No Evidence of a Genetic Causal Relationship between Ankylosing Spondylitis and Gut Microbiota: A Two-Sample Mendelian Randomization Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. GWAS Summary Data for Gut Microbiota
2.3. GWAS Summary Data for AS
2.4. IV Selection
2.5. Statistical Analysis
3. Results
3.1. IVs Selection
3.2. MR Analysis
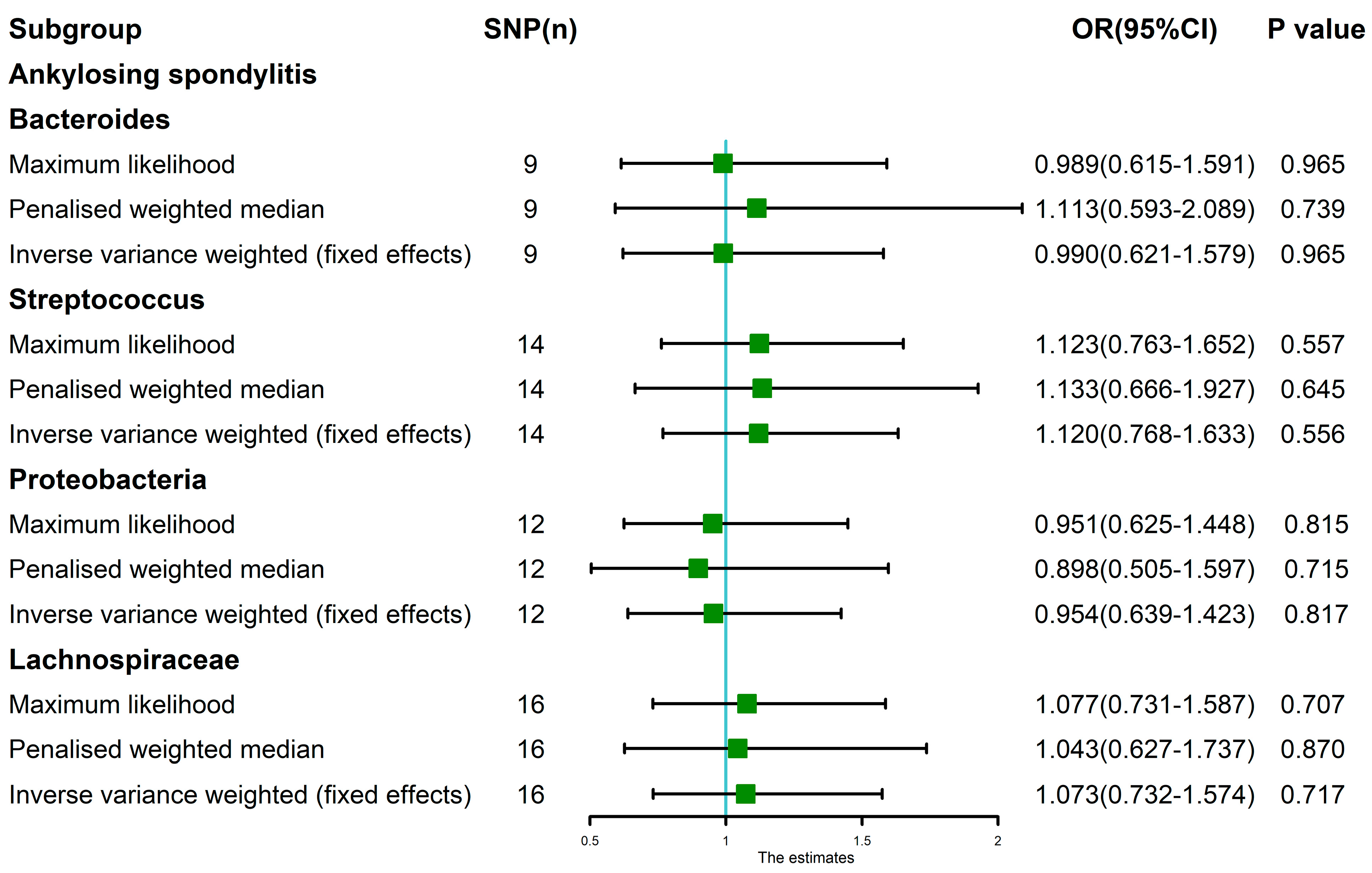
3.3. MR Analysis after Removing Outliers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, W.; He, X.; Cheng, K.; Zhang, L.; Chen, D.; Wang, X.; Qiu, G.; Cao, X.; Weng, X. Ankylosing spondylitis: Etiology, pathogenesis, and treatments. Bone Res. 2019, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Exarchou, S.; Lindström, U.; Askling, J.; Eriksson, J.K.; Forsblad-d’Elia, H.; Neovius, M.; Turesson, C.; Kristensen, L.E.; Jacobsson, L.T. The prevalence of clinically diagnosed ankylosing spondylitis and its clinical manifestations: A nationwide register study. Arthritis Res. Ther. 2015, 17, 118. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.J.; Maksymowych, W.P. The Pathogenesis of Ankylosing Spondylitis: An Update. Curr. Rheumatol. Rep. 2019, 21, 58. [Google Scholar] [CrossRef]
- Sieper, J.; Poddubnyy, D. Axial spondyloarthritis. Lancet 2017, 390, 73–84. [Google Scholar] [CrossRef]
- Kim, J.O.; Lee, J.S.; Choi, J.Y.; Lee, K.H.; Kim, Y.B.; Yoo, D.H.; Kim, T.H. The relationship between peripheral arthritis and anti-cyclic citrullinated peptide antibodies in ankylosing spondylitis. Joint Bone Spine 2013, 80, 399–401. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Lin, Z.; Gu, J.; Huang, F.; Li, T.; Wei, Q.; Liao, Z.; Cao, S.; Jiang, Y.; Huang, J. Abnormal high-expression of CD154 on T lymphocytes of ankylosing spondylitis patients is down-regulated by etanercept treatment. Rheumatol. Int. 2010, 30, 317–323. [Google Scholar] [CrossRef]
- Sieper, J.; Poddubnyy, D.; Miossec, P. The IL-23-IL-17 pathway as a therapeutic target in axial spondyloarthritis. Nat. Rev. Rheumatol. 2019, 15, 747–757. [Google Scholar] [CrossRef]
- Furue, K.; Ito, T.; Furue, M. Differential efficacy of biologic treatments targeting the TNF-α/IL-23/IL-17 axis in psoriasis and psoriatic arthritis. Cytokine 2018, 111, 182–188. [Google Scholar] [CrossRef]
