Effects of Endurance Exercise Intensities on Autonomic and Metabolic Controls in Children with Obesity: A Feasibility Study Employing Online Exercise Training
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Evaluations
2.1.1. Clinical, Auxological, and Hemodynamic Assessment
2.1.2. Metabolic Assessment
2.1.3. Lifestyle Assessment
- -
- -
- The lifestyle questionnaire inquired also about hours of sleep/day, hours of sedentariness/week, and perceptions of quality of sleep, health, and school performance (assessed using evaluation scales from 0 (‘worst quality’) to 10 (‘best quality’) for each measure).
- -
- Physical activity (total activity volume) was assessed by a modified version of the commonly employed short version of the International Physical Activity Questionnaire (IPAQ) [52,53], which focuses on intensity (nominally estimated in metabolic equivalents (METs) according to the type of activity) and duration (in min) of physical activity. We decided to employ this questionnaire, even if it was designed for adults, because it has the advantage of furnishing a numeric parameter of exercise volume (expressed in METs) capable of reflecting the total exercise volume.
- ▪
- (METsTOT) Total weekly physical activity volume [MET·min/week] = (3.3 × minutes of brisk walking × days of brisk walking) + (4.0 × minutes of other moderate intensity activity × days of other moderate intensity activities) + (8.0 × minutes of vigorous intensity activity × days of vigorous intensity activity).
- ▪
- (METsMV) Weekly physical activity volume calculated only considering other activities of moderate intensity and activities of vigorous intensity [MET·min/week] = (4.0 × minutes of other moderate intensity activity × days of other moderate intensity activities) + (8.0 × minutes of vigorous intensity activity × days of vigorous intensity activity). METsMV may be considered as the total weekly volume of structured exercise.
- -
- Subdivision A considered the total weekly physical activity volume (METsTOT), i.e., considering both time spent walking (at least for 10 min consecutively) and time spent performing structured exercise (other moderate intensity activities and vigorous intensity activities)
- -
- Subdivision B considered only the weekly volume of structured exercise (METsMV) (only other moderate intensity activities and vigorous intensity activities).
2.1.4. Physical Fitness (PF) Assessment
- -
- Six-minute walk test (6MWT). This field test is considered a valid and reliable tool for measuring PF in children and is widespread, has inexpensive equipment, and is easy to administer in a clinical setting [56]. The 6MWT was performed according to international administration guidelines [57]. The children were instructed by the trainers to walk the greatest distance possible while maintaining their own pace. Standardized encouragement and information about the remaining time were given to the children every minute; for example, “you are doing well” or “keep up the good work” [58]. Children were permitted to stop (if required) during the test but were instructed to resume walking once able and the covered distance was registered in meters. Test-retest reliability was undertaken, and the intraclass correlation coefficient (95% confidence interval) was calculated as 0.94 (0.89–0.96).
- -
- After an adequate recovery time, children were interviewed by the same investigator to assess perceived physical fitness and physical activity level, respectively, using the International Fitness Enjoyment Scale (IFIS) and Physical Activity Questionnaire for Older Children (PAQ-C) questionnaires.
- -
- The International Fitness Enjoyment Scale (IFIS) questionnaire is a self-reported, easy, and rapid fitness scale previously validated in several European countries and languages. It describes physical fitness as an indicator of physical competence [59]. The IFIS is composed of a 5-point Likert scale (from 1 ‘very poor’ to 5 ‘very good’), with questions focused on five areas of fitness: general fitness, cardiorespiratory, strength, speed-agility, and flexibility. The IFIS has high validity and moderate-to-good reliability (average weighted Kappa: 0.70 and 0.59) for school-aged children.
- -
