampshire, A.; Schreiber, L.R.N.; Kim, S.W. A proof of concept study of tolcapone for pathological gambling: Relationships with COMT genotype and brain activation. Eur. Neuropsychopharmacol. 2013, 23, 1587–1596. [Google Scholar] [CrossRef]
- Chung, S.K.; You, I.H.; Cho, G.H.; Chung, G.H.; Shin, Y.C.; Kim, D.J.; Choi, S.W. Changes of functional MRI findings in a patient whose pathological gambling improved with fluvoxamine. Yonsei Med. J. 2009, 50, 441–444. [Google Scholar] [CrossRef]
- Pallanti, S.; Haznedar, M.M.; Hollander, E.; Licalzi, E.M.; Bernardi, S.; Newmark, R.; Buchsbaum, M.S. Basal ganglia activity in pathological gambling: A fluorodeoxyglucose- positron emission tomography study. Neuropsychobiology 2010, 62, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Hollander, E.; Buchsbaum, M.S.; Haznedar, M.M.; Berenguer, J.; Berlin, H.A.; Chaplin, W.; Goodman, C.R.; LiCalzi, E.M.; Newmark, R.; Pallanti, S. FDG-PET study in pathological gamblers: 1. Lithium increases orbitofrontal, dorsolateral and cingulate metabolism. Neuropsychobiology 2008, 58, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Potenza, M.N.; Balodis, I.M.; Franco, C.A.; Bullock, S.; Xua, J.; Chung, T.; Grant, J.E. Neurobiological considerations in understanding behavioral treatments for pathological gambling. Psychol. Addict Behav 2013, 27, 380–392. [Google Scholar] [CrossRef]
- Spagnolo, P.A.; Gómez Pérez, L.J.; Terraneo, A.; Gallimberti, L.; Bonci, A. Neural correlates of cue- and stress-induced craving in gambling disorders: Implications for transcranial magnetic stimulation interventions. Eur. J. Neurosci. 2019, 50, 2370–2383. [Google Scholar] [CrossRef]
- Gay, A.; Boutet, C.; Sigaud, T.; Kamgoue, A.; Sevos, J.; Brunelin, J.; Massoubre, C. A single session of repetitive transcranial magnetic stimulation of the prefrontal cortex reduces cue-induced craving in patients with gambling disorder. Eur. Psychiatry 2017, 41, 68–74. [Google Scholar] [CrossRef]
- Gay, A.; Cabe, J.; De Chazeron, I.; Lambert, C.; Defour, M.; Bhoowabul, V.; Charpeaud, T.; Tremey, A.; Llorca, P.-M.; Pereira, B.; et al. Repetitive Transcranial Magnetic Stimulation (rTMS) as a Promising Treatment for Craving in Stimulant Drugs and Behavioral Addiction: A Meta-Analysis. J. Clin. Med. 2022, 11, 624. [Google Scholar] [CrossRef]
- Soyata, A.Z.; Aksu, S.; Woods, A.J.; İşçen, P.; Saçar, K.T.; Karamürsel, S. Effect of transcranial direct current stimulation on decision making and cognitive flexibility in gambling disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2019, 269, 275–284. [Google Scholar] [CrossRef]
- Pettorruso, M.; Di Giuda, D.; Martinotti, G.; Cocciolillo, F.; De Risio, L.; Montemitro, C.; Camardese, G.; Di Nicola, M.; Janiri, L.; di Giannantonio, M.; et al. Dopaminergic and clinical correlates of high-frequency repetitive transcranial magnetic stimulation in gambling addiction: A SPECT case study. Addict. Behav. 2019, 93, 246–249. [Google Scholar] [CrossRef]
- Martinotti, G.; Chillemi, E.; Lupi, M.; De Risio, L.; Pettorruso, M.; Giannantonio, M. Di Gambling disorder and bilateral transcranial direct current stimulation: A case report. J. Behav. Addict. 2018, 7, 834–837. [Google Scholar] [CrossRef]
