A Murine Model of Food Allergy by Epicutaneous Adjuvant-Free Allergen Sensitization Followed by Oral Allergen Challenge Combined with Aspirin for Enhanced Detection of Hypersensitivity Manifestations and Immunotherapy Monitoring
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Antigens and Reagents
2.3. Epicutaneous Sensitization
2.4. Measurement of Allergen sIgE, sIgG1 and Mouse Mast Cell Protease-1
2.5. Spleen Cell Culture and Cytokine Productions
2.6. Assessment of Anaphylaxis by Fall in Rectal Temperature
2.7. Oral Administration of ASA to Amplify Mild Allergic Symptoms Induced by Oral Allergen Challenge
2.8. Assessment of Allergic Hypersensitivity Reactions Using a Modified Symptom Scoring System
2.9. Statistical Analysis
3. Results
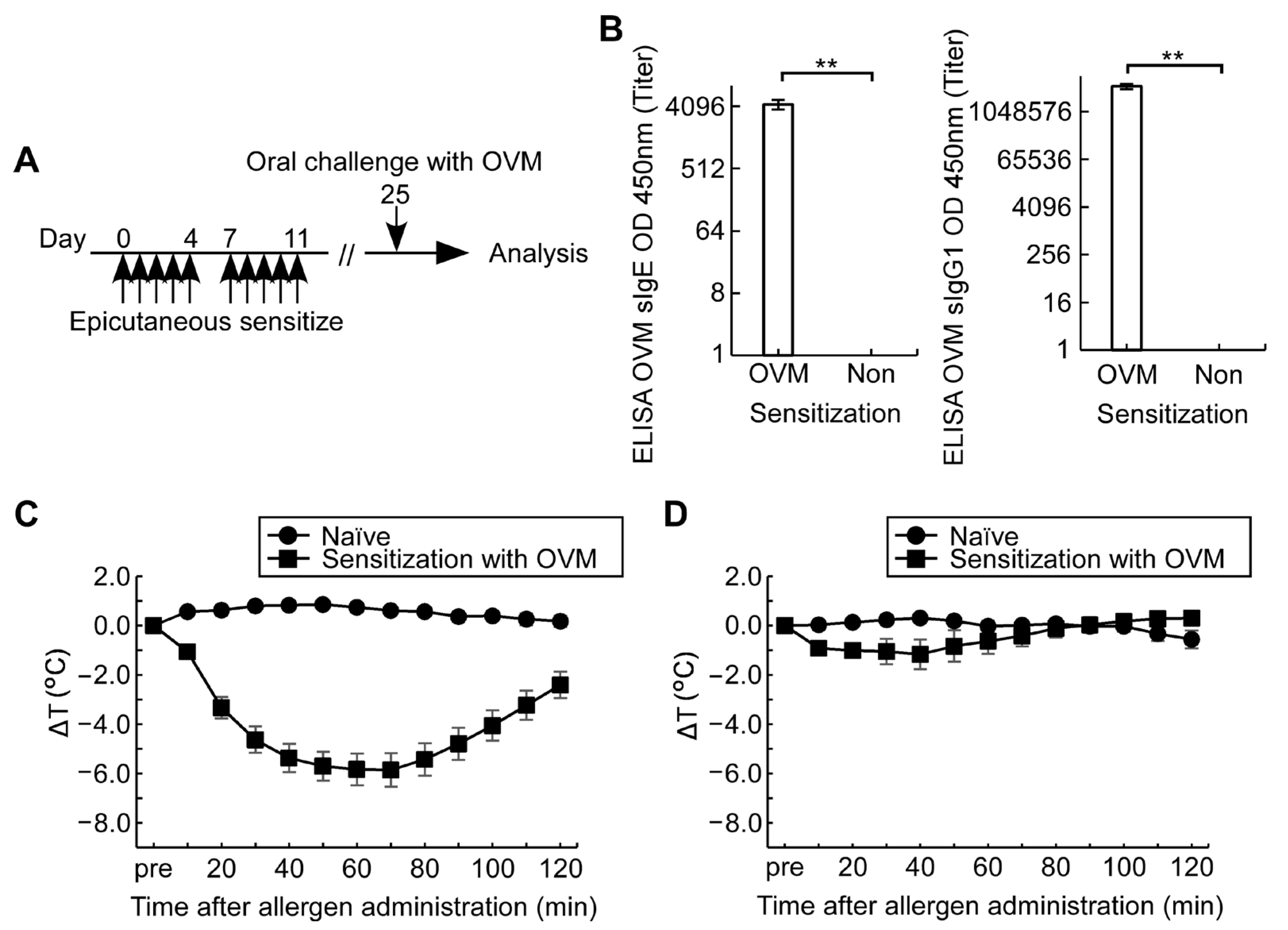
3.1. Epicutaneous OVM Sensitization Induces Severe Hypersensitivity Reactions by Intraperitoneal OVM Challenge but Not by Oral Challenge
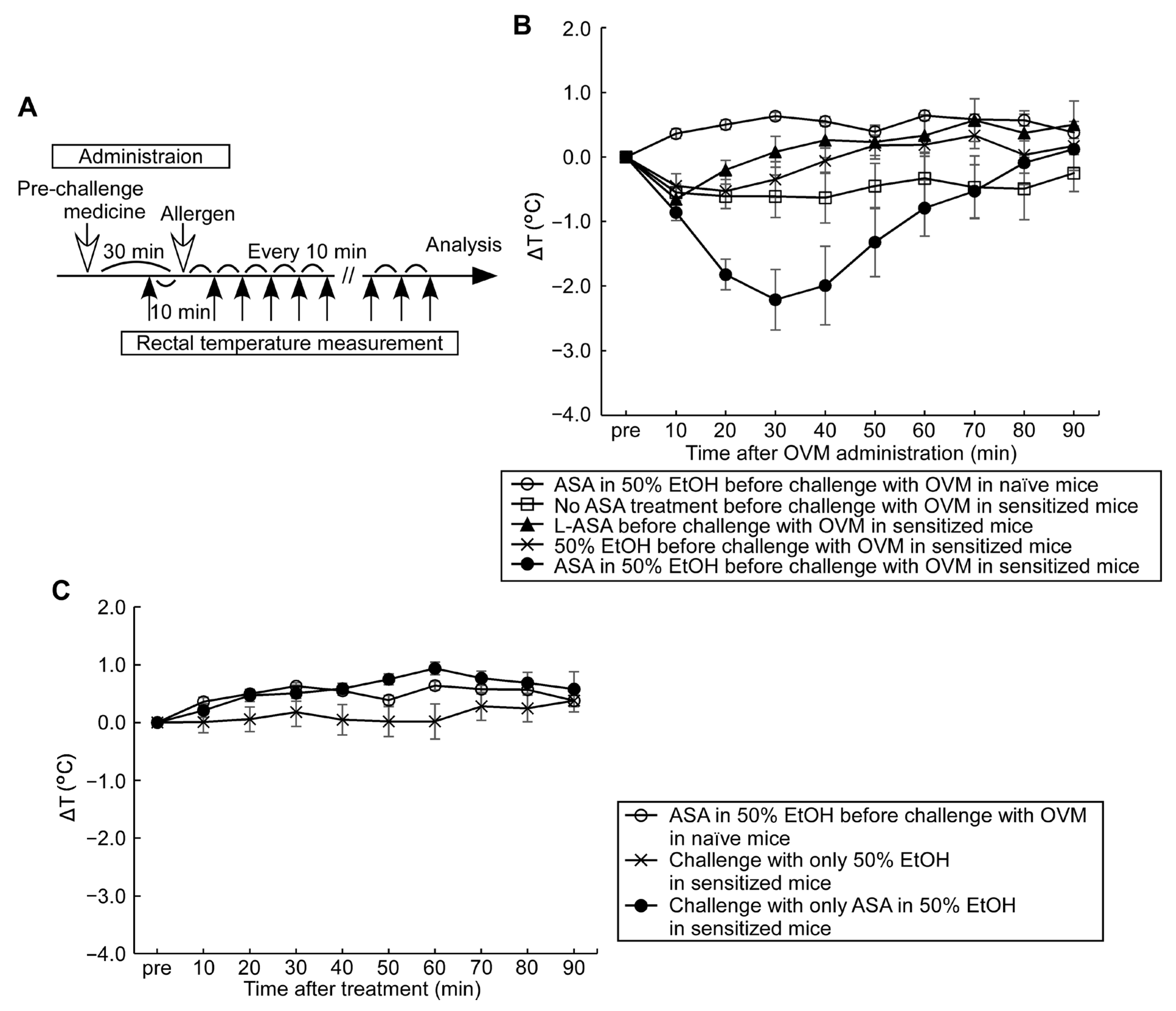
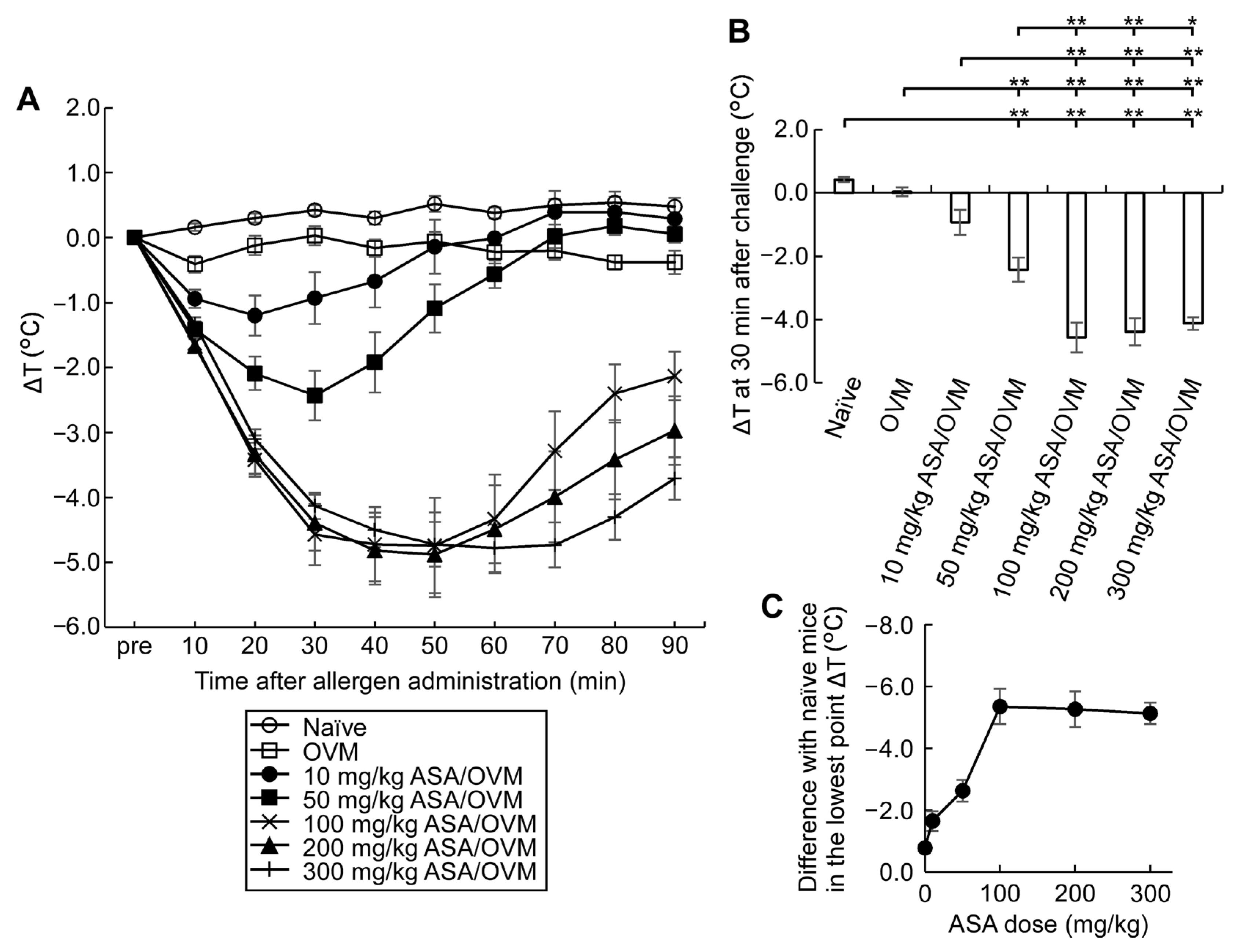
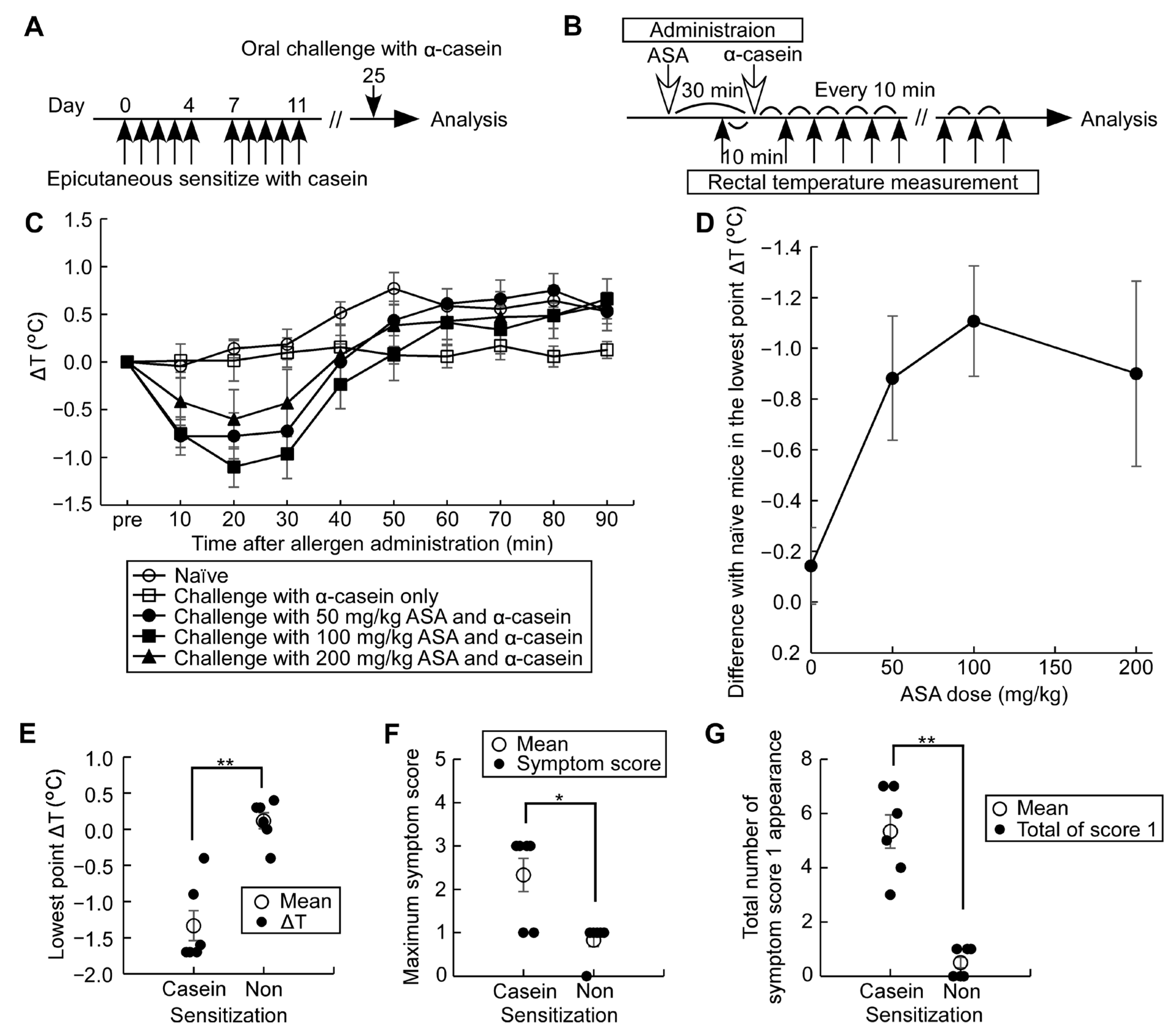
3.2. Compound Effect of Oral ASA Administration on Allergic Hypersensitivity Reactions
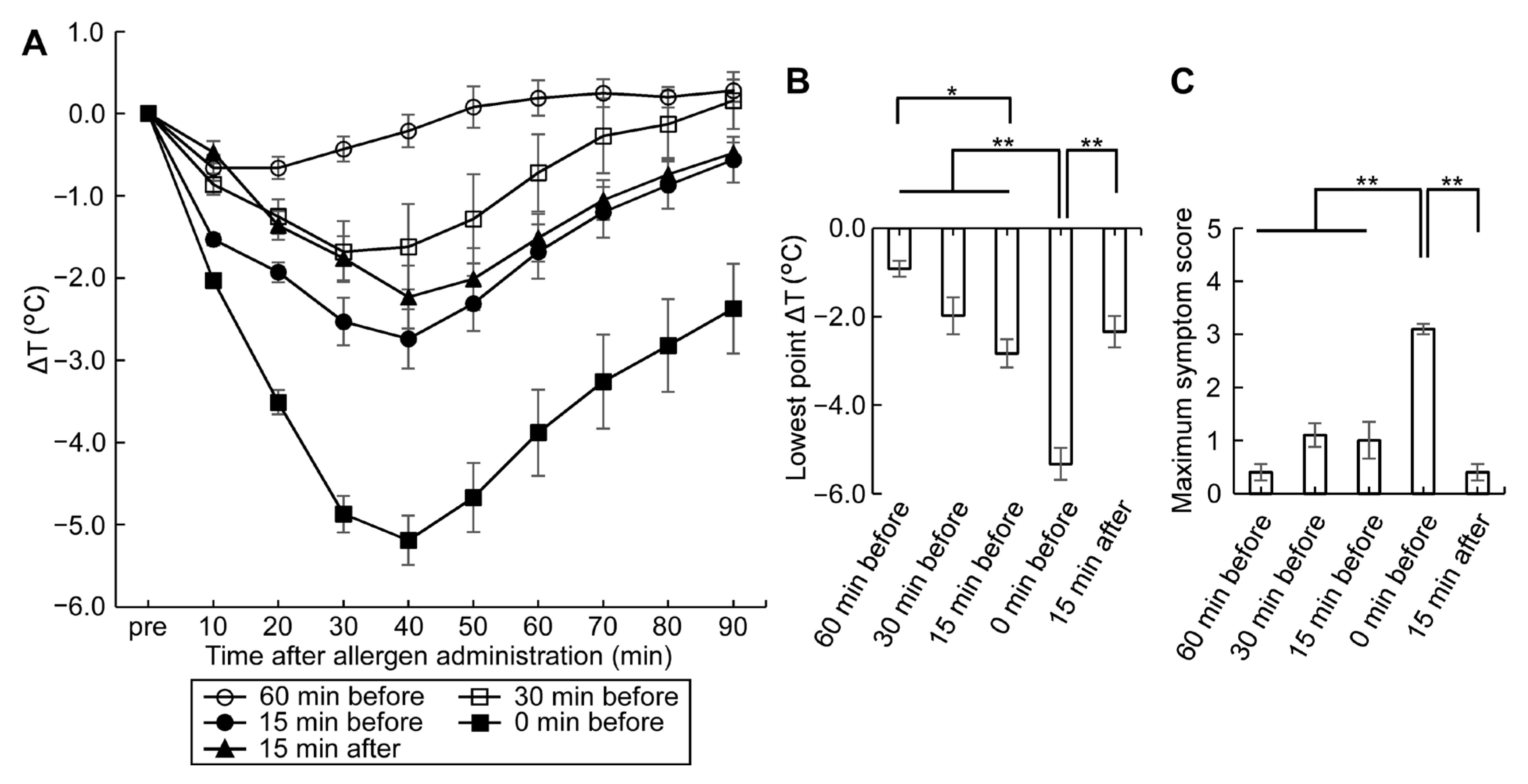
3.3. ASA Amplifies Symptom Score, a Useful Tool for Monitoring Allergic Hypersensitivity Reactions
3.4. High mMCPT-1 Blood Levels Induced by Combination of Oral OVM Challenge Plus ASA and Splenocyte Cytokine Productions
3.5. Establishment of an Adjuvant-Free Casein-Sensitized Cow’s Milk Allergy Mouse Model and Analysis of Allergic Hypersensitivity Reactions
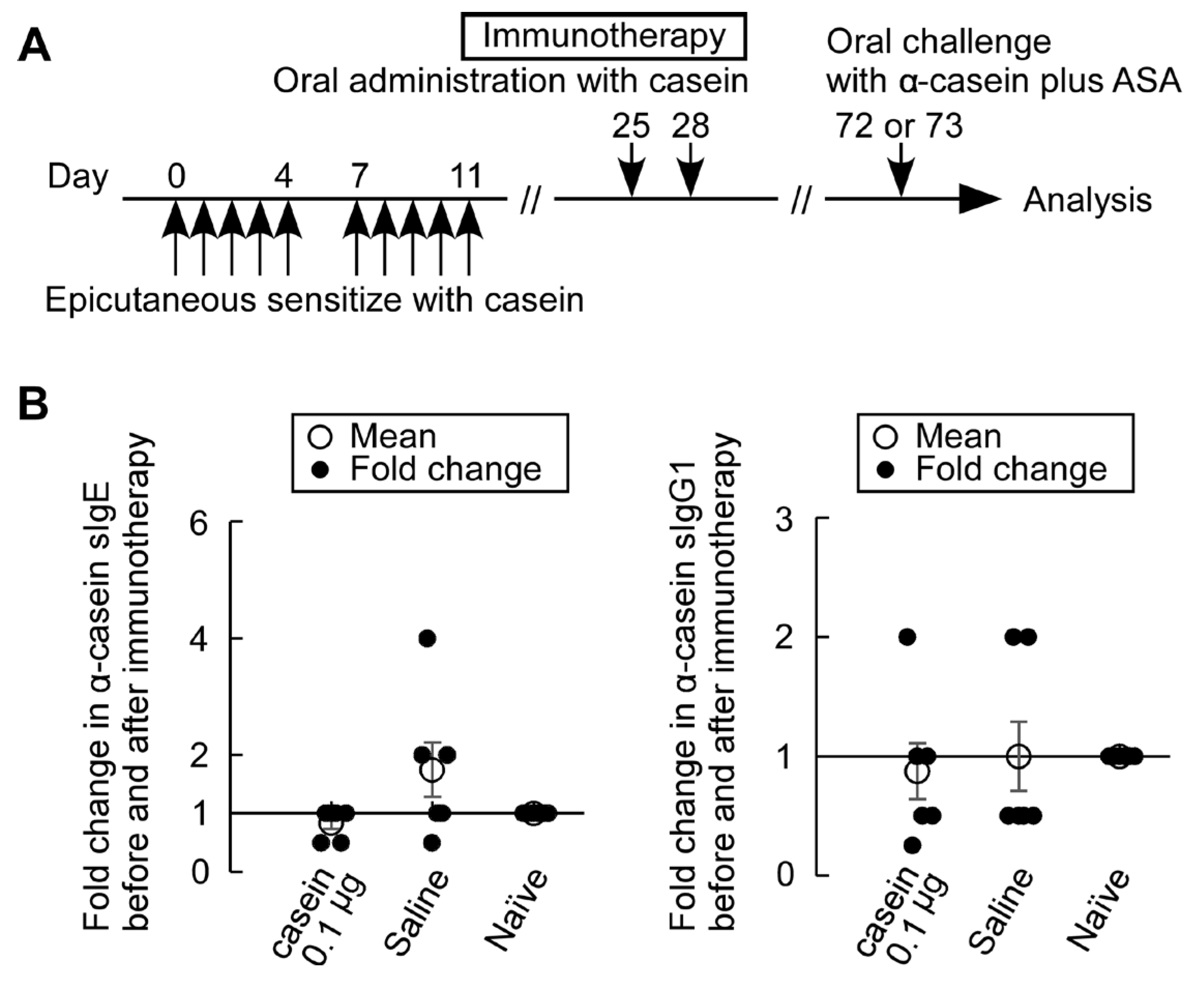
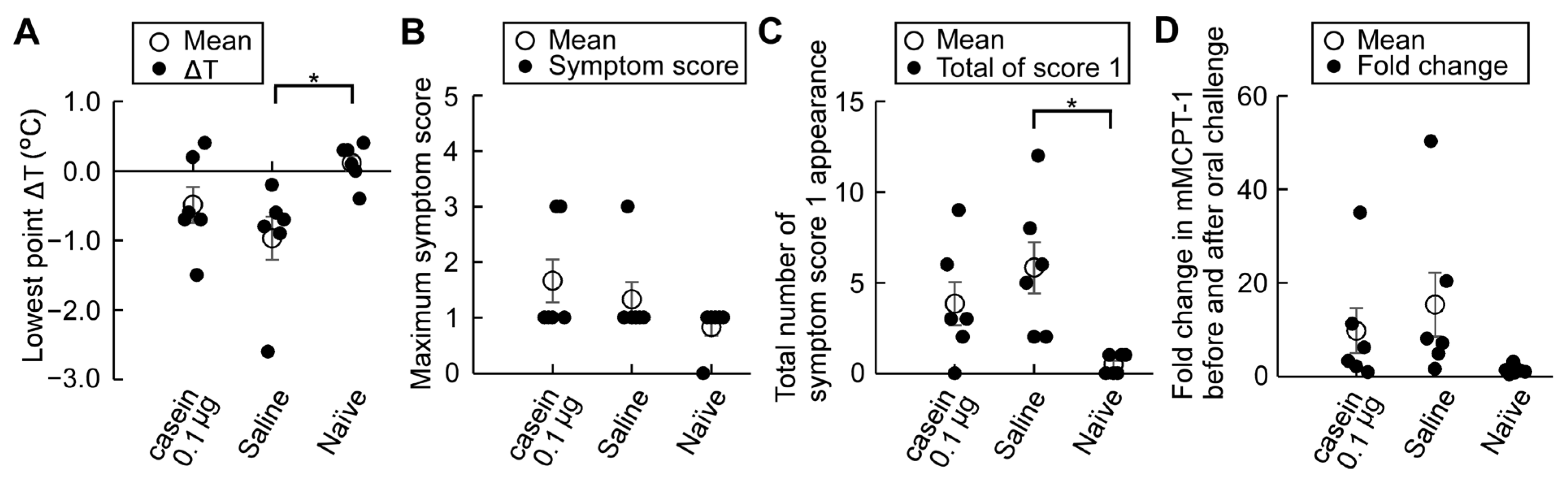
3.6. Casein Immunotherapy Monitoring