- Song, I.H.; Poddubnyy, D.A.; Rudwaleit, M.; Sieper, J. Benefits and risks of ankylosing spondylitis treatment with nonsteroidal antiinflammatory drugs. Arthritis Rheum. 2008, 58, 929–938. [Google Scholar] [CrossRef]
- Chiu, Y.M.; Chen, D.Y. Infection risk in patients undergoing treatment for inflammatory arthritis: Non-biologics versus biologics. Expert Rev. Clin. Immunol. 2020, 16, 207–228. [Google Scholar] [CrossRef]
- Siu, S.; Haraoui, B.; Bissonnette, R.; Bessette, L.; Roubille, C.; Richer, V.; Starnino, T.; McCourt, C.; McFarlane, A.; Fleming, P.; et al. Meta-analysis of tumor necrosis factor inhibitors and glucocorticoids on bone density in rheumatoid arthritis and ankylosing spondylitis trials. Arthritis Care Res. 2015, 67, 754–764. [Google Scholar] [CrossRef]
- Lin, D.; Charalambous, A.; Hanna, S.A. Bilateral total hip arthroplasty in ankylosing spondylitis: A systematic review. EFORT Open Rev. 2019, 4, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zheng, G.; Wang, Y.; Zhang, X.; Hu, F.; Wang, Y. Comparison of 2 Surgeries in Correction of Severe Kyphotic Deformity Caused by Ankylosing Spondylitis: Vertebral Column Decancellation and Pedicle Subtraction Osteotomy. World Neurosurg. 2019, 127, e972–e978. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Luo, P.; Zhang, F.; Xu, K.; Feng, R.; Xu, P. Large-scale correlation analysis of deep venous thrombosis and gut microbiota. Front. Cardiovasc. Med. 2022, 9, 1025918. [Google Scholar] [CrossRef]
- Gosalbes, M.J.; Durban, A.; Pignatelli, M.; Abellan, J.J.; Jimenez-Hernandez, N.; Perez-Cobas, A.E.; Latorre, A.; Moya, A. Metatranscriptomic approach to analyze the functional human gut microbiota. PLoS ONE 2011, 6, e17447. [Google Scholar] [CrossRef] [PubMed]
- Mangiola, F.; Ianiro, G.; Franceschi, F.; Fagiuoli, S.; Gasbarrini, G.; Gasbarrini, A. Gut microbiota in autism and mood disorders. World J. Gastroenterol. 2016, 22, 361–368. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, W.; Wang, H.L.; Dai, L.L.; Zong, W.H.; Wang, Y.Z.; Bi, J.; Han, W.; Dong, G.J. Gut microbiota and obesity-associated osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1257–1265. [Google Scholar] [CrossRef]
- Mei, L.; Yang, Z.; Zhang, X.; Liu, Z.; Wang, M.; Wu, X.; Chen, X.; Huang, Q.; Huang, R. Sustained Drug Treatment Alters the Gut Microbiota in Rheumatoid Arthritis. Front. Immunol. 2021, 12, 704089. [Google Scholar] [CrossRef]