- Dickler, M.; Lenglos, C.; Renauld, E.; Ferland, F.; Edden, R.; Leblond, J.; Fecteau, S. Online effects of transcranial direct current stimulation on prefrontal metabolites in gambling disorder. Neuropharmacology 2018, 131, 51–57. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, S.; Turel, O.; Bechara, A.; He, Q. A tripartite neurocognitive model of internet gaming disorder. Front. Psychiatry 2017, 8, 285. [Google Scholar] [CrossRef]
- Turel, O.; He, Q.; Wei, L.; Bechara, A. The role of the insula in internet gaming disorder. Addict. Biol. 2021, 26, e12894. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, P.; Gong, Y.; Wang, Y.; Wu, Y.; Wang, C.; Guo, X. Altered neural responses to missed chance contribute to the risk-taking behaviour in individuals with Internet gaming disorder. Addict. Biol. 2022, 27, e13124. [Google Scholar] [CrossRef]
- Liu, L.; Xue, G.; Potenza, M.N.; Zhang, J.T.; Yao, Y.W.; Xia, C.C.; Lan, J.; Ma, S.S.; Fang, X.Y. Dissociable neural processes during risky decision-making in individuals with Internet-gaming disorder. NeuroImage Clin. 2017, 14, 741–749. [Google Scholar] [CrossRef]
- Young, K.S.; Brand, M. Merging theoretical models and therapy approaches in the context of internet gaming disorder: A personal perspective. Front. Psychol. 2017, 8, 1853. [Google Scholar] [CrossRef]
- Vollstädt-Klein, S.; Wichert, S.; Rabinstein, J.; Bühler, M.; Klein, O.; Ende, G.; Hermann, D.; Mann, K. Initial, habitual and compulsive alcohol use is characterized by a shift of cue processing from ventral to dorsal striatum. Addiction 2010, 105, 1741–1749. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.-J.; Telang, F.; Fowler, J.S.; Logan, J.; Childress, A.-R.; Jayne, M.; Ma, Y.; Wong, C. Cocaine cues and dopamine in dorsal striatum: Mechanism of craving in cocaine addiction. J. Neurosci. 2006, 26, 6583–6588. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.-J.; Telang, F.; Fowler, J.S.; Logan, J.; Childress, A.-R.; Jayne, M.; Ma, Y.; Wong, C. Dopamine increases in striatum do not elicit craving in cocaine abusers unless they are coupled with cocaine cues. Neuroimage 2008, 39, 1266–1273. [Google Scholar] [CrossRef]
- Liu, L.; Yip, S.W.; Zhang, J.-T.; Wang, L.-J.; Shen, Z.-J.; Liu, B.; Ma, S.-S.; Yao, Y.-W.; Fang, X.-Y. Activation of the ventral and dorsal striatum during cue reactivity in Internet gaming disorder. Addict. Biol. 2017, 22, 791–801. [Google Scholar] [CrossRef]
- Yuan, K.; Yu, D.; Cai, C.; Feng, D.; Li, Y.; Bi, Y.; Liu, J.; Zhang, Y.; Jin, C.; Li, L.; et al. Frontostriatal circuits, resting state functional connectivity and cognitive control in internet gaming disorder. Addict. Biol. 2017, 22, 813–822. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, H.; Zhou, W.; Jiang, Q.; Dong, G.-H. Persistent dependent behaviour is accompanied by dynamic switching between the ventral and dorsal striatal connections in internet gaming disorder. Addict. Biol. 2021, 26, e13046. [Google Scholar] [CrossRef]
- Brand, M.; Wegmann, E.; Stark, R.; Müller, A.; Wölfling, K.; Robbins, T.W.; Potenza, M.N. The Interaction of Person-Affect-Cognition-Execution (I-PACE) model for addictive behaviors: Update, generalization to addictive behaviors beyond internet-use disorders, and specification of the process character of addictive behaviors. Neurosci. Biobehav. Rev. 2019, 104, 1–10. [Google Scholar] [CrossRef]