- The Physical Activity Questionnaire for Older Children (PAQ-C) evaluates the weekly amount of physical activity reported by children. This questionnaire was verified to be adequate for school-aged children (approximate ages between 8 and 14). The PAQ-C is recognized as a valid and reliable measurement of general physical activity level from childhood to adolescence. The PAQ-C utilizes cues such as break time at school and evening physical activity to ameliorate the recall ability of children. The PAQ-C is cost- and time-efficient, simple to administer, and displays normal distribution properties. The PAQ-C is shown to have good reliability and an intraclass correlation (ICC) = 0.96 [60].
2.1.5. Cardiac Autonomic Regulation (CAR) Assessment
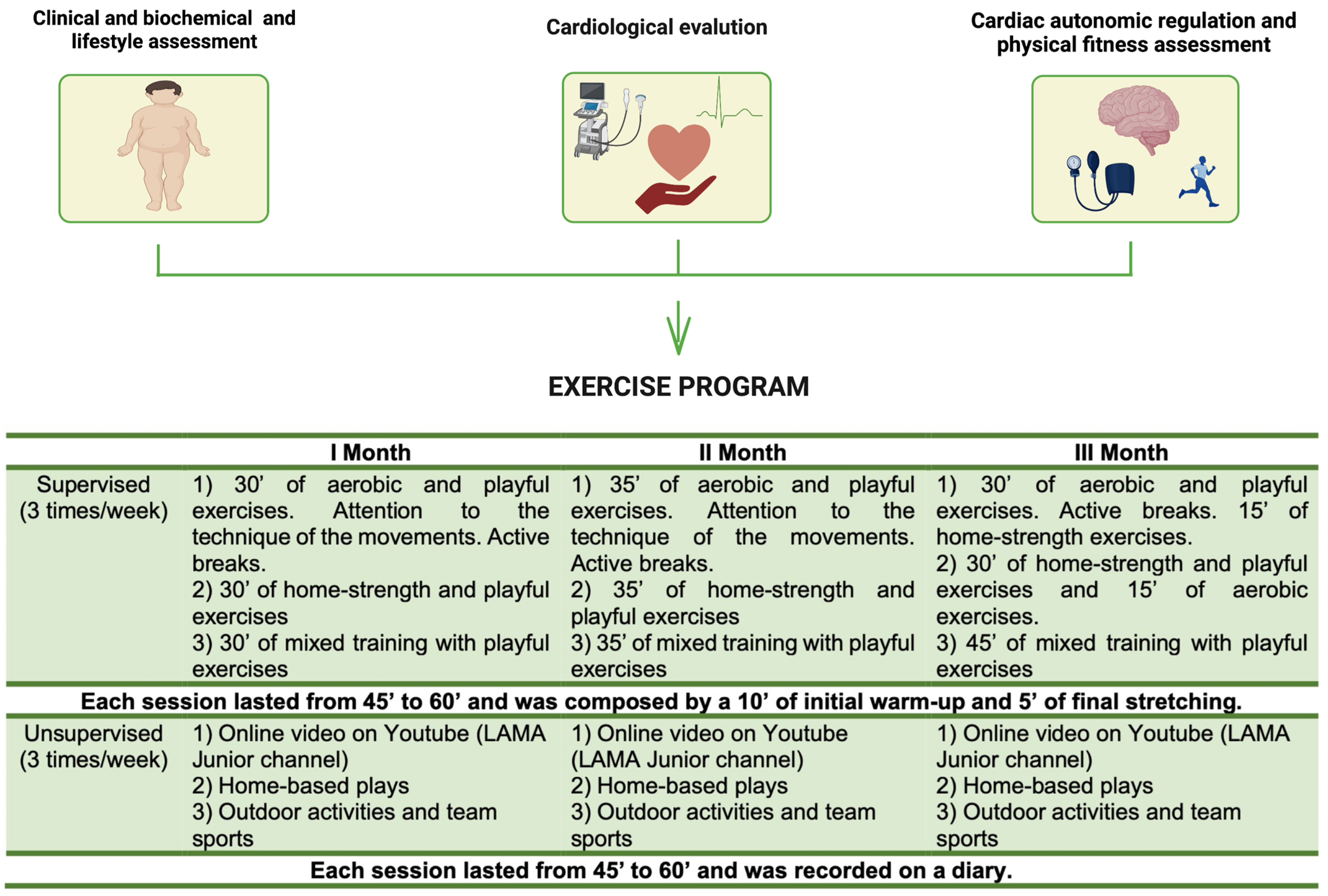
2.2. Exercise Training Protocol
2.3. Statistical Analysis
3. Results
3.1. Clinical, Auxological, Hemodynamical and Metabolic Data
3.2. Lifestyle Data
3.3. Physical Fitness Assessment Data
3.4. Cardiac Autonomic Regulation Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Turco, J.V.; Inal-Veith, A.; Fuster, V. Cardiovascular health promotion: An issue that can no longer wait. J. Am. Coll. Cardiol. 2018, 72, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Zhang, Y.; Yu, J.; Zha, M.; Zhu, Y.; Rahimi, K.; Rudan, I. Global Prevalence of Hypertension in Children: A Systematic Review and Meta-analysis. JAMA Pediatr. 2019, 173, 1154–1163. [Google Scholar] [CrossRef]
- Hampl, S.E.; Hassink, S.G.; Skinner, A.C.; Armstrong, S.C.; Barlow, S.E.; Bolling, C.F.; Avila Edwards, K.C.; Eneli, I.; Hamre, R.; Joseph, M.M.; et al. Clinical Practice Guideline for the Evaluation and Treatment of Children and Adolescents With Obesity. Pediatrics 2023, 151, e2022060640. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics—2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Wang, Q.; Piernas, C.; Astbury, N.M.; Jebb, S.A.; Holmes, M.V.; Aveyard, P. Associations between body composition, fat distribution and metabolic consequences of excess adiposity with severe COVID-19 outcomes: Observational study and Mendelian randomisation analysis. Int. J. Obes. 2022, 46, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Sallis, R.; Young, D.R.; Tartof, S.Y.; Sallis, J.F.; Sall, J.; Li, Q.; Smith, G.N.; Cohen, D.A. Physical inactivity is associated with a higher risk for severe COVID-19 outcomes: A study in 48 440 adult patients. Br. J. Sports Med. 2021, 55, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Artinian, N.T.; Fletcher, G.F.; Mozaffarian, D.; Kris-Etherton, P.; Van Horn, L.; Lichtenstein, A.H.; Kumanyika, S.; Kraus, W.E.; Fleg, J.L.; Redeker, N.S.; et al. Interventions to Promote Physical Activity and Dietary Lifestyle Changes for Cardiovascular Risk Factor Reduction in Adults: A scientific statement from the American Heart Association. Circulation 2010, 122, 406–441. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.M.; Hong, Y.; Labarthe, D.; Mozaffarian, D.; Appel, L.J.; Van Horn, L.; Greenlund, K.; Daniels, S.; Nichol, G.; Tomaselli, G.F.; et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: The American heart association’s strategic impact goal through 2020 and beyond. Circulation 2010, 121, 586–613. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. The Association Between School-Based Physical Activity, Including Physical Education, and Academic Performance; US Department of Health and Human Services: Atlanta, GA, USA, 2010.
- Jackson, T.; Dixon, J. The New Zealand Resource Management Act: An exercise in delivering sustainable development through an ecological modernisation agenda. Environ. Plan. B Plan. Des. 2007, 34, 107–120. [Google Scholar] [CrossRef]
- Chaput, J.-P.; Willumsen, J.; Bull, F.; Chou, R.; Ekelund, U.; Firth, J.; Jago, R.; Ortega, F.B.; Katzmarzyk, P.T. 2020 WHO guidelines on physical activity and sedentary behaviour for children and adolescents aged 5–17 years: Summary of the evidence. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 141. [Google Scholar] [CrossRef]
- Wyszyńska, J.; Ring-Dimitriou, S.; Thivel, D.; Weghuber, D.; Hadjipanayis, A.; Grossman, Z.; Ross-Russell, R.; Dereń, K.; Mazur, A. Physical Activity in the Prevention of Childhood Obesity: The Position of the European Childhood Obesity Group and the European Academy of Pediatrics. Front. Pediatr. 2020, 8, 662. [Google Scholar] [CrossRef] [PubMed]
- Steinberger, J.; Daniels, S.R.; Hagberg, N.; Isasi, C.R.; Kelly, A.S.; Lloyd-Jones, D.; Pate, R.R.; Pratt, C.; Shay, C.M.; Towbin, J.A.; et al. Cardiovascular Health Promotion in Children: Challenges and Opportunities for 2020 and Beyond: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e236–e255. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.euro.who.int/__data/assets/pdf_file/0009/513756/Physical-activity-2021-Italy-eng.pdf (accessed on 12 January 2023).