- Hill, C.J.; Lynch, D.B.; Murphy, K.; Ulaszewska, M.; Jeffery, I.B.; O’Shea, C.A.; Watkins, C.; Dempsey, E.; Mattivi, F.; Tuohy, K.; et al. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome 2017, 5, 4. [Google Scholar] [CrossRef]
- Zhou, C.; Zhao, H.; Xiao, X.Y.; Chen, B.D.; Guo, R.J.; Wang, Q.; Chen, H.; Zhao, L.D.; Zhang, C.C.; Jiao, Y.H.; et al. Metagenomic profiling of the pro-inflammatory gut microbiota in ankylosing spondylitis. J. Autoimmun. 2020, 107, 102360. [Google Scholar] [CrossRef]
- Zhang, L.; Han, R.; Zhang, X.; Fang, G.; Chen, J.; Li, J.; Xu, S.; Qian, L.; Chen, W.; Pan, F. Fecal microbiota in patients with ankylosing spondylitis: Correlation with dietary factors and disease activity. Clin. Chim. Acta 2019, 497, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Ma, C.; Zhang, B.; Bi, L. Dynamic changes in gut microbiota under the influence of smoking and TNF-alpha-blocker in patients with ankylosing spondylitis. Clin. Rheumatol. 2020, 39, 2653–2661. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yang, L.; Cui, Z.; Zheng, J.; Huang, J.; Zhao, Q.; Su, Z.; Wang, M.; Zhang, W.; Liu, J.; et al. Anti-TNF-α therapy alters the gut microbiota in proteoglycan-induced ankylosing spondylitis in mice. Microbiologyopen 2019, 8, e927. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, B.; Zheng, J.; Huang, J.; Zhao, Q.; Liu, J.; Su, Z.; Wang, M.; Cui, Z.; Wang, T.; et al. Rifaximin Alters Intestinal Microbiota and Prevents Progression of Ankylosing Spondylitis in Mice. Front. Cell. Infect. Microbiol. 2019, 9, 44. [Google Scholar] [CrossRef]
- Wen, C.; Zheng, Z.; Shao, T.; Liu, L.; Xie, Z.; Le Chatelier, E.; He, Z.; Zhong, W.; Fan, Y.; Zhang, L.; et al. Quantitative metagenomics reveals unique gut microbiome biomarkers in ankylosing spondylitis. Genome Biol. 2017, 18, 142. [Google Scholar] [CrossRef]
- Sternes, P.R.; Brett, L.; Phipps, J.; Ciccia, F.; Kenna, T.; de Guzman, E.; Zimmermann, K.; Morrison, M.; Holtmann, G.; Klingberg, E.; et al. Distinctive gut microbiomes of ankylosing spondylitis and inflammatory bowel disease patients suggest differing roles in pathogenesis and correlate with disease activity. Arthritis Res. Ther. 2022, 24, 163. [Google Scholar] [CrossRef]
- Liu, B.; Ding, Z.; Xiong, J.; Heng, X.; Wang, H.; Chu, W. Gut Microbiota and Inflammatory Cytokine Changes in Patients with Ankylosing Spondylitis. Biomed. Res. Int. 2022, 2022, 1005111. [Google Scholar] [CrossRef]