- Weinstein, A.M. Computer and video game addiction-a comparison between game users and non-game users. Am. J. Drug Alcohol. Abus. 2010, 36, 268–276. [Google Scholar] [CrossRef]
- Kühn, S.; Romanowski, A.; Schilling, C.; Lorenz, R.; Mörsen, C.; Seiferth, N.; Banaschewski, T.; Barbot, A.; Barker, G.J.; Büchel, C.; et al. The neural basis of video gaming. Transl. Psychiatry 2011, 1, e53. [Google Scholar] [CrossRef]
- Weinstein, A.; Livny, A.; Weizman, A. New developments in brain research of internet and gaming disorder. Neurosci. Biobehav. Rev. 2017, 75, 314–330. [Google Scholar] [CrossRef]
- Cai, C.; Yuan, K.; Yin, J.; Feng, D.; Bi, Y.; Li, Y.; Yu, D.; Jin, C.; Qin, W.; Tian, J. Striatum morphometry is associated with cognitive control deficits and symptom severity in internet gaming disorder. Brain Imaging Behav. 2016, 10, 12–20. [Google Scholar] [CrossRef]
- Hou, H.; Jia, S.; Hu, S.; Fan, R.; Sun, W.; Sun, T.; Zhang, H. Reduced striatal dopamine transporters in people with internet addiction disorder. J. Biomed. Biotechnol. 2012, 2012, 854524. [Google Scholar] [CrossRef]
- Palaus, M.; Marron, E.M.; Viejo-Sobera, R.; Redolar-Ripoll, D. Neural basis of video gaming: A systematic review. Front. Hum. Neurosci. 2017, 11, 248. [Google Scholar] [CrossRef]
- Lorenz, R.C.; Krüger, J.-K.; Neumann, B.; Schott, B.H.; Kaufmann, C.; Heinz, A.; Wüstenberg, T. Cue reactivity and its inhibition in pathological computer game players. Addict. Biol. 2013, 18, 134–146. [Google Scholar] [CrossRef]
- Dong, G.-H.; Dong, H.; Wang, M.; Zhang, J.; Zhou, W.; Du, X.; Potenza, M.N. Dorsal and ventral striatal functional connectivity shifts play a potential role in internet gaming disorder. Commun. Biol. 2021, 4, 866. [Google Scholar] [CrossRef]
- Shin, Y.B.; Kim, H.; Kim, S.J.; Kim, J.J. A neural mechanism of the relationship between impulsivity and emotion dysregulation in patients with Internet gaming disorder. Addict. Biol. 2021, 26, e12916. [Google Scholar] [CrossRef]
- Chun, J.W.; Park, C.H.; Kim, J.Y.; Choi, J.; Cho, H.; Jung, D.J.; Ahn, K.J.; Choi, J.S.; Kim, D.J.; Choi, I.Y. Altered core networks of brain connectivity and personality traits in internet gaming disorder. J. Behav. Addict. 2020, 9, 298–311. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, H.; Kang, E. Impaired Feedback Processing for Symbolic Reward in Individuals with Internet Game Overuse. Front. Psychiatry 2017, 8, 195. [Google Scholar] [CrossRef]
- Dong, H.; Wang, M.; Zhang, J.; Hu, Y.; Potenza, M.N.; Dong, G.-H. Reduced frontostriatal functional connectivity and associations with severity of Internet gaming disorder. Addict. Biol. 2021, 26, e12985. [Google Scholar] [CrossRef]
- Gong, L.; Zhou, H.; Su, C.; Geng, F.; Xi, W.; Teng, B.; Yuan, K.; Zhao, M.; Hu, Y. Self-control impacts symptoms defining Internet gaming disorder through dorsal anterior cingulate-ventral striatal pathway. Addict. Biol. 2022, 27, e13210. [Google Scholar] [CrossRef]
- Kim, J.; Kang, E. Internet Game Overuse Is Associated With an Alteration of Fronto-Striatal Functional Connectivity During Reward Feedback Processing. Front. Psychiatry 2018, 9, 371. [Google Scholar] [CrossRef]