- Burden, S.J.; Weedon, B.D.; Turner, A.; Whaymand, L.; Meaney, A.; Dawes, H.; Jones, A. Intensity and Duration of Physical Activity and Cardiorespiratory Fitness. Pediatrics 2022, 150, e2021056003. [Google Scholar] [CrossRef]
- Ross, R.; Blair, S.N.; Arena, R.; Church, T.S.; Despres, J.P.; Franklin, B.A.; Haskell, W.L.; Kaminsky, L.A.; Levine, B.D.; Lavie, C.J.; et al. Importance of Assessing Cardiorespiratory Fitness in Clinical Practice: A Case for Fitness as a Clinical Vital Sign: A Scientific Statement from the American Heart Association. Circulation 2016, 134, e653–e699. [Google Scholar] [CrossRef]
- Dempsey, P.C.; Musicha, C.; Rowlands, A.V.; Davies, M.; Khunti, K.; Razieh, C.; Timmins, I.; Zaccardi, F.; Codd, V.; Nelson, C.P.; et al. Investigation of a UK biobank cohort reveals causal associations of self-reported walking pace with telomere length. Commun. Biol. 2022, 5, 381. [Google Scholar] [CrossRef] [PubMed]
- Fiuza-Luces, C.; Santos-Lozano, A.; Joyner, M.; Carrera-Bastos, P.; Picazo, O.; Zugaza, J.L.; Izquierdo, M.; Ruilope, L.M.; Lucia, A. Exercise benefits in cardiovascular disease: Beyond attenuation of traditional risk factors. Nat. Rev. Cardiol. 2018, 15, 731–743. [Google Scholar] [CrossRef]
- Joyner, M.J.; Green, D.J. Exercise protects the cardiovascular system: Effects beyond traditional risk factors. J. Physiol. 2009, 587, 5551–5558. [Google Scholar] [CrossRef] [PubMed]
- Esler, M.; Rumantir, M.; Wiesner, G.; Kaye, D.; Hastings, J.; Lambert, G. Sympathetic nervous system and insulin resistance: From obesity to diabetes. Am. J. Hypertens. 2001, 14, S304–S309. [Google Scholar] [CrossRef]
- Lucini, D.; Mela, G.S.; Malliani, A.; Pagani, M. Impairment in cardiac autonomic regulation preceding arterial hypertension in humans: Insights from spectral analysis of beat-by-beat cardiovascular variability. Circulation 2002, 106, 2673–2679. [Google Scholar] [CrossRef]
- Lucini, D.; Zuccotti, G.; Malacarne, M.; Scaramuzza, A.; Riboni, S.; Palombo, C.; Pagani, M. Early Progression of the Autonomic Dysfunction Observed in Pediatric Type 1 Diabetes Mellitus. Hypertension 2009, 54, 987–994. [Google Scholar] [CrossRef]
- Calcaterra, V.; Palombo, C.; Malacarne, M.; Pagani, M.; Federico, G.; Kozakova, M.; Zuccotti, G.; Lucini, D. Interaction between Autonomic Regulation, Adiposity Indexes and Metabolic Profile in Children and Adolescents with Overweight and Obesity. Children 2021, 8, 686. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Seravalle, G.; Colombo, M.; Bolla, G.; Cattaneo, B.M.; Cavagnini, F.; Mancia, G. Body Weight Reduction, Sympathetic Nerve Traffic, and Arterial Baroreflex in Obese Normotensive Humans. Circulation 1998, 97, 2037–2042. [Google Scholar] [CrossRef] [PubMed]
- Soares-Miranda, L.; Sattelmair, J.; Chaves, P.; Duncan, G.E.; Siscovick, D.S.; Stein, P.K.; Mozaffarian, D. Physical Activity and Heart Rate Variability in Older Adults: The Cardiovascular Health Study. Circulation 2014, 129, 2100–2110. [Google Scholar] [CrossRef] [PubMed]
- Lucini, D.; Milani, R.V.; Costantino, G.; Lavie, C.J.; Porta, A.; Pagani, M. Effects of cardiac rehabilitation and exercise training on autonomic regulation in patients with coronary artery disease. Am. Heart J. 2002, 143, 977–983. [Google Scholar] [CrossRef]
- Lucini, D.; Zuccotti, G.V.; Scaramuzza, A.; Malacarne, M.; Gervasi, F.; Pagani, M. Exercise might improve cardiovascular autonomic regulation in adolescents with type 1 diabetes. Acta Diabetol. 2012, 50, 341–349. [Google Scholar] [CrossRef]
- Calcaterra, V.; Vandoni, M.; Rossi, V.; Berardo, C.; Grazi, R.; Cordaro, E.; Tranfaglia, V.; Pellino, V.C.; Cereda, C.; Zuccotti, G. Use of Physical Activity and Exercise to Reduce Inflammation in Children and Adolescents with Obesity. Int. J. Environ. Res. Public Health 2022, 19, 6908. [Google Scholar] [CrossRef]
- Lucini, D.; Pagani, M. Exercise Prescription to Foster Health and Well-Being: A Behavioral Approach to Transform Barriers into Opportunities. Int. J. Environ. Res. Public Health 2021, 18, 968. [Google Scholar] [CrossRef]
- Abu-Omar, K.; Messing, S.; Sarkadi-Nagy, E.; Kovács, V.A.; Kaposvari, C.; Brukało, K.; Hassapidou, M.; Janssen, D.; Sandu, P.; Tecklenburg, E.; et al. Barriers, facilitators and capacities for childhood obesity prevention in 12 European Union Member States: Results of a policy-maker survey. Public Health Panor. 2018, 4, 360–367. [Google Scholar]
- Shahsanai, A.; Bahreynian, M.; Fallah, Z.; Hovsepian, S.; Kelishadi, R. Perceived barriers to healthy lifestyle from the parental perspective of overweight and obese students. J. Educ. Health Promot. 2019, 8, 79. [Google Scholar] [CrossRef]
- Zabinski, M.F.; Saelens, B.E.; Stein, R.; Hayden-Wade, H.A.; Wilfley, D.E. Overweight Children’s Barriers to and Support for Physical Activity. Obes. Res. 2003, 11, 238–246. [Google Scholar] [CrossRef]
- Kirwan, M.; Chiu, C.L.; Laing, T.; Chowdhury, N.; Gwynne, K. A Web-Delivered, Clinician-Led Group Exercise Intervention for Older Adults with Type 2 Diabetes: Single-Arm Pre-Post Intervention. J. Med. Internet Res. 2022, 24, e39800. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, V.; Iafusco, D.; Pellino, V.C.; Mameli, C.; Tornese, G.; Chianese, A.; Cascella, C.; Macedoni, M.; Redaelli, F.; Zuccotti, G.; et al. “CoVidentary”: An online exercise training program to reduce sedentary behaviours in children with type 1 diabetes during the COVID-19 pandemic. J. Clin. Transl. Endocrinol. 2021, 25, 100261. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, V.; Verduci, E.; Vandoni, M.; Rossi, V.; Di Profio, E.; Pellino, V.C.; Tranfaglia, V.; Pascuzzi, M.C.; Borsani, B.; Bosetti, A.; et al. Telehealth: A Useful Tool for the Management of Nutrition and Exercise Programs in Pediatric Obesity in the COVID-19 Era. Nutrients 2021, 13, 3689. [Google Scholar] [CrossRef] [PubMed]
- Vandoni, M.; Pellino, V.C.; Gatti, A.; Lucini, D.; Mannarino, S.; Larizza, C.; Rossi, V.; Tranfaglia, V.; Pirazzi, A.; Biino, V.; et al. Effects of an Online Supervised Exercise Training in Children with Obesity during the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2022, 19, 9421. [Google Scholar] [CrossRef] [PubMed]
- Showell, N.N.; Fawole, O.; Segal, J.; Wilson, R.F.; Cheskin, L.J.; Bleich, S.N.; Wu, Y.; Lau, B.; Wang, Y. A Systematic Review of Home-Based Childhood Obesity Prevention Studies. Pediatrics 2013, 132, e193–e200. [Google Scholar] [CrossRef]
- Available online: https://www.who.int/tools/child-growth-standards (accessed on 12 January 2023).