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sampath, V.; Sindher, S.B.; Alvarez Pinzon, A.M.; Nadeau, K.C. Can food allergy be cured? What are the future prospects? Allergy 2020, 75, 1316–1326. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/all.14116 (accessed on 6 May 2022). [CrossRef] [PubMed]
- McClain, S.; Bannon, G.A. Animal models of food allergy: Opportunities and barriers. Curr. Allergy Asthma Rep. 2006, 6, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Tsakok, T.; Marrs, T.; Mohsin, M.; Baron, S.; du Toit, G.; Till, S.; Flohr, C. Does atopic dermatitis cause food allergy? A systematic review. J. Allergy Clin. Immunol. 2016, 137, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Lack, G.; Fox, D.; Northstone, K.; Golding, J.; the Avon Longitudinal Study of Parents and Children Study Team. Factors Associated with the Development of Peanut Allergy in Childhood. N. Engl. J. Med. 2003, 348, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Natsume, O.; Kabashima, S.; Nakazato, J.; Yamamoto-Hanada, K.; Narita, M.; Kondo, M.; Saito, M.; Kishino, A.; Takimoto, T.; Inoue, E.; et al. Two-step egg introduction for prevention of egg allergy in high-risk infants with eczema (PETIT): A randomised, double-blind, placebo-controlled trial. Lancet 2016, 389, 276–286. [Google Scholar] [CrossRef]
- Larsen, J.M.; Bøgh, K.L. Animal models of allergen-specific immunotherapy in food allergy: Overview and opportunities. Clin. Exp. Allergy 2018, 48, 1255–1274. [Google Scholar] [CrossRef]
- Bowman, C.C.; Selgrade, M.K. Differences in Allergenic Potential of Food Extracts following Oral Exposure in Mice Reflect Differences in Digestibility: Potential Approaches to Safety Assessment. Toxicol. Sci. 2008, 102, 100–109. [Google Scholar] [CrossRef]