- Dong, G.H.; Wang, M.; Zhang, J.; Du, X.; Potenza, M.N. Functional neural changes and altered cortical–subcortical connectivity associated with recovery from Internet gaming disorder. J. Behav. Addict. 2019, 8, 692–702. [Google Scholar] [CrossRef]
- Dong, G.; Wang, M.; Liu, X.; Liang, Q.; Du, X.; Potenza, M.N. Cue-elicited craving-related lentiform activation during gaming deprivation is associated with the emergence of Internet gaming disorder. Addict Biol. 2020, 25, e12713. [Google Scholar] [CrossRef]
- Zajac, K.; Ginley, M.K.; Chang, R. Treatments of internet gaming disorder: A systematic review of the evidence. Expert Rev. Neurother. 2020, 20, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Zajac, K.; Ginley, M.K.; Chang, R.; Petry, N.M. Treatments for Internet Gaming Disorder and Internet Addiction: A Systematic Review. Psychol. Addict Behav. 2017, 31, 979–994. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, Y.S.; Sohn, J.H.; Han, D.H. Effectiveness of atomoxetine and methylphenidate for problematic online gaming in adolescents with attention deficit hyperactivity disorder. Hum. Psychopharmacol. 2016, 31, 427–432. [Google Scholar] [CrossRef]
- Han, D.H.; Hwang, J.W.; Renshaw, P.F. Bupropion sustained release treatment decreases craving for video games and cue-induced brain activity in patients with internet video game addiction. Exp. Clin. Psychopharmacol. 2010, 18, 297–304. [Google Scholar] [CrossRef]
- Seo, E.H.; Yang, H.J.; Kim, S.G.; Park, S.C.; Lee, S.K.; Yoon, H.J. A Literature Review on the Efficacy and Related Neural Effects of Pharmacological and Psychosocial Treatments in Individuals With Internet Gaming Disorder. Psychiatry Investig. 2021, 18, 1149–1163. [Google Scholar] [CrossRef]
- Nam, B.; Bae, S.; Kim, S.M.; Hong, J.S.; Han, D.H. Comparing the Effects of Bupropion and Escitalopram on Excessive Internet Game Play in Patients with Major Depressive Disorder. Clin. Psychopharmacol. Neurosci. 2017, 15, 361–368. [Google Scholar] [CrossRef]
- Konova, A.B.; Moeller, S.J.; Goldstein, R.Z. Common and distinct neural targets of treatment: Changing brain function in substance addiction. Neurosci. Biobehav. Rev. 2013, 37, 2806–2817. [Google Scholar] [CrossRef]
- Garland, E.L.; Froeliger, B.; Howard, M.O. Neurophysiological evidence for remediation of reward processing deficits in chronic pain and opioid misuse following treatment with Mindfulness-Oriented Recovery Enhancement: Exploratory ERP findings from a pilot RCT. J. Behav. Med. 2015, 38, 327–336. [Google Scholar] [CrossRef]
- Vollstädt-Klein, S.; Loeber, S.; Kirsch, M.; Bach, P.; Richter, A.; Bhler, M.; Von Der Goltz, C.; Hermann, D.; Mann, K.; Kiefer, F. Effects of cue-exposure treatment on neural cue reactivity in alcohol dependence: A randomized trial. Biol. Psychiatry 2011, 69, 1060–1066. [Google Scholar] [CrossRef]
- Han, X.; Wang, Y.; Jiang, W.; Bao, X.; Sun, Y.; Ding, W.; Cao, M.; Wu, X.; Du, Y.; Zhou, Y. Resting-state activity of prefrontal-striatal circuits in internet gaming disorder: Changes with cognitive behavior therapy and predictors of treatment response. Front. Psychiatry 2018, 9, 341. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, S.M.; Roh, S.; Soh, M.A.; Lee, S.H.; Kim, H.; Lee, Y.S.; Han, D.H. The effects of a virtual reality treatment program for online gaming addiction. Comput. Methods Programs Biomed. 2016, 129, 99–108. [Google Scholar] [CrossRef]