- Marshall, W.A.; Tanner, J.M. Variations in the Pattern of Pubertal Changes in Boys. Arch. Dis. Child. 1970, 45, 13–23. [Google Scholar] [CrossRef]
- Marshall, W.A.; Tanner, J.M. Variations in pattern of pubertal changes in girls. Arch. Dis. Child. 1969, 44, 291–303. [Google Scholar] [CrossRef]
- Maffeis, C.; Banzato, C.; Talamini, G.; Obesity Study Group of the Italian Society of Pediatric Endocrinology and Diabetology. Waist–to–Height Ratio, a Useful Index to Identify High Metabolic Risk in Overweight Children. J. Pediatr. 2008, 152, 207–213. [Google Scholar] [CrossRef]
- Ramírez-Vélez, R.; Correa-Bautista, J.E.; Carrillo, H.A.; González-Jiménez, E.; Schmidt-RioValle, J.; Correa-Rodríguez, M.; García-Hermoso, A.; González-Ruíz, K. Tri-Ponderal Mass Index vs. Fat Mass/Height3 as a Screening Tool for Metabolic Syndrome Prediction in Colombian Children and Young People. Nutrients 2018, 10, 412. [Google Scholar] [CrossRef]
- Mameli, C.; Krakauer, N.Y.; Krakauer, J.C.; Bosetti, A.; Ferrari, C.M.; Moiana, N.; Schneider, L.; Borsani, B.; Genoni, T.; Zuccotti, G. The association between a body shape index and cardiovascular risk in overweight and obese children and adolescents. PLoS ONE 2018, 13, e0190426. [Google Scholar] [CrossRef]
- Calcaterra, V.; Verduci, E.; Schneider, L.; Cena, H.; De Silvestri, A.; Vizzuso, S.; Vinci, F.; Mameli, C.; Zuccotti, G. Sex-Specific Differences in the Relationship between Insulin Resistance and Adiposity Indexes in Children and Adolescents with Obesity. Children 2021, 8, 449. [Google Scholar] [CrossRef] [PubMed]
- National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 2004, 114, 555–576. [Google Scholar] [CrossRef]
- Rosner, B.; Cook, N.; Portman, R.; Daniels, S.; Falkner, B. Determination of Blood Pressure Percentiles in Normal-Weight Children: Some Methodological Issues. Am. J. Epidemiol. 2008, 167, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.T.; Kaelber, D.C.; Baker-Smith, C.M.; Blowey, D.; Carroll, A.E.; Daniels, S.R.; De Ferranti, S.D.; Dionne, J.M.; Falkner, B.; Flinn, S.K.; et al. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics 2017, 140, e20171904. [Google Scholar] [CrossRef]
- Available online: https://www.bcm.edu/bodycomplab/BPappZjs/BPvAgeAPPz.html (accessed on 12 January 2023).
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Vieira-Ribeiro, S.A.; Fonseca, P.C.; Andreoli, C.S.; Ribeiro, A.Q.; Hermsdorff, H.H.; Pereira, P.F.; Priore, S.E.; Franceschini, S.C. The TyG index cutoff point and its association with body adiposity and lifestyle in children. J. Pediatr. 2019, 95, 217–223. [Google Scholar] [CrossRef]
- Lucini, D.; Solaro, N.; Lesma, A.; Gillet, V.B.; Pagani, M.; Dickerson, J.; Ivannikov, M. Health Promotion in the Workplace: Assessing Stress and Lifestyle with an Intranet Tool. J. Med. Internet Res. 2011, 13, e88. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.L.; Yngve, A.; Sallis, J.F.; et al. International Physical Activity Questionnaire: 12-Country Reliability and Validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Minetto, M.A.; Motta, G.; Gorji, N.E.; Lucini, D.; Biolo, G.; Pigozzi, F.; Portincasa, P.; Maffiuletti, N.A. Reproducibility and validity of the Italian version of the International Physical Activity Questionnaire in obese and diabetic patients. J. Endocrinol. Investig. 2017, 41, 343–349. [Google Scholar] [CrossRef]
- Lucini, D.; Pagani, E.; Capria, F.; Galliano, M.; Marchese, M.; Cribellati, S. Evidence of Better Psychological Profile in Working Population Meeting Current Physical Activity Recommendations. Int. J. Environ. Res. Public Health 2021, 18, 8991. [Google Scholar] [CrossRef]
- Lucini, D.; Zanuso, S.; Blair, S.; Pagani, M. A simple healthy lifestyle index as a proxy of wellness: A proof of concept. Acta Diabetol. 2014, 52, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.R.; Castro-Pinero, J.; Espana-Romero, V.; Artero, E.G.; Ortega, F.B.; Cuenca, M.M.; Jimenez-Pavon, D.; Chillon, P.; Girela-Rejon, M.J.; Mora, J.; et al. Field-based fitness assessment in young people: The ALPHA health-related fitness test battery for children and adolescents. Br. J. Sports Med. 2011, 45, 518–524. [Google Scholar] [CrossRef]