- Ladics, G.; Knippels, L.; Penninks, A.; Bannon, G.; Goodman, R.; Herouet-Guicheney, C. Review of animal models designed to predict the potential allergenicity of novel proteins in genetically modified crops. Regul. Toxicol. Pharmacol. 2010, 56, 212–224. [Google Scholar] [CrossRef]
- Sicherer, S.H. Epidemiology of food allergy. J. Allergy Clin. Immunol. 2011, 127, 594–602. [Google Scholar] [CrossRef]
- Osborne, N.J.; Koplin, J.J.; Martin, P.E.; Gurrin, L.C.; Lowe, A.J.; Matheson, M.C.; Ponsonby, A.-L.; Wake, M.; Tang, M.L.; Dharmage, S.C. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. J. Allergy Clin. Immunol. 2011, 127, 668–676.e2. [Google Scholar] [CrossRef]
- Li, X.-M.; Schofield, B.H.; Huang, C.-K.; Kleiner, G.I.; Sampson, H.A. A murine model of IgE-mediated cow’s milk hypersensitivity. J. Allergy Clin. Immunol. 1999, 103, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-M.; Serebrisky, D.; Lee, S.-Y.; Huang, C.-K.; Bardina, L.; Schofield, B.H.; Stanley, J.; Burks, A.; Bannon, G.A.; Sampson, H.A. A murine model of peanut anaphylaxis: T- and B-cell responses to a major peanut allergen mimic human responses. J. Allergy Clin. Immunol. 2000, 106, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Schülke, S.; Albrecht, M. Mouse models for food allergies: Where do we stand? Cells 2019, 8, 546. [Google Scholar] [CrossRef] [PubMed]
- Xian, M.; Wawrzyniak, P.; Rückert, B.; Duan, S.; Meng, Y.; Sokolowska, M.; Globinska, A.; Zhang, L.; Akdis, M.; Akdis, C.A. Anionic surfactants and commercial detergents decrease tight junction barrier integrity in human keratinocytes. J. Allergy Clin. Immunol. 2016, 138, 890–893.e9. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tan, G.; Eljaszewicz, A.; Meng, Y.; Wawrzyniak, P.; Acharya, S.; Altunbulakli, C.; Westermann, P.; Dreher, A.; Yan, L.; et al. Laundry detergents and detergent residue after rinsing directly disrupt tight junction barrier integrity in human bronchial epithelial cells. J. Allergy Clin. Immunol. 2019, 143, 1892–1903. [Google Scholar] [CrossRef]