- Han, D.H.; Kim, S.M.; Lee, Y.S.; Renshaw, P.F. The effect of family therapy on the changes in the severity of on-line game play and brain activity in adolescents with on-line game addiction. Psychiatry Res. Neuroimaging 2012, 202, 126–131. [Google Scholar] [CrossRef]
- Zhang, J.T.; Ma, S.S.; Li, C.S.R.; Liu, L.; Xia, C.C.; Lan, J.; Wang, L.-J.; Liu, B.; Yao, Y.W.; Fang, X.-Y. Craving Behavioral Intervention for Internet Gaming Disorder: Remediation of Functional Connectivity of the Ventral Striatum. Addict Biol. 2018, 23, 337–346. [Google Scholar] [CrossRef]
- Zhang, J.T.; Yao, Y.W.; Potenza, M.N.; Xia, C.C.; Lan, J.; Liu, L.; Wang, L.J.; Liu, B.; Ma, S.S.; Fang, X.Y. Effects of craving behavioral intervention on neural substrates of cue-induced craving in Internet gaming disorder. NeuroImage Clin. 2016, 12, 591–599. [Google Scholar] [CrossRef]
- Wang, Z.L.; Potenza, M.N.; Song, K.R.; Fang, X.Y.; Liu, L.; Ma, S.S.; Xia, C.C.; Lan, J.; Yao, Y.W.; Zhang, J.T. Neural classification of internet gaming disorder and prediction of treatment response using a cue-reactivity fMRI task in young men. J. Psychiatr. Res. 2020, 145, 309–316. [Google Scholar] [CrossRef]
- Zheng, H.; Hu, Y.; Wang, Z.; Wang, M.; Du, X.; Dong, G. Meta-analyses of the functional neural alterations in subjects with Internet gaming disorder: Similarities and differences across different paradigms. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 94, 109656. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Potenza, M.N.; Lacadie, C.M.; Zhang, J.T.; Yip, S.W.; Xia, C.C.; Lan, J.; Yao, Y.W.; Deng, L.Y.; Park, S.Q.; et al. Altered intrinsic connectivity distribution in internet gaming disorder and its associations with psychotherapy treatment outcomes. Addict. Biol. 2021, 26, e12917. [Google Scholar] [CrossRef]
- Kang, K.D.; Jung, T.W.; Park, I.H.; Han, D.H. Effects of equine-assisted activities and therapies on the affective network of adolescents with internet gaming disorder. J. Altern. Complement. Med. 2018, 24, 841–849. [Google Scholar] [CrossRef]
- Lee, J.; Jang, J.H.; Choi, A.R.; Chung, S.J.; Kim, B.; Park, M.; Oh, S.; Jung, M.H.; Choi, J. Neuromodulatory Effect of Transcranial Direct Current Stimulation on Resting-State EEG Activity in Internet Gaming Disorder: A Randomized, Double-Blind, Sham-Controlled Parallel Group Trial. Cereb. Cortex Commun. 2021, 2, tgaa095. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.L.; Potenza, M.N.; Zhou, N.; Kober, H.; Shi, X.H.; Yip, S.W.; Xu, J.H.; Zhu, L.; Wang, R.; Liu, G.Q.; et al. Efficacy of single-session transcranial direct current stimulation on addiction-related inhibitory control and craving: A randomized trial in males with internet gaming disorder. J. Psychiatry Neurosci. 2021, 46, E111–E118. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Potenza, M.N.; Zhou, N.; Kober, H.; Shi, X.; Yip, S.W.; Xu, J.; Zhu, L.; Liu, G.; Zhang, J.; et al. A Role for the Right Dorsolateral Prefrontal Cortex in Enhancing Regulation of both Craving and Negative Emotions in Internet Gaming Disorder: A Randomized Trial. Eur. Neuropsychopharmacol. 2021, 36, 29–37. [Google Scholar] [CrossRef]