- Li, M.; Dai, B.; Tang, Y.; Lei, L.; Li, N.; Liu, C.; Ge, T.; Zhang, L.; Xu, Y.; Hu, Y.; et al. Altered Bacterial-Fungal Interkingdom Networks in the Guts of Ankylosing Spondylitis Patients. mSystems 2019, 4, e00176-18. [Google Scholar] [CrossRef]
- Klingberg, E.; Magnusson, M.K.; Strid, H.; Deminger, A.; Stahl, A.; Sundin, J.; Simren, M.; Carlsten, H.; Ohman, L.; Forsblad-d’Elia, H. A distinct gut microbiota composition in patients with ankylosing spondylitis is associated with increased levels of fecal calprotectin. Arthritis Res. Ther. 2019, 21, 248. [Google Scholar] [CrossRef] [PubMed]
- Costello, M.E.; Ciccia, F.; Willner, D.; Warrington, N.; Robinson, P.C.; Gardiner, B.; Marshall, M.; Kenna, T.J.; Triolo, G.; Brown, M.A. Brief Report: Intestinal Dysbiosis in Ankylosing Spondylitis. Arthritis Rheumatol. 2015, 67, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Qi, J.; Wei, Q.; Zheng, X.; Wu, X.; Li, X.; Liao, Z.; Lin, Z.; Gu, J. Variations in gut microbial profiles in ankylosing spondylitis: Disease phenotype-related dysbiosis. Ann. Transl. Med. 2019, 7, 571. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Shao, M.; Wu, X. Vitamin D and risk of ankylosing spondylitis: A two-sample mendelian randomization study. Hum. Immunol. 2022, 83, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Hou, G.; Meng, X.; Feng, H.; He, B.; Tian, Y. Bidirectional Causal Associations Between Inflammatory Bowel Disease and Ankylosing Spondylitis: A Two-Sample Mendelian Randomization Analysis. Front. Genet. 2020, 11, 587876. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Xing, H.; Wang, X.; Zhang, N.; Xu, Q. Causal Relationships Between Total Physical Activity and Ankylosing Spondylitis: A Mendelian Randomization Study. Front. Immunol. 2022, 13, 887326. [Google Scholar] [CrossRef]
- McCarthy, S.; Das, S.; Kretzschmar, W.; Delaneau, O.; Wood, A.R.; Teumer, A.; Kang, H.M.; Fuchsberger, C.; Danecek, P.; Sharp, K.; et al. A reference panel of 64,976 haplotypes for genotype imputation. Nat. Genet. 2016, 48, 1279–1283. [Google Scholar]
- Pers, T.H.; Timshel, P.; Hirschhorn, J.N. SNPsnap: A Web-based tool for identification and annotation of matched SNPs. Bioinformatics 2015, 31, 418–420. [Google Scholar] [CrossRef]