- Kimoto, T.; Kim, H.; Sakai, S.; Takahashi, E.; Kido, H. Oral vaccination with influenza hemagglutinin combined with human pulmonary surfactant-mimicking synthetic adjuvant SF-10 induces efficient local and systemic immunity compared with nasal and subcutaneous vaccination and provides protective immunity in mice. Vaccine 2019, 37, 612–622. [Google Scholar] [CrossRef]
- Harada, S.; Horikawa, T.; Ashida, M.; Kamo, T.; Nishioka, E.; Ichihashi, M. Aspirin enhances the induction of type I allergic symptoms when combined with food and exercise in patients with food-dependent exercise-induced anaphylaxis. Br. J. Dermatol. 2001, 145, 336–339. [Google Scholar] [CrossRef]
- Feldweg, A.M. Food-Dependent, Exercise-Induced Anaphylaxis: Diagnosis and Management in the Outpatient Setting. J. Allergy Clin. Immunol. Pract. 2017, 5, 283–288. [Google Scholar] [CrossRef]
- Matsuo, H.; Kaneko, S.; Tsujino, Y.; Honda, S.; Kohno, K.; Takahashi, H.; Mihara, S.; Hide, M.; Aburatani, K.; Honjoh, T.; et al. Effects of non-steroidal anti-inflammatory drugs (NSAIDs) on serum allergen levels after wheat ingestion. J. Dermatol. Sci. 2009, 53, 241–243. [Google Scholar] [CrossRef]
- Yokooji, T.; Fukushima, T.; Hamura, K.; Ninomiya, N.; Ohashi, R.; Taogoshi, T.; Matsuo, H. Intestinal absorption of the wheat allergen gliadin in rats. Allergol. Int. 2019, 68, 247–253. [Google Scholar] [CrossRef]
- Fuc, E.; Złotkowska, D.; Stachurska, E.; Wróblewska, B. Immunoreactive properties of alpha-casein and kappa-casein: Ex vivo and in vivo studies. J. Dairy Sci. 2018, 101, 10703–10713. [Google Scholar] [CrossRef] [PubMed]