- Cuppone, D.; Perez, L.J.G.; Cardullo, S.; Cellini, N.; Sarlo, M.; Soldatesca, S.; Chindamo, S.; Madeo, G.; Gallimberti, L. The role of repetitive transcranial magnetic stimulation (rTMS) in the treatment of behavioral addictions: Two case reports and review of the literature. J. Behav. Addict. 2021, 10, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Potenza, M.N. Obesity, food, and addiction: Emerging neuroscience and clinical and public health implications. Neuropsychopharmacology 2014, 39, 249–250. [Google Scholar] [CrossRef]
- Stoeckel, L.E.; Weller, R.E.; Cook, E.W.; Twieg, D.B.; Knowlton, R.C.; Cox, J.E. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage 2008, 41, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Davids, S.; Lauffer, H.; Thoms, K.; Jagdhuhn, M.; Hirschfeld, H.; Domin, M.; Hamm, A.; Lotze, M. Increased dorsolateral prefrontal cortex activation in obese children during observation of food stimuli. Int. J. Obes. 2010, 34, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Pelchat, M.L.; Johnson, A.; Chan, R.; Valdez, J.; Ragland, J.D. Images of desire: Food-craving activation during fMRI. Neuroimage 2004, 23, 1486–1493. [Google Scholar] [CrossRef] [PubMed]
- Hommer, R.E.; Seo, D.; Lacadie, C.M.; Chaplin, T.M.; Mayes, L.C.; Sinha, R.; Potenza, M.N. Neural correlates of stress and favorite-food cue exposure in adolescents: A functional magnetic resonance imaging study. Hum. Brain Mapp. 2013, 34, 2561–2573. [Google Scholar] [CrossRef]
- Contreras-Rodriguez, O.; Burrows, T.; Pursey, K.M.; Stanwell, P.; Parkes, L.; Soriano-Mas, C.; Verdejo-Garcia, A. Food addiction linked to changes in ventral striatum functional connectivity between fasting and satiety. Appetite 2019, 133, 18–23. [Google Scholar] [CrossRef]
- Romer, A.L.; Su Kang, M.; Nikolova, Y.S.; Gearhardt, A.N.; Hariri, A.R. Dopamine genetic risk is related to food addiction and body mass through reduced reward-related ventral striatum activity. Appetite 2019, 133, 24–31. [Google Scholar] [CrossRef]
- Smith, D.G.; Robbins, T.W. The neurobiological underpinnings of obesity and binge eating: A rationale for adopting the food addiction model. Biol. Psychiatry 2013, 73, 804–810. [Google Scholar] [CrossRef]
- Tomasi, D.; Volkow, N.D. Striatocortical pathway dysfunction in addiction and obesity: Differences and similarities. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 1–19. [Google Scholar] [CrossRef]
- Romei, A.; Voigt, K.; Verdejo-Garcia, A. A Perspective on Candidate Neural Underpinnings of Binge Eating Disorder: Reward and Homeostatic Systems. Curr. Pharm. Des. 2020, 26, 2327–2333. [Google Scholar] [CrossRef]
- Hutson, P.H.; Balodis, I.M.; Potenza, M.N. Binge-eating disorder: Clinical and therapeutic advances. Pharmacol. Ther. 2018, 182, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Balodis, I.M.; Grilo, C.M.; Kober, H.; Worhunsky, P.D.; White, M.A.; Stevens, M.C.; Pearlson, G.D.; Potenza, M.N. A pilot study linking reduced fronto-Striatal recruitment during reward processing to persistent bingeing following treatment for binge-eating disorder. Int. J. Eat. Disord. 2014, 47, 376–384. [Google Scholar] [CrossRef]