- Westra, H.J.; Peters, M.J.; Esko, T.; Yaghootkar, H.; Schurmann, C.; Kettunen, J.; Christiansen, M.W.; Fairfax, B.P.; Schramm, K.; Powell, J.E.; et al. Systematic identification of trans eQTLs as putative drivers of known disease associations. Nat. Genet. 2013, 45, 1238–1243. [Google Scholar] [CrossRef]
- Kurilshikov, A.; Medina-Gomez, C.; Bacigalupe, R.; Radjabzadeh, D.; Wang, J.; Demirkan, A.; Le Roy, C.I.; Raygoza Garay, J.A.; Finnicum, C.T.; Liu, X.; et al. Large-scale association analyses identify host factors influencing human gut microbiome composition. Nat. Genet. 2021, 53, 156–165. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, J.; Wu, Y.; Ni, M.; Deng, Y.; Sun, X.; Wang, X.; Zhang, T.; Pan, F.; Tang, Z. Tea consumption and risk of lower respiratory tract infections: A two-sample mendelian randomization study. Eur. J. Nutr. 2022, 62, 385–393. [Google Scholar] [CrossRef]
- Xu, Q.; Ni, J.J.; Han, B.X.; Yan, S.S.; Wei, X.T.; Feng, G.J.; Zhang, H.; Zhang, L.; Li, B.; Pei, Y.F. Causal Relationship Between Gut Microbiota and Autoimmune Diseases: A Two-Sample Mendelian Randomization Study. Front. Immunol. 2021, 12, 746998. [Google Scholar] [CrossRef]
- Ni, J.J.; Xu, Q.; Yan, S.S.; Han, B.X.; Zhang, H.; Wei, X.T.; Feng, G.J.; Zhao, M.; Pei, Y.F.; Zhang, L. Gut Microbiota and Psychiatric Disorders: A Two-Sample Mendelian Randomization Study. Front. Microbiol. 2021, 12, 737197. [Google Scholar] [CrossRef]
- Shu, M.J.; Li, J.R.; Zhu, Y.C.; Shen, H. Migraine and Ischemic Stroke: A Mendelian Randomization Study. Neurol. Ther. 2022, 11, 237–246. [Google Scholar] [CrossRef]
- Dulger, S.; Aykurt Karlibel, I.; Kasapoglu Aksoy, M.; Altan, L.; Sengoren Dikis, O.; Yildiz, T. How Does Smoking Cessation Affect Disease Activity, Function Loss, and Quality of Life in Smokers With Ankylosing Spondylitis? J. Clin. Rheumatol. 2019, 25, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.F.; Kuo, Y.H.; Lai, S.W. Diabetes mellitus in ankylosing spondylitis. Ann. Rheumatic Dis. 2021, 80, e134. [Google Scholar] [CrossRef] [PubMed]
- Ortolan, A.; Lorenzin, M.; Felicetti, M.; Ramonda, R. Do Obesity and Overweight Influence Disease Activity Measures in Axial Spondyloarthritis? A Systematic Review and Meta-Analysis. Arthritis Care Res. 2021, 73, 1815–1825. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Mi, S.; Zhu, J.; Jin, W.; Li, Y.; Wang, T.; Li, Y.; Fan, C. No Causal Association Between Adiponectin and the Risk of Rheumatoid Arthritis: A Mendelian Randomization Study. Front. Genet. 2021, 12, 670282. [Google Scholar] [CrossRef] [PubMed]