- Wróblewska, B.; Kaliszewska-Suchodoła, A.; Fuc, E.; Markiewicz, L.; Ogrodowczyk, A.; Złotkowska, D.; Wasilewska, E. Effect of Low-Immunogenic Yogurt Drinks and Probiotic Bacteria on Immunoreactivity of Cow’s Milk Proteins and Tolerance Induction—In Vitro and In Vivo Studies. Nutrients 2020, 12, 3390. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.A.; Martos, G.; Wang, W.; Nowak-Węgrzyn, A.; Berin, M.C. Oral immunotherapy induces local protective mechanisms in the gastrointestinal mucosa. J Allergy Clin. Immunol. 2012, 129, 1579–1587.e1. [Google Scholar] [CrossRef]
- Akdis, C.A. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat. Rev. Immunol. 2021, 21, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Bartnikas, L.M.; Gurish, M.F.; Burton, O.T.; Leisten, S.; Janssen, E.; Oettgen, H.C.; Beaupré, J.; Lewis, C.N.; Austen, K.F.; Schulte, S.; et al. Epicutaneous sensitization results in IgE-dependent intestinal mast cell expansion and food-induced anaphylaxis. J. Allergy Clin. Immunol. 2013, 131, 451–460.e6. [Google Scholar] [CrossRef]
- Morin, S.; Bernard, H.; Przybylski-Nicaise, L.; Corthier, G.; Rabot, S.; Wal, J.M.; Hazebrouck, S. Allergenic and immunogenic potential of cow’s milk ꞵ-lactoglobulin and caseins evidenced without adjuvant in germ-free mice. Mol. Nutr. Food Res. 2011, 55, 1700–1707. [Google Scholar] [CrossRef]
- Babu, K.S.; Salvi, S.S. Aspirin and Asthma. Chest 2000, 118, 1470–1476. [Google Scholar] [CrossRef]
- Sakakibara, H.; Tsuda, M.; Suzuki, M.; Handa, M.; Saga, T.; Umeda, H.; Suetsugu, S.; Konishi, Y. A new method of diagnosis of aspirin-induced asthma by inhalation test with water-soluble aspirin (aspirin DL-lysine, Venopirin®). Jap. J. Thorac. Dis. 1988, 26, 275–283. [Google Scholar] [CrossRef]