- Holland, A.E.; Spruit, M.A.; Troosters, T.; Puhan, M.A.; Pepin, V.; Saey, D.; McCormack, M.C.; Carlin, B.W.; Sciurba, F.C.; Pitta, F.; et al. An official European Respiratory Society/American Thoracic Society technical standard: Field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1428–1446. [Google Scholar] [CrossRef] [PubMed]
- Vandoni, M.; Correale, L.; Puci, M.V.; Galvani, C.; Codella, R.; Togni, F.; La Torre, A.; Casolo, F.; Passi, A.; Orizio, C.; et al. Six minute walk distance and reference values in healthy Italian children: A cross-sectional study. PLoS ONE 2018, 13, e0205792. [Google Scholar] [CrossRef]
- Ortega, F.B.; Ruiz, J.R.; España-Romero, V.; Vicente-Rodriguez, G.; Martínez-Gómez, D.; Manios, Y.; Béghin, L.; Molnar, D.; Widhalm, K.; Moreno, L.A.; et al. The International Fitness Scale (IFIS): Usefulness of self-reported fitness in youth. Int. J. Epidemiol. 2011, 40, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Benítez-Porres, J.; López-Fernández, I.; Raya, J.F.; Carnero, S.; Alvero-Cruz, J.R.; Carnero, E. Reliability and Validity of the PAQ-C Questionnaire to Assess Physical Activity in Children. J. Sch. Heal. 2016, 86, 677–685. [Google Scholar] [CrossRef]
- La Rovere, M.T.; Bigger, J.T., Jr.; Marcus, F.I.; Mortara, A.; Schwartz, P.J. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet 1998, 351, 478–484. [Google Scholar] [CrossRef]
- Solaro, N.; Pagani, M.; Lucini, D. Altered Cardiac Autonomic Regulation in Overweight and Obese Subjects: The Role of Age-and-Gender-Adjusted Statistical Indicators of Heart Rate Variability and Cardiac Baroreflex. Front. Physiol. 2021, 11, 567312. [Google Scholar] [CrossRef]
- Cicone, Z.S.; Holmes, C.J.; Fedewa, M.V.; MacDonald, H.V.; Esco, M.R. Age-Based Prediction of Maximal Heart Rate in Children and Adolescents: A Systematic Review and Meta-Analysis. Res. Q. Exerc. Sport 2019, 90, 417–428. [Google Scholar] [CrossRef]
- Calcaterra, V.; Vandoni, M.; Pellino, V.C.; Cena, H. Special Attention to Diet and Physical Activity in Children and Adolescents With Obesity During the Coronavirus Disease-2019 Pandemic. Front. Pediatr. 2020, 8, 407. [Google Scholar] [CrossRef]
- Paluch, A.E.; Bajpai, S.; Bassett, D.R.; Carnethon, M.R.; Ekelund, U.; Evenson, K.R.; Galuska, D.A.; Jefferis, B.J.; Kraus, W.E.; Lee, I.M.; et al. Daily steps and all-cause mortality: A meta-analysis of 15 international cohorts. Lancet Public Health 2022, 7, e219–e228. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, V.; Verduci, E.; Vandoni, M.; Rossi, V.; Fiore, G.; Massini, G.; Berardo, C.; Gatti, A.; Baldassarre, P.; Bianchi, A.; et al. The Effect of Healthy Lifestyle Strategies on the Management of Insulin Resistance in Children and Adolescents with Obesity: A Narrative Review. Nutrients 2022, 14, 4692. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-S.; Li, B.; Wang, G.-X.; Ke, Y.-Z.; Meng, S.-Q.; Li, Y.-X.; Cui, Z.-L.; Tong, W.-X. Physical Fitness, Exercise Behaviors, and Sense of Self-Efficacy Among College Students: A Descriptive Correlational Study. Front. Psychol. 2022, 13, 932014. [Google Scholar] [CrossRef] [PubMed]
- Pamungkas, R.A.; Chamroonsawasdi, K. Home-Based Interventions to Treat and Prevent Childhood Obesity: A Systematic Review and Meta-Analysis. Behav. Sci. 2019, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Lucini, D.; Malacarne, M.; Solaro, N.; Busin, S.; Pagani, M. Complementary medicine for the management of chronic stress: Superiority of active versus passive techniques. J. Hypertens. 2009, 27, 2421–2428. [Google Scholar] [CrossRef]
- Lucini, D.; Riva, S.; Pizzinelli, P.; Pagani, M. Stress management at the worksite: Reversal of symptoms profile and cardiovascular dysregulation. Hypertension 2007, 49, 291–297. [Google Scholar] [CrossRef]
| Indices | Groups | Significance | |||
|---|---|---|---|---|---|
| Group 1 n = 12 | Group 2 n = 23 | ||||
| Below 1200 METs | Above 1200 METs | Between Groups | Between T0–T1 | Interaction | |
| Median (Percentile 25°; 75°) | Median (Percentile 25°; 75°) | ||||
| HR T0 [b/min] | 90.76 (83.38; 97.48) | 82.15 (75.67; 85.90) | 0.283 | 0.843 | 0.163 |
| HR T1 | 90.80 (78.83; 95.34) | 83.52 (77.86; 95.90) | |||