- Balodis, I.M.; Kober, H.; Worhunsky, P.D.; White, M.A.; Stevens, M.C.; Pearlson, G.D.; Sinha, R.; Grilo, C.M.; Potenza, M.N. Monetary reward processing in obese individuals with and without binge eating disorder. Biol. Psychiatry 2013, 73, 877–886. [Google Scholar] [CrossRef]
- Griffiths, K.R.; Yang, J.; Touyz, S.W.; Hay, P.J.; Clarke, S.D.; Korgaonkar, M.S.; Gomes, L.; Anderson, G.; Foster, S.; Kohn, M.R. Understanding the neural mechanisms of lisdexamfetamine dimesylate (LDX) pharmacotherapy in Binge Eating Disorder (BED): A study protocol. J. Eat. Disord. 2019, 7, 23. [Google Scholar] [CrossRef]
- Fleck, D.E.; Eliassen, J.C.; Guerdjikova, A.I.; Mori, N.; Williams, S.; Blom, T.J.; Beckwith, T.; Tallman, M.J.; Adler, C.M.; DelBello, M.P.; et al. Effect of lisdexamfetamine on emotional network brain dysfunction in binge eating disorder. Psychiatry Res. Neuroimaging 2019, 286, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Schneider, E.; Martin, E.; Rotshtein, P.; Qureshi, K.L.; Chamberlain, S.R.; Spetter, M.S.; Dourish, C.T.; Higgs, S. The effects of lisdexamfetamine dimesylate on eating behaviour and homeostatic, reward and cognitive processes in women with binge-eating symptoms: An experimental medicine study. Transl. Psychiatry 2022, 12, 9. [Google Scholar] [CrossRef]
- Cambridge, V.C.; Ziauddeen, H.; Nathan, P.J.; Subramaniam, N.; Dodds, C.; Chamberlain, S.R.; Koch, A.; Maltby, K.; Skeggs, A.L.; Napolitano, A.; et al. Neural and behavioral effects of a novel mu opioid receptor antagonist in binge-eating obese people. Biol. Psychiatry 2013, 73, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Juarascio, A.S.; Presseller, E.K.; Wilkinson, M.L.; Kelkar, A.; Srivastava, P.; Chen, J.Y.; Dengler, J.; Manasse, S.M.; Medaglia, J. Correcting the reward imbalance in binge eating: A pilot randomized trial of reward re-training treatment. Appetite 2022, 176, 106103. [Google Scholar] [CrossRef]
- Linardon, J.; Wade, T.D.; de la Piedad Garcia, X.; Brennan, L. The efficacy of cognitive-behavioral therapy for eating disorders: A systematic review and meta-analysis. J. Consult. Clin. Psychol. 2017, 85, 1080–1094. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, K.; Woodside, B.; Lam, E.; Olmsted, M.; Colton, P.; Giacobbe, P.; Downar, J. Increases in frontostriatal connectivity are associated with response to dorsomedial repetitive transcranial magnetic stimulation in refractory binge/purge behaviors. NeuroImage Clin. 2015, 8, 611–618. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mestre-Bach, G.; Potenza, M.N. Potential Biological Markers and Treatment Implications for Binge Eating Disorder and Behavioral Addictions. Nutrients 2023, 15, 827. https://doi.org/10.3390/nu15040827
Mestre-Bach G, Potenza MN. Potential Biological Markers and Treatment Implications for Binge Eating Disorder and Behavioral Addictions. Nutrients. 2023; 15(4):827. https://doi.org/10.3390/nu15040827
Chicago/Turabian StyleMestre-Bach, Gemma, and Marc N. Potenza. 2023. "Potential Biological Markers and Treatment Implications for Binge Eating Disorder and Behavioral Addictions" Nutrients 15, no. 4: 827. https://doi.org/10.3390/nu15040827
APA StyleMestre-Bach, G., & Potenza, M. N. (2023). Potential Biological Markers and Treatment Implications for Binge Eating Disorder and Behavioral Addictions. Nutrients, 15(4), 827. https://doi.org/10.3390/nu15040827