- Phillips, G.; Foord, R.; Holgate, S. Inhaled lysine-aspirin as a bronchoprovocation procedure in aspirin-sensitive asthma: Its repeatability, absence of a late-phase reaction, and the role of histamine. J. Allergy Clin. Immunol. 1989, 84, 232–241. [Google Scholar] [CrossRef]
- Park, H.S. Early and late onset asthmatic responses following lysine-aspirin inhalation in aspirin-sensitive asthmatic patients. Clin. Exp. Allergy 1995, 25, 38–40. [Google Scholar] [CrossRef]
- Bayer Yakuhin, Ltd. Bayaspirin Tablets 100 mg Pharmaceutical Interview Form, 19th ed.; Bayer Yakuhin, Ltd.: Osaka, Japan, 2022; pp. 28–36. Available online: https://www.pmda.go.jp/PmdaSearch/iyakuSearch (accessed on 5 September 2022).
- Graham, D.Y.; Smith, J.L. Aspirin and the stomach. Ann. Intern. Med. 1986, 104, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Bretagne, J.F.; Feuillu, A.; Gosselin, M.; Gastard, J. Aspirin and gastroduodenal toxicity. A double-blind endoscopic study of the effects of placebo, aspirin and lysine acetylsalicylate in healthy subjects. Gastroenterol. Clin. Biol 1984, 8, 28–32. Available online: https://pubmed.ncbi.nlm.nih.gov/6698337/ (accessed on 5 September 2022). [PubMed]
- Yokooji, T.; Nouma, H.; Matsuo, H. Characterization of Ovalbumin Absorption Pathways in the Rat Intestine, Including the Effects of Aspirin. Biol. Pharm. Bull. 2014, 37, 1359–1365. [Google Scholar] [CrossRef] [PubMed]
- Longo, G.; Barbi, E.; Berti, I.; Meneghetti, R.; Pittalis, A.; Ronfani, L.; Ventura, A. Specific oral tolerance induction in children with very severe cow’s milk-induced reactions. J. Allergy Clin. Immunol. 2008, 121, 343–347. [Google Scholar] [CrossRef]