- Dan, Y.L.; Wang, P.; Cheng, Z.; Wu, Q.; Wang, X.R.; Wang, D.G.; Pan, H.F. Circulating adiponectin levels and systemic lupus erythematosus: A two-sample Mendelian randomization study. Rheumatology 2021, 60, 940–946. [Google Scholar] [CrossRef]
- Cao, Z.; Wu, Y.; Li, Q.; Li, Y.; Wu, J. A causal relationship between childhood obesity and risk of osteoarthritis: Results from a two-sample Mendelian randomization analysis. Ann. Med. 2022, 54, 1636–1645. [Google Scholar] [CrossRef]
- Lee, Y.H. Causal association between smoking behavior and the decreased risk of osteoarthritis: A Mendelian randomization. Z. Rheumatol. 2019, 78, 461–466. [Google Scholar] [CrossRef]
- Zheng, C.; He, M.H.; Huang, J.R.; He, Y. Causal Relationships Between Social Isolation and Osteoarthritis: A Mendelian Randomization Study in European Population. Int. J. Gen. Med. 2021, 14, 6777–6786. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Niu, M.; Guo, Z.; Liu, P.; Zheng, Y.; Liu, D.; Yang, S.; Wang, W.; Li, Y.; Hou, H. A Mild Causal Relationship Between Tea Consumption and Obesity in General Population: A Two-Sample Mendelian Randomization Study. Front. Genet. 2022, 13, 795049. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, F.P.; Davey Smith, G.; Bowden, J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int. J. Epidemiol. 2017, 46, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Hemani, G.; Zheng, J.; Elsworth, B.; Wade, K.H.; Haberland, V.; Baird, D.; Laurin, C.; Burgess, S.; Bowden, J.; Langdon, R.; et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife 2018, 7, e34408. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Jiang, L.; Song, Z.; Wang, F. Causal Associations of Circulating Lipids with Osteoarthritis: A Bidirectional Mendelian Randomization Study. Nutrients 2022, 14, 1327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J. Mendelian Randomization Study Implies Causal Linkage Between Telomere Length and Juvenile Idiopathic Arthritis in a European Population. J. Inflamm. Res. 2022, 15, 977–986. [Google Scholar] [CrossRef]