| RR T0 [msec] | 661.20 (615.73; 719.99) | 730.38 (698.48; 792.92) | 0.186 | 0.898 | 0.289 |
| RR T1 | 660.93 (629.34; 761.20) | 718.41 (625.64; 770.63) | |||
| RRTP T0 [msec2] | 2015.38 (700.66; 4175.22) | 2070.68 (1174.64; 4358.51) | 0.726 | 0.694 | 0.604 |
| RRTP T1 | 1941.73 (1036.30; 2996.04) | 1974.04 (880.01; 3978.67) | |||
| RRLFa T0 [msec2] | 278.57 (152.21; 1536.34) | 569.95 (324.55; 1064.11) | 0.613 | 0.911 | 0.795 |
| RRLFa T1 | 414.05 (246.20; 1126.03) | 679.74 (218.92; 1833.57) | |||
| RRHFa T0 [msec2] | 565.22 (124.40; 1610.27) | 600.91 (198.91; 1718.47) | 0.917 | 0.423 | 0.331 |
| RRHFa T1 | 461.04 (252.52; 1177.21) | 630.36 (235.87; 1128.62) | |||
| RRLFnu T0 [nu] | 30.66 (25.04; 48.65) | 46.38 (31.23; 55.80) | 0.267 | 0.264 | 0.727 |
| RRLFnu T1 | 41.86 (34.10; 44.14) | 41.52 (33.52; 65.68) | |||
| RRHFnu T0 [nu] | 52.41 (37.54; 61.06) | 46.00 (28.39; 59.02) | 0.808 | 0.310 | 0.367 |
| RRHFnu T1 | 47.47 (32.09; 56.59) | 43.79 (28.31; 57.69) | |||
| RRLF/HF T0 [.] | 0.58 (0.41; 1.30) | 1.01 (0.46; 2.27) | 0.299 | 0.660 | 0.691 |
| RRLF/HF T1 | 0.91 (0.55; 1.46) | 0.95 (0.59; 2.32) | |||
| SAPpc T0 [%] | 78.00 (35.50; 87.00) | 80.00 (63.00; 92.00) | 0.058 | 0.464 | 0.895 |
| SAPpc T1 | 58.50 (49.50; 65.00) | 80.00 (51.00; 93.00) | |||
| DAPpc T0 [%] | 75.00 (56.00; 92.00) | 71.00 (59.00; 95.00) | 0.348 | 0.376 | 0.068 |
| DAPpc T1 | 61.50 (42.50; 84.50) | 78.00 (59.00; 91.00) | |||
| AHA score T0 [.] | 1.50 (1.00; 2.50) | 2.00 (1.00; 3.00) | 0.157 | 0.180 | 0.180 |
| AHA score T1 | 2.00 (1.00; 2.50) | 3.00 (2.00; 3.00) | |||
| Hours of sleep T0 [h/day] | 8.00 (8.00; 9.00) | 8.50 (8.00; 9.00) | 0.156 | 0.208 | 0.402 |
| Hours of sleep T1 | 9.00 (7.00; 9.00) | 9.00 (8.00; 9.00) | |||
| Quality of Sleep T0 [.] | 8.50 (6.00; 10.00) | 10.00 (8.00; 10.00) | 0.020 | 0.351 | 0.829 |
| Quality of SleepT1 | 8.50 (4.50; 9.50) | 9.00 (9.00; 10.00) | |||
| Health T0 [.] | 7.00 (6.00; 9.00) | 8.00 (6.00; 10.00) | 0.051 | 0.215 | 0.215 |
| Health T1 | 6.50 (5.00; 9.00) | 8.00 (6.00; 9.00) | |||
| School Performance T0 [.] | 8.00 (7.00; 10.00) | 9.00 (7.00; 10.00) | 0.450 | 0.215 | 0.907 |
| School Performance T1 | 8.50 (7.00; 9.00) | 8.00 (7.00; 9.00) | |||
| Sedentariness T0 [h/week] | 68.00 (61.00; 82;00) | 56.00 (49.00; 68.00) | 0.038 | 0.229 | 0.775 |
| Sedentariness T1 | 66.50 (52.00; 84.50) | 61.00 (28; 68.00) | |||
| METsMV T0 [MET·min/week] | 240.00 (0.00; 880.00) | 480.00 (0.00; 720.00) | 0.056 | 0.119 | 0.117 |
| METsMV T1 | 480.00 (330.00; 670.00) | 960.00 (720.00; 1800.00) | |||
| METsTOT T0 [MET·min/week] | 361.50 (153.00; 966.25) | 918.00 (495.00; 1635.00) | 0.000 | 0.008 | 0.030 |
| METsTOT T1 | 752.25 (538.50; 960.50) | 1860.00 (1395.00; 2580.00) | |||
| BMI z-score T0 [.] | 2.13 (1.65; 2.52) | 2.04 (1.84; 2.41) | 0.678 | 0.004 | 0.200 |
| BMI z-score T1 | 2.00 (1.35; 2.54) | 1.97 (1.82; 2.49) | |||
| WHtR T0 [.] | 0.59 (0.57; 0.62) | 0.59 (0.57; 0.64) | 0.702 | 0.223 | 0.142 |
| WHtR T1 | 0.58 (0.54; 0.60) | 0.58 (0.55; 0.60) | |||
| FBG T0 [mg/dL] | 89.50 (87.00; 91.50) | 87.00 (85.00; 95.00) | 0.638 | 0.683 | 0.406 |
| FBG T1 | 93.00 (86.50; 99.00) | 89.00 (85.00; 95.00) | |||
| Insulin T0 [mg/dL] | 17.55 (11.25; 25.85) | 17.40 (13.14; 30.70) | 0.343 | 0.515 | 0.375 |
| Insulin T1 | 15.40 (8.80; 30.75) | 18.00 (12.00; 30.10) | |||
| HOMA-IR T0 [.] | 3.97 (2.50; 5.59) | 3.87 (2.76; 6.64) | 0.354 | 0.596 | 0.363 |
| HOMA-IR T1 | 3.78 (1.86; 7.02) | 3.78 (2.77; 5.72) | |||
| TG T0 [mg/dL] | 93.00 (64.50; 117.50) | 113.00 (73.00; 150.00) | 0.281 | 0.886 | 0.331 |
| TG T1 | 91.00 (60.00; 119.00) | 110.00 (65.00; 148.00) | |||
| TOT Chol T0 [mg/dL] | 149.00 (123.00; 156.00) | 170.00 (150.00; 190.00) | 0.048 | 0.314 | 0.068 |
| TOT Chol T1 | 143.00 (129.00; 183.00) | 167.00 (144.00; 172.00) | |||
| HDL C T0 [mg/dL] | 47.00 (37.00; 53.50) | 45.00 (41.00; 50.00) | 0.990 | 0.427 | 0.567 |
| HDL C T1 | 49.00 (43.00; 52.00) | 47.00 (40.00; 50.00) | |||
| TMI T0 [.] | 19.13 (18.17; 21.30) | 18.64 (17.24; 20.53) | 0.637 | 0.000 | 0.160 |
| TMI T1 | 18.62 (17.47; 20.91) | 18.32 (16.72; 19.68) | |||
| VAI T0 [.] | 3.33 (1.60; 4.90) | 3.30 (1.58; 4.96) | 0.330 | 0.137 | 0.392 |