- Meglio, P.; Bartone, E.; Plantamura, M.; Arabito, E.; Giampietro, P.G. A protocol for oral desensitization in children with IgE-mediated cow’s milk allergy. Allergy 2004, 59, 980–987. [Google Scholar] [CrossRef]
- Staden, U.; Blumchen, K.; Blankenstein, N.; Dannenberg, N.; Ulbricht, H.; Dobberstein, K.; Ziegert, M.; Niggemann, B.; Wahn, U.; Beyer, K. Rush oral immunotherapy in children with persistent cow’s milk allergy. J. Allergy Clin. Immunol. 2008, 122, 418–419. [Google Scholar] [CrossRef]
- Staden, U.; Rolinck-Werninghaus, C.; Brewe, F.; Wahn, U.; Niggemann, B.; Beyer, K. Specific oral tolerance induction in food allergy in children: Efficacy and clinical patterns of reaction. Allergy 2007, 62, 1261–1269. [Google Scholar] [CrossRef]
- Skripak, J.M.; Nash, S.D.; Rowley, H.; Brereton, N.H.; Oh, S.; Hamilton, R.G.; Matsui, E.C.; Burks, A.W.; Wood, R.A. A randomized, double-blind, placebo-controlled study of milk oral immunotherapy for cow’s milk allergy. J. Allergy Clin. Immunol. 2008, 122, 1154–1160. [Google Scholar] [CrossRef]
- Maeda, M.; Imai, T.; Ishikawa, R.; Nakamura, T.; Kamiya, T.; Kimura, A.; Fujita, S.; Akashi, K.; Tada, H.; Morita, H.; et al. Effect of oral immunotherapy in children with milk allergy: The ORIMA study. Allergol. Int. 2020, 70, 223–228. [Google Scholar] [CrossRef]
- Calatayud, C.M.; García, A.M.; Aragonés, A.M.; de la Hoz Caballer, B. Safety and efficacy profile and immunological changes associated with oral immunotherapy for IgE-mediated cow’s milk allergy in children: Systematic review and meta-analysis. J. Investig. Allergol. Clin. Immunol. 2014, 24, 298–307. Available online: https://pubmed.ncbi.nlm.nih.gov/25345300/ (accessed on 5 September 2022).