- Brook, I.; Frazier, E.H. Aerobic and anaerobic microbiology in intra-abdominal infections associated with diverticulitis. J. Med. Microbiol. 2000, 49, 827–830. [Google Scholar] [CrossRef]
- Stoll, M.L.; Weiss, P.F.; Weiss, J.E.; Nigrovic, P.A.; Edelheit, B.S.; Bridges, S.L., Jr.; Danila, M.I.; Spencer, C.H.; Punaro, M.G.; Schikler, K.; et al. Age and fecal microbial strain-specific differences in patients with spondyloarthritis. Arthritis Res. Ther. 2018, 20, 14. [Google Scholar] [CrossRef]
- Purcell, R.V.; Pearson, J.; Aitchison, A.; Dixon, L.; Frizelle, F.A.; Keenan, J.I. Colonization with enterotoxigenic Bacteroides fragilis is associated with early-stage colorectal neoplasia. PLoS ONE 2017, 12, e0171602. [Google Scholar] [CrossRef]
- Erturk-Hasdemir, D.; Kasper, D.L. Finding a needle in a haystack: Bacteroides fragilis polysaccharide A as the archetypical symbiosis factor. Ann. N. Y. Acad. Sci. 2018, 1417, 116–129. [Google Scholar] [CrossRef]
- Andam, C.P.; Hanage, W.P. Mechanisms of genome evolution of Streptococcus. Infect. Genet. Evol. 2015, 33, 334–342. [Google Scholar] [CrossRef]
- Briles, D.E.; Paton, J.C.; Mukerji, R.; Swiatlo, E.; Crain, M.J. Pneumococcal Vaccines. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Mostefaoui, Y.; Bart, C.; Frenette, M.; Rouabhia, M. Candida albicans and Streptococcus salivarius modulate IL-6, IL-8, and TNF-alpha expression and secretion by engineered human oral mucosa cells. Cell Microbiol. 2004, 6, 1085–1096. [Google Scholar] [CrossRef]
- Kaci, G.; Lakhdari, O.; Dore, J.; Ehrlich, S.D.; Renault, P.; Blottiere, H.M.; Delorme, C. Inhibition of the NF-kappaB pathway in human intestinal epithelial cells by commensal Streptococcus salivarius. Appl. Environ. Microbiol. 2011, 77, 4681–4684. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Sun, L.; Zhang, X. Integration of microbiome and epigenome to decipher the pathogenesis of autoimmune diseases. J. Autoimmun. 2017, 83, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Haghikia, A.; Jorg, S.; Duscha, A.; Berg, J.; Manzel, A.; Waschbisch, A.; Hammer, A.; Lee, D.H.; May, C.; Wilck, N.; et al. Dietary Fatty Acids Directly Impact Central Nervous System Autoimmunity via the Small Intestine. Immunity 2015, 43, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Y.; Zhang, P.; Song, C.; Pan, F.; Li, G.; Peng, L.; Yang, Y.; Wei, Z.; Huang, F. Gut microbiota changes in patients with spondyloarthritis: A systematic review. Semin. Arthritis Rheum. 2022, 52, 151925. [Google Scholar] [CrossRef]