| VAI T1 | 3.01 (2.01; 4.53) | 4.46 (2.48; 5.58) | |||
| TyG T0 [.] | 8.26 (8.01; 8.54) | 8.56 (7.90; 8.82) | 0.523 | 0.722 | 0.306 |
| TyG T1 | 8.35 (7.94; 8.47) | 8.47 (7.93; 8.80) | |||
| 6MWT T0 [m] | 464.00 (427.00; 540.00) | 472.00 (438.00; 504.00) | 0.596 | 0.000 | 0.122 |
| 6MWT T1 | 516.00 (482.00. 560.00) | 540.00 (500.00; 574.00) | |||
| PAQ-C score T0 [.] | 1.92 (1.74; 2.17) | 1.97 (1.57; 2.30) | 0.840 | 0.043 | 0.858 |
| PAQ-C score T1 | 2.39 (1.90; 2.53) | 2.25 (1.77; 2.69) | |||
| IFIS score T0 [.] | 3.40 (3.20; 4.20) | 3.00 (2.80; 3.80) | 0.159 | 0.543 | 0.653 |
| IFIS score T1 | 3.80 (3.20; 4.00) | 3.40 (2.80; 4.00) | |||
| Indices | Groups | Significance | |||
|---|---|---|---|---|---|
| Below 1200 METs | Above 1200 METs | Between Groups | Between T0–T1 | Interaction | |
| Median (Percentile 25°; 75°) | Median (Percentile 25°; 75°) | ||||
| HR T0 [b/min] | 86.61 (81.86; 95.46) | 77.07 (73.44; 83.37) | 0.139 | 0.555 | 0.344 |
| HR T1 | 89.55 (80.08; 95.83) | 80.87 (68.25; 94.11) | |||
| RR T0 [msec] | 692.84 (628.57; 732.96) | 778.47 (719.76; 816.99) | 0.062 | 0.721 | 0.675 |
| RR T1 | 670.07 (626.13; 749.24) | 741.94 (637.58; 879.14) | |||
| RRTP T0 [msec2] | 1602.01 (775.36; 3131.09) | 3215.74 (2070.68; 4532.33) | 0.686 | 0.996 | 0.552 |
| RRTP T1 | 1711.46 (1036.30; 2996.04) | 2342.19 (815.39; 4338.19) | |||
| RRLFa.T0 [msec2] | 392.29 (165.69; 1052.10) | 952.41 (465.37; 1154.68) | 0.995 | 0.740 | 0.702 |
| RRLFa T1 | 498.63 (246.86; 1390.14) | 679.74 (138.87; 1833.57) | |||
| RRHFa. T0 [msec2] | 551.61 (158.16; 1360.03) | 773.98 (269.76; 2289.90) | 0.754 | 0.942 | 0.266 |
| RRHFa T1 | 474.34 (252.52; 1092.48) | 777.43 (235.87; 1485.89) | |||
| RRLFnu T0 [nu] | 44.24 (27.10; 51.30) | 48.20 (26.04; 69.70) | 0.947 | 0.735 | 0.063 |
| RRLFnu.T1 | 42.13 (34.23; 57.47) | 34.69 (22.89; 67.94) | |||
| RRHFnu T0 [nu] | 49.16 (40.03; 59.66) | 49.43 (20.20; 67.22) | 0.651 | 0.952 | 0.032 |
| RRHFnu. T1 | 42.55 (32.09; 55.36) | 55.05 (27.20; 70.76) | |||
| RRLF/HF T0 [.] | 0.87 (0.48; 1.29) | 0.98 (0.44; 3.53) | 0.450 | 0.546 | 0.015 |
| RRLF/HF T1 | 1.09 (0.59; 1.68) | 0.60 (0.32; 2.58) | |||
| SAPpc T0 [%] | 77.00 (46.00; 87) | 86.00 (65.00; 94.00) | 0.315 | 0.243 | 0.197 |
| SAPpc T1 | 64.5 (56.00; 91.00) | 83.00 (50.00; 92.00) | |||
| DAPpc T0 [%] | 75.00 (60.00; 92.00) | 65.00 (58.00; 95.00) | 0.987 | 0.864 | 0.768 |
| DAPpc T1 | 79.5 (49.5; 90.5) | 76.00 (55.00; 89.00) | |||
| AHA score T0 [.] | 1.00 (1.00; 2.00) | 2.00 (2.00; 3.00) | 0.019 | 0.045 | 0.359 |
| AHA score T1 | 2.00 (1.00; 3.00) | 3.00 (2.00; 4.00) | |||
| Hours of sleep T0 [h] | 8.5 (8.00; 9.00) | 8.00 (8.00; 9.00) | 0.507 | 0.575 | 0.141 |
| Hours of sleep.T1 | 8.00 (7.50; 9.00) | 9.00 (8.00; 9.00) | |||
| Quality of Sleep T0 [.] | 9.00 (8.00; 10.00) | 10.00 (8.00;1 0.00) | 0.233 | 0.414 | 0.935 |
| Quality of Sleep T1 | 9.00 (7.00; 10.00) | 9.00 (9.00; 10.00) | |||
| Health T0 [.] | 8.00 (6.00; 10.00) | 7.00 (6.00; 9.00) | 0.744 | 0.703 | 0.229 |
| Health T1 | 8.00 (5.50; 9.00) | 7.00 (6.00; 9.00) | |||
| School Performance T0 [.] | 9.00 (7.00; 10.00) | 8.00 (7.00; 10.00) | 0.921 | 0.160 | 0.560 |
| School Performance T1 | 8.00 (7.00; 9.00) | 8.00 (6.00; 9.00) | |||
| Sedentariness T0 [h/week] | 66.00 (56.00; 78.50) | 53.00 (48.00; 56.00) | 0.010 | 0.162 | 0.708 |
| Sedentariness T1 | 63.00 (52.00; 79.50) | 54.00 (26.00; 67.00) | |||
| METsMV T0 [MET·min/week] | 240.00 (0.00; 480.00) | 720.00 (0.00;1 680.00) | 0.000 | 0.002 | 0.007 |
| METsMV T1 | 600.00 (480.00; 720.00) | 1800 (1200.00; 2080.00) | |||
| METsTOT T0 [MET·min/week] | 648.75 (268.50; 1333.00) | 819.00 (0.00; 1920.00) | 0.012 | 0.000 | 0.012 |
| METsTOT T1 | 1207.50 (752.25; 1644.00) | 2493.00 (1860.00; 2773.00) | |||
| BMI z-score T0 [.] | 2.11 (1.91; 2.38) | 1.99 (1.69; 2.55) | 0.885 | 0.033 | 0.282 |
| BMI z-score T1 | 1.99 (1.63; 2.35) | 1.90 (1.58; 2.52) | |||
| WHtR T0 [.] | 0.59 (0.57; 0.63) | 0.59 (0.56; 0.64) | 0.647 | 0.848 | 0.142 |
| WHtR T1 | 0.57 (0.56; 0.60) | 0.59 (0.55; 0.63) | |||
| FBG T0 [mg/dL] | 89.00 (86.00; 92.50) | 87.00 (86.00; 96.00) | 0.708 | 0.939 | 0.594 |
| FBG T1 | 90.00 (85.50; 96.00) | 88.00 (87.00; 96.00) | |||