- Noti, M.; Kim, B.S.; Siracusa, M.C.; Rak, G.D.; Kubo, M.; Moghaddam, A.E.; Sattentau, Q.A.; Comeau, M.R.; Spergel, J.M.; Artis, D. Exposure to food allergens through inflamed skin promotes intestinal food allergy through the thymic stromal lymphopoietin–basophil axis. J. Allergy Clin. Immunol. 2014, 133, 1390–1399.e6. [Google Scholar] [CrossRef] [PubMed]
- Tordesillas, L.; Goswami, R.; Benedé, S.; Grishina, G.; Dunkin, D.; Järvinen, K.M.; Maleki, S.J.; Sampson, H.A.; Berin, M.C. Skin exposure promotes a Th2-dependent sensitization to peanut allergens. J. Clin. Investig. 2014, 124, 4965–4975. [Google Scholar] [CrossRef] [PubMed]


| Cytokine 1 | OVM Stimulation | OVM-Sensitized Mice (n = 6) | Naïve Mice (n = 6) |
|---|---|---|---|
| IFN-γ | − | 1286.2 ± 337.5 | 2197.7 ± 1217.5 |
| + | 4818.3 ± 1023.4 | 1570.5 ± 919.3 | |
| IL-4 | − | 17.3 ± 3.2 | 11.0 ± 2.5 |
| + | 26.0 ± 1.8 ** | 7.5 ± 2.7 | |
| IL-5 | − | 7.2 ± 2.2 | 1.0 ± 0.6 |
| + | 378.5 ± 82.1 ** | 1.8 ± 1.1 | |
| IL-6 | − | 147.2 ± 28.4 | 72.4 ± 25.8 |
| + | 759.5 ± 133.0 ** | 58.5 ± 22.1 | |
| IL-9 | − | 74.9 ± 14.8 | 54.4 ± 28.0 |
| + | 800.8 ± 135.5 ** | 50.5 ± 26.6 | |
| IL-13 | − | 33.6 ± 5.1 | 26.7 ± 11.2 |
| + | 314.8 ± 55.1 ** | 18.5 ± 8.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kameda, K.; Takahashi, E.; Kimoto, T.; Morita, R.; Sakai, S.; Nagao, M.; Fujisawa, T.; Kido, H. A Murine Model of Food Allergy by Epicutaneous Adjuvant-Free Allergen Sensitization Followed by Oral Allergen Challenge Combined with Aspirin for Enhanced Detection of Hypersensitivity Manifestations and Immunotherapy Monitoring. Nutrients 2023, 15, 757. https://doi.org/10.3390/nu15030757
Kameda K, Takahashi E, Kimoto T, Morita R, Sakai S, Nagao M, Fujisawa T, Kido H. A Murine Model of Food Allergy by Epicutaneous Adjuvant-Free Allergen Sensitization Followed by Oral Allergen Challenge Combined with Aspirin for Enhanced Detection of Hypersensitivity Manifestations and Immunotherapy Monitoring. Nutrients. 2023; 15(3):757. https://doi.org/10.3390/nu15030757
Chicago/Turabian StyleKameda, Keiko, Etsuhisa Takahashi, Takashi Kimoto, Ryoko Morita, Satoko Sakai, Mizuho Nagao, Takao Fujisawa, and Hiroshi Kido. 2023. "A Murine Model of Food Allergy by Epicutaneous Adjuvant-Free Allergen Sensitization Followed by Oral Allergen Challenge Combined with Aspirin for Enhanced Detection of Hypersensitivity Manifestations and Immunotherapy Monitoring" Nutrients 15, no. 3: 757. https://doi.org/10.3390/nu15030757
APA StyleKameda, K., Takahashi, E., Kimoto, T., Morita, R., Sakai, S., Nagao, M., Fujisawa, T., & Kido, H. (2023). A Murine Model of Food Allergy by Epicutaneous Adjuvant-Free Allergen Sensitization Followed by Oral Allergen Challenge Combined with Aspirin for Enhanced Detection of Hypersensitivity Manifestations and Immunotherapy Monitoring. Nutrients, 15(3), 757. https://doi.org/10.3390/nu15030757