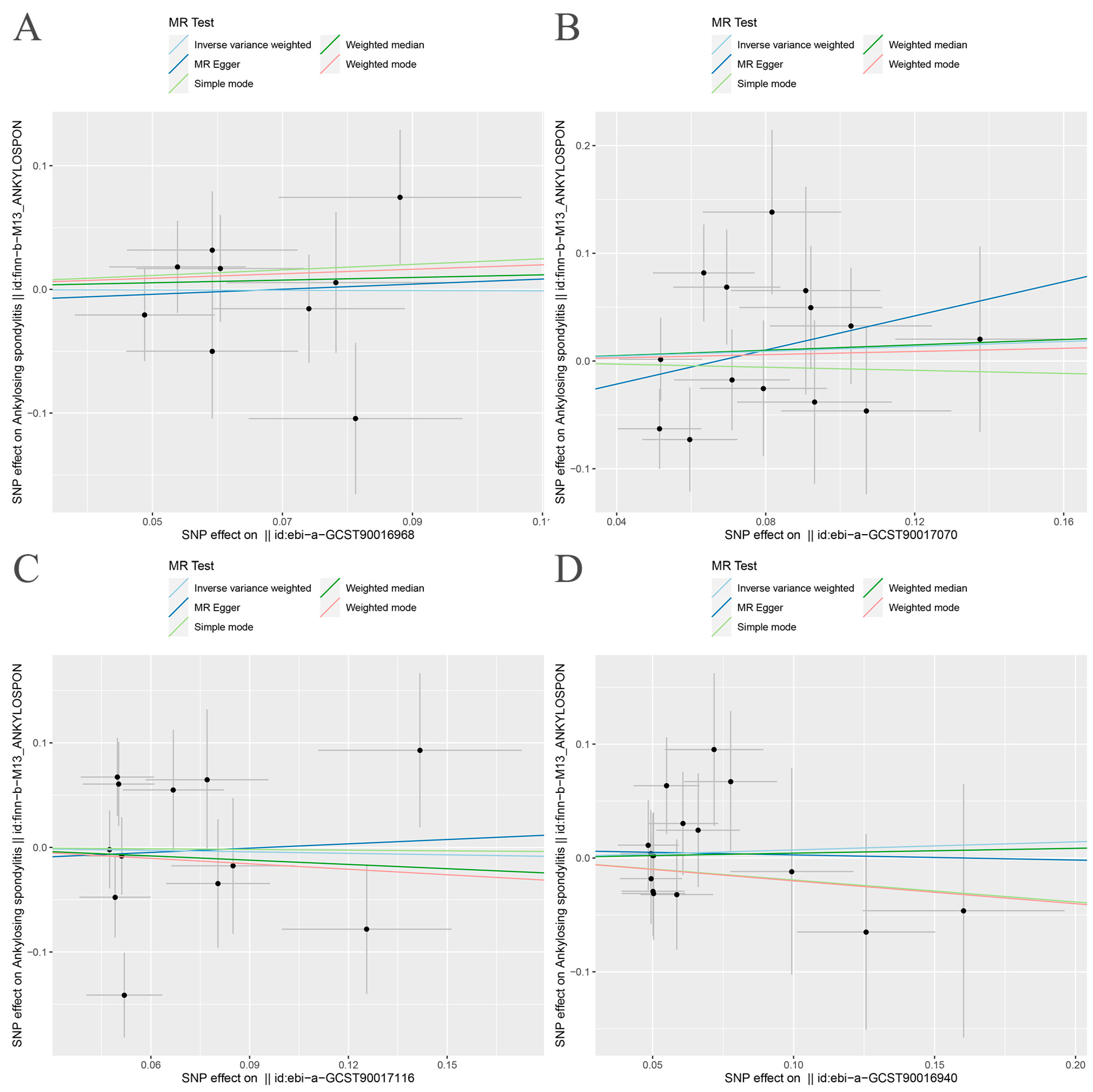
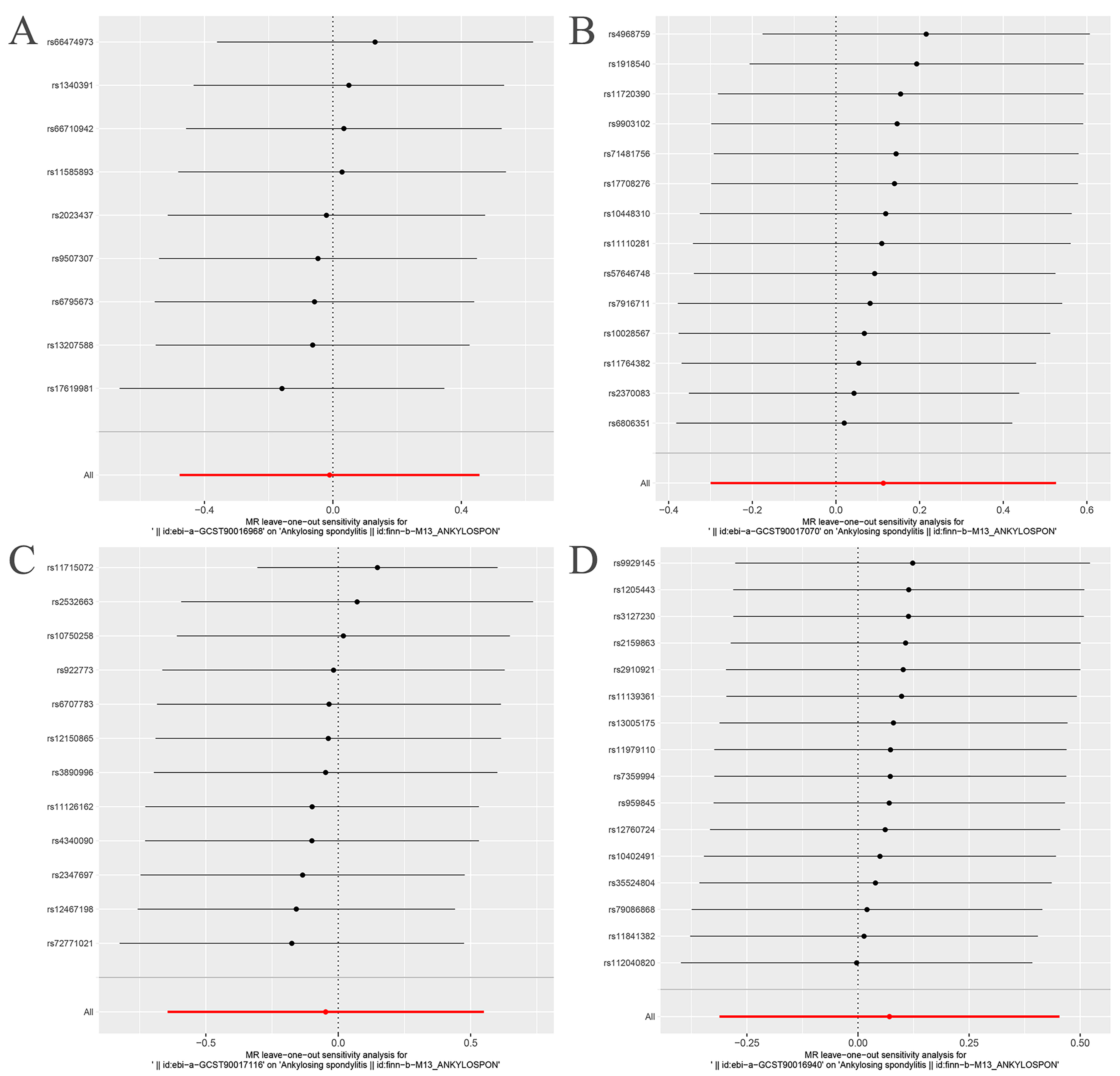

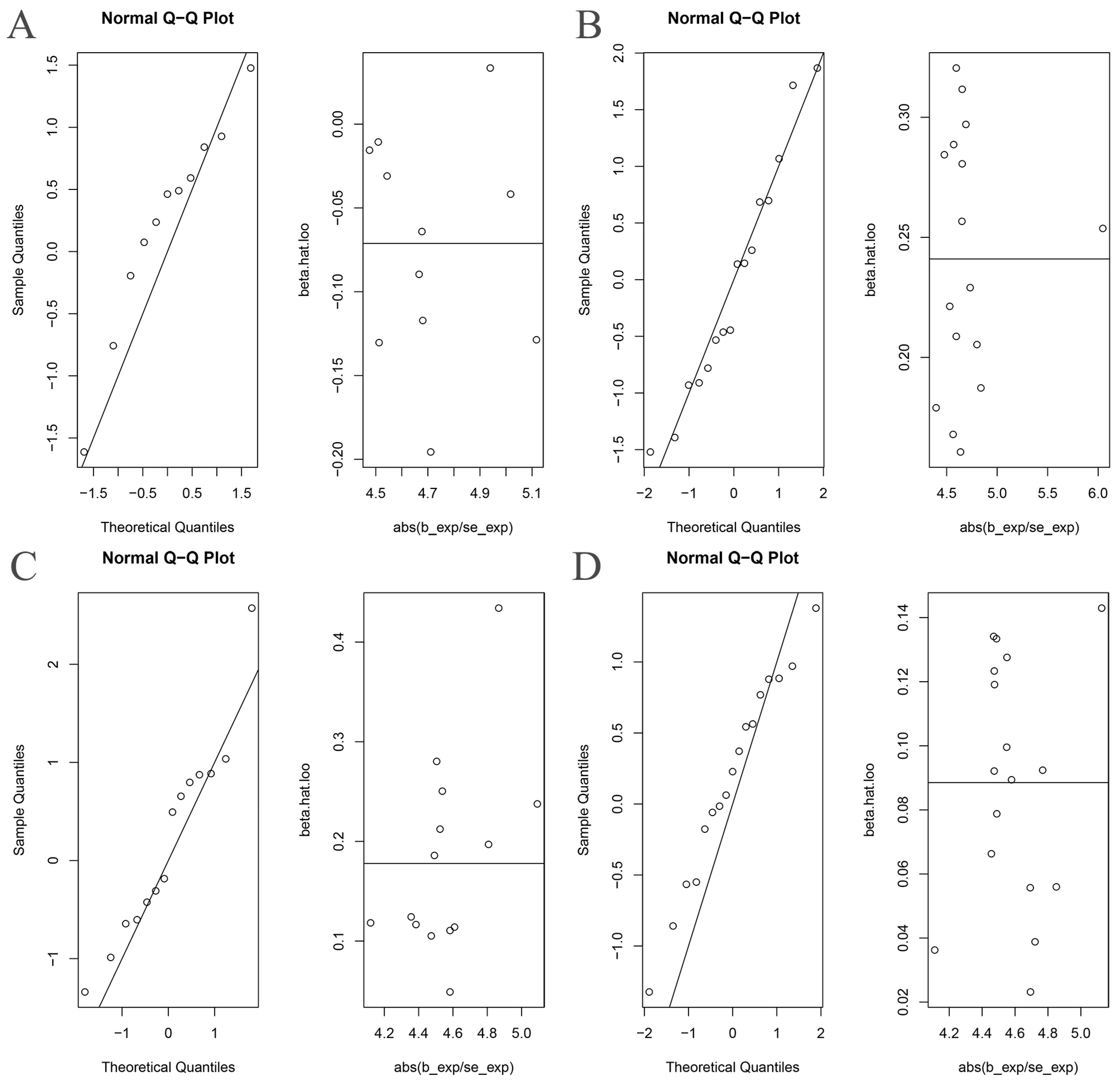
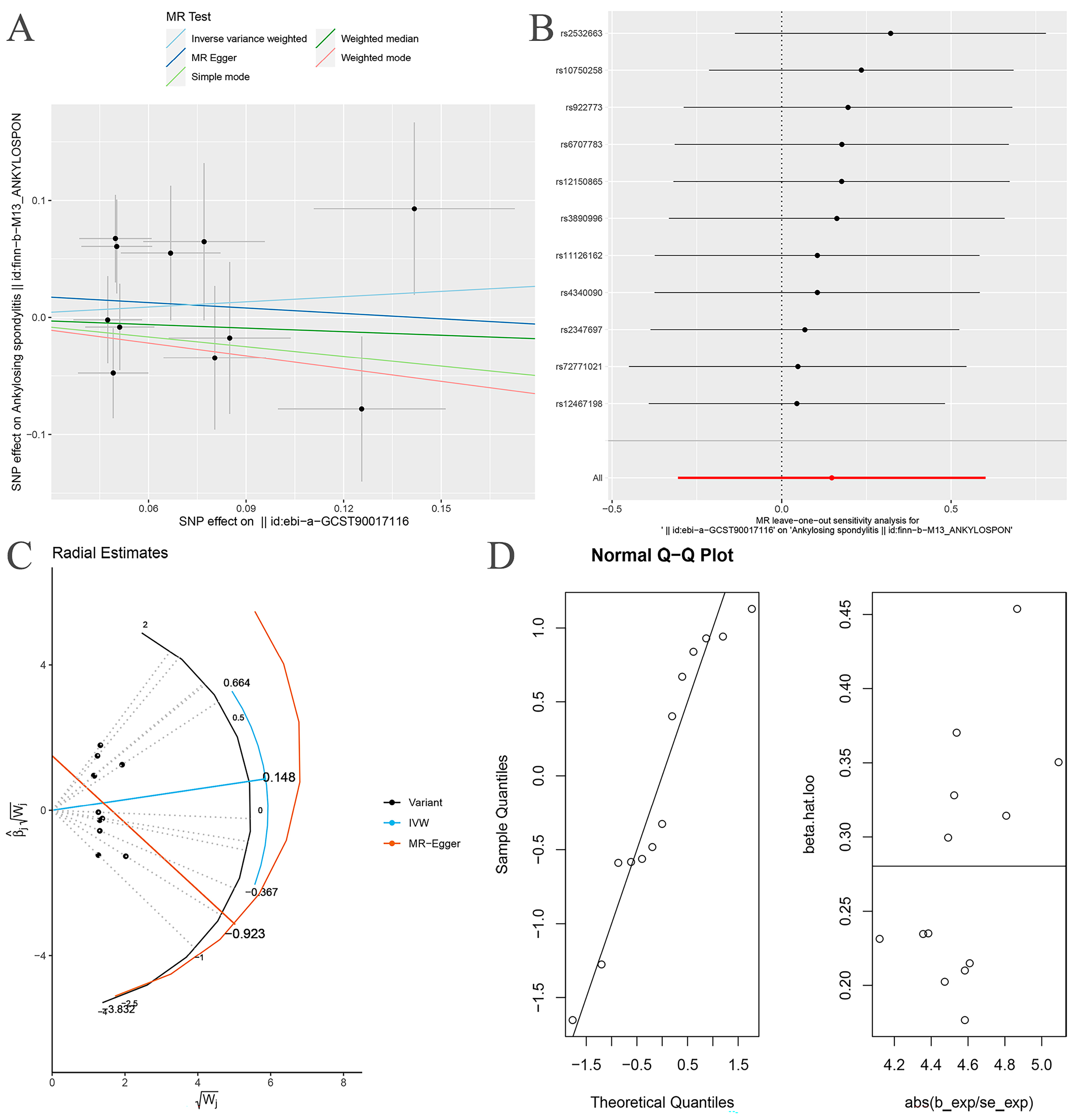
| Exposure | Outcome | Heterogeneity Test | Pleiotropy Test | MR-PRESSO | MR-RAPS | ||||
|---|---|---|---|---|---|---|---|---|---|
| Cochran’s Q Test (p Value) | Rucker’s Q Test (p Value) | Egger Intercept (p Value) | Distortion Test | Global Test | MR Analysis | Normal Distribution | |||
| IVW | MR-Egger | MR-Egger | Outliers | p Value | OR | p Value | p Value | ||
| Bacteroides | AS | 0.547 | 0.443 | 0.863 | NA | 0.667 | 0.931 | 0.765 | 0.569 |
| Streptococcus | AS | 0.272 | 0.266 | 0.376 | NA | 0.330 | 1.273 | 0.232 | 0.548 |
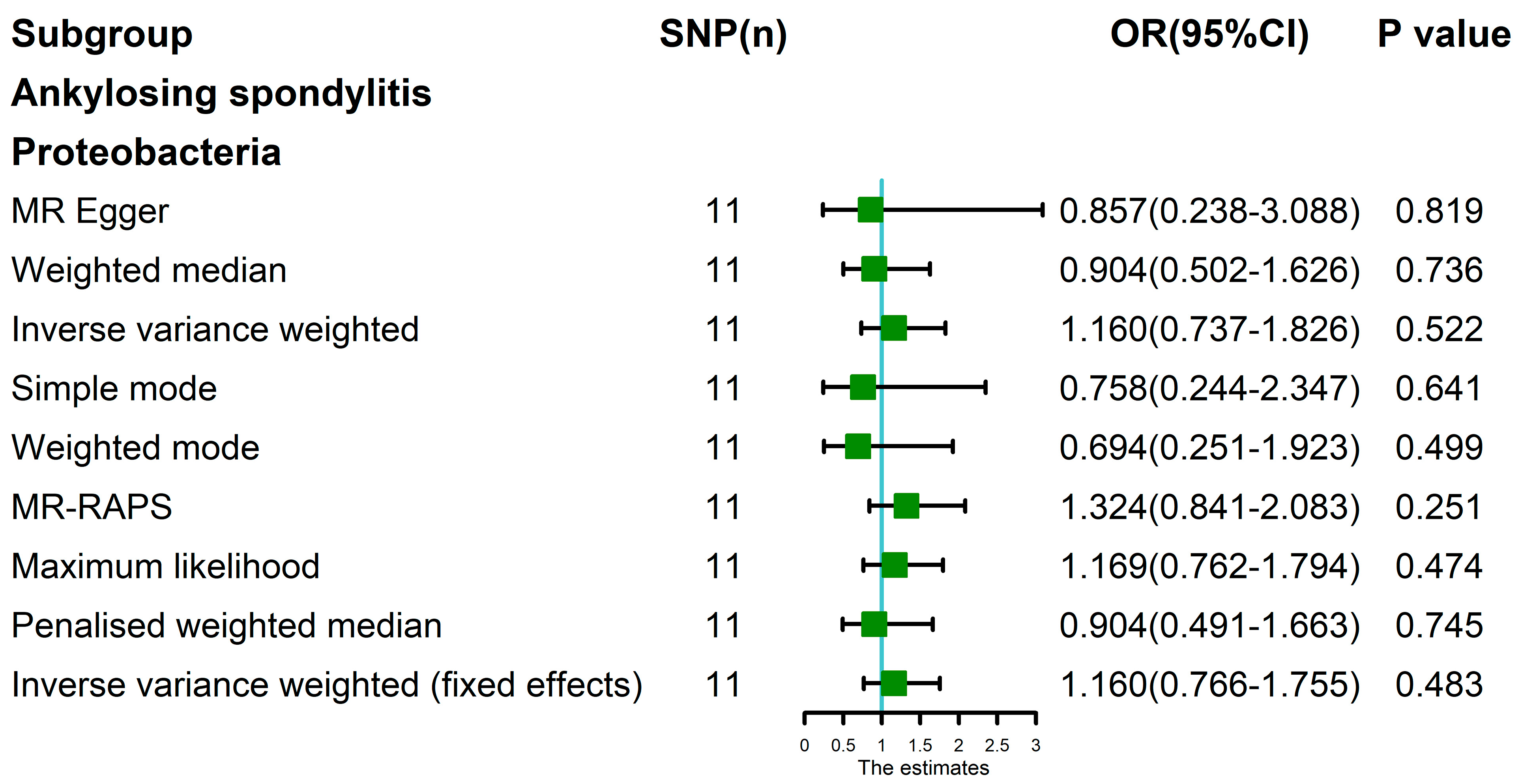
| Proteobacteria | AS | 0.011 | 0.007 | 0.828 | 1 | 0.014 | 1.195 | 0.502 | 0.416 |
| Proteobacteria * | AS | 0.286 | 0.233 | 0.631 | NA | 0.298 | 1.324 | 0.251 | 0.187 |
| Lachnospiraceae | AS | 0.897 | 0.857 | 0.861 | NA | 0.908 | 1.093 | 0.668 | 0.905 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Wan, X.; Zheng, H.; Xu, K.; Xie, J.; Yu, H.; Wang, J.; Xu, P. No Evidence of a Genetic Causal Relationship between Ankylosing Spondylitis and Gut Microbiota: A Two-Sample Mendelian Randomization Study. Nutrients 2023, 15, 1057. https://doi.org/10.3390/nu15041057
Yang M, Wan X, Zheng H, Xu K, Xie J, Yu H, Wang J, Xu P. No Evidence of a Genetic Causal Relationship between Ankylosing Spondylitis and Gut Microbiota: A Two-Sample Mendelian Randomization Study. Nutrients. 2023; 15(4):1057. https://doi.org/10.3390/nu15041057
Chicago/Turabian StyleYang, Mingyi, Xianjie Wan, Haishi Zheng, Ke Xu, Jiale Xie, Hui Yu, Jiachen Wang, and Peng Xu. 2023. "No Evidence of a Genetic Causal Relationship between Ankylosing Spondylitis and Gut Microbiota: A Two-Sample Mendelian Randomization Study" Nutrients 15, no. 4: 1057. https://doi.org/10.3390/nu15041057
APA StyleYang, M., Wan, X., Zheng, H., Xu, K., Xie, J., Yu, H., Wang, J., & Xu, P. (2023). No Evidence of a Genetic Causal Relationship between Ankylosing Spondylitis and Gut Microbiota: A Two-Sample Mendelian Randomization Study. Nutrients, 15(4), 1057. https://doi.org/10.3390/nu15041057