| Insulin T0 [mg/dL] | 16.80 (11.75; 23.80) | 18.00 (13.14; 35.00) | 0.420 | 0.088 | 0.061 |
| Insulin T1 | 16.00 (10.24; 30.15) | 18.20 (14.80; 30.10) | |||
| HOMA-IR T0 [.] | 3.80 (2.51; 5.36) | 3.87 (2.76; 7.78) | 0.372 | 0.103 | 0.053 |
| HOMA-IR T1 | 3.75 (2.22; 6.46) | 4.27 (3.22; 5.72) | |||
| TG T0 [mg/dL] | 96.00 (69.50; 122.50) | 121.00 (62.00; 169.00) | 0.362 | 0.876 | 0.991 |
| TG T1 | 91.00 (63.00; 120.00) | 112.00 (75.00; 156.00) | |||
| TOT Chol T0 [mg/dL] | 156.00 (139.00; 187.00) | 165.00 (150.00; 190.00) | 0.782 | 0.048 | 0.199 |
| TOT Chol T1 | 155.50 (133.00; 176.00) | 151.00 (144.00; 168.00) | |||
| HDL C T0 [mg/dL] | 47.50 (40.50; 55.50) | 43.00 (39.00; 48.00) | 0.390 | 0.445 | 0.675 |
| HDL C T1 | 48.00 (43.00; 52.00) | 46.00 (39.00; 48.00) | |||
| TMI T0 [.] | 19.08 (18.30; 21.03) | 17.58 (17.15; 19.93) | 0.285 | 0.003 | 0.760 |
| TMI T1 | 19.07 (17.69; 20.91) | 17.48 (16.38; 19.68) | |||
| VAI T0 [.] | 3.26 (1.79; 4.85) | 3.42 (1.51; 5.53) | 0.611 | 0.012 | 0.048 |
| VAI T1 | 3.61 (2.07; 4.73) | 5.34 (2.65; 5.85) | |||
| TyG T0 [.] | 8.35 (7.99; 8.66) | 8.58 (7.88; 8.97) | 0.454 | 0.998 | 0.938 |
| TyG T1 | 8.35 (7.92; 8.62) | 8.61 (8.09; 8.84) | |||
| 6MWT T0 [m] | 464.00 (432.00; 509.00) | 490.00 (440.00; 509.00) | 0.532 | 0.000 | 0.263 |
| 6MWT T1 | 520.00 (492.00; 560.00) | 564.00 (520.00; 580.00) | |||
| PAQ-C score T0 [.] | 1.97 (1.73; 2.17) | 1.84 (1.57; 2.39) | 0.895 | 0.046 | 0.731 |
| PAQ-C score T1 | 2.30 (1.77; 2.53) | 2.25 (1.81; 2.69) | |||
| IFIS score T0 [.] | 3.00 (2.80; 3.60) | 3.20 (3.00; 4.20) | 0.693 | 0.578 | 0.214 |
| IFIS score T1 | 3.60 (3.20; 4.00) | 3.40 (2.80; 4.00) | |||
| Δ METsMV | 0.844 ** | |||||||
|---|---|---|---|---|---|---|---|---|
| 0.000 | ||||||||
| Δ RR | 0.227 | 0.423 * | ||||||
| 0.189 | 0.011 | |||||||
| Δ RRTP | 0.405 * | 0.509 ** | 0.686 ** | |||||
| 0.016 | 0.002 | 0.000 | ||||||
| Δ RRLFnu | −0.117 | −0.204 | −0.086 | −0.012 | ||||
| 0.504 | 0.239 | 0.622 | 0.946 | |||||
| Δ RRHFnu | 0.216 | 0.335 * | 0.061 | 0.088 | −0.872 ** | |||
| 0.213 | 0.049 | 0.729 | 0.617 | 0.000 | ||||
| Δ LF/HF | −0.137 | −0.238 | −0.004 | 0.069 | 0.721 ** | 0.716 ** | ||
| 0.433 | 0.168 | 0.983 | 0.692 | 0.000 | 0.000 | |||
| Δ SAPpc | −0.136 | −0.400 * | −0.512 ** | −0.368 * | 0.214 | −0.187 | 0.061 | |
| 0.436 | 0.017 | 0.002 | 0.030 | 0.217 | 0.281 | 0.729 | ||
| Δ BMI z-score | −0.088 | −0.008 | −0.189 | −0.243 | 0.099 | −0.103 | −0.079 | 0.094 |
| 0.617 | 0.963 | 0.278 | 0.160 | 0.572 | 0.555 | 0.654 | 0.590 | |
| Δ METsTOT | Δ METsMV | Δ RR | Δ RRTP | Δ RRLFnu | Δ RRHFnu | Δ LF/HF | Δ SAPpc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calcaterra, V.; Bernardelli, G.; Malacarne, M.; Vandoni, M.; Mannarino, S.; Pellino, V.C.; Larizza, C.; Pagani, M.; Zuccotti, G.; Lucini, D. Effects of Endurance Exercise Intensities on Autonomic and Metabolic Controls in Children with Obesity: A Feasibility Study Employing Online Exercise Training. Nutrients 2023, 15, 1054. https://doi.org/10.3390/nu15041054
Calcaterra V, Bernardelli G, Malacarne M, Vandoni M, Mannarino S, Pellino VC, Larizza C, Pagani M, Zuccotti G, Lucini D. Effects of Endurance Exercise Intensities on Autonomic and Metabolic Controls in Children with Obesity: A Feasibility Study Employing Online Exercise Training. Nutrients. 2023; 15(4):1054. https://doi.org/10.3390/nu15041054
Chicago/Turabian StyleCalcaterra, Valeria, Giuseppina Bernardelli, Mara Malacarne, Matteo Vandoni, Savina Mannarino, Vittoria Carnevale Pellino, Cristiana Larizza, Massimo Pagani, Gianvincenzo Zuccotti, and Daniela Lucini. 2023. "Effects of Endurance Exercise Intensities on Autonomic and Metabolic Controls in Children with Obesity: A Feasibility Study Employing Online Exercise Training" Nutrients 15, no. 4: 1054. https://doi.org/10.3390/nu15041054
APA StyleCalcaterra, V., Bernardelli, G., Malacarne, M., Vandoni, M., Mannarino, S., Pellino, V. C., Larizza, C., Pagani, M., Zuccotti, G., & Lucini, D. (2023). Effects of Endurance Exercise Intensities on Autonomic and Metabolic Controls in Children with Obesity: A Feasibility Study Employing Online Exercise Training. Nutrients, 15(4), 1054. https://doi.org/10.3390/nu15041054









