The Association between Water Consumption and Hyperuricemia and Its Relation with Early Arterial Aging in Middle-Aged Lithuanian Metabolic Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Assessment of the Study Population
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ye, C.; Huang, X.; Wang, R.; Halimulati, M.; Aihemaitijiang, S.; Zhang, Z. Dietary Inflammatory Index and the Risk of Hyperuricemia: A Cross-Sectional Study in Chinese Adult Residents. Nutrients 2021, 13, 4504. [Google Scholar] [CrossRef] [PubMed]
- Benn, C.L.; Dua, P.; Gurrell, R.; Loudon, P.; Pike, A.; Storer, R.I.; Vangjeli, C. Physiology of Hyperuricemia and Urate-Lowering Treatments. Front. Med. 2018, 5, 160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-Z. Why Does Hyperuricemia Not Necessarily Induce Gout? Biomolecules 2021, 11, 280. [Google Scholar] [CrossRef] [PubMed]
- Borghi, C.; Rosei, E.A.; Bardin, T.; Dawson, J.; Dominiczak, A.; Kielstein, J.T.; Manolis, A.J.; Perez-Ruiz, F.; Mancia, G. Serum uric acid and the risk of cardiovascular and renal disease. J. Hypertens. 2015, 33, 1729–1741. [Google Scholar] [CrossRef]
- Billiet, L.; Doaty, S.; Katz, J.D.; Velasquez, M.T. Review of Hyperuricemia as New Marker for Metabolic Syndrome. ISRN Rheumatol. 2014, 2014, 852954. [Google Scholar] [CrossRef]
- Yokose, C.; McCormick, N.; Choi, H.K. The role of diet in hyperuricemia and gout. Curr. Opin. Rheumatol. 2021, 33, 135–144. [Google Scholar] [CrossRef]
- Mazzali, M.; Hughes, J.; Kim, Y.-G.; Jefferson, J.A.; Kang, D.-H.; Gordon, K.L.; Lan, H.Y.; Kivlighn, S.; Johnson, R. Elevated Uric Acid Increases Blood Pressure in the Rat by a Novel Crystal-Independent Mechanism. Hypertension 2001, 38, 1101–1106. [Google Scholar] [CrossRef]
- Castro-Barquero, S.; Ruiz-León, A.M.; Sierra-Pérez, M.; Estruch, R.; Casas, R. Dietary Strategies for Metabolic Syndrome: A Comprehensive Review. Nutrients 2020, 12, 2983. [Google Scholar] [CrossRef]
- Yu, K.-H.; See, L.-C.; Huang, Y.-C.; Yang, C.-H.; Sun, J.-H. Dietary Factors Associated with Hyperuricemia in Adults. Semin. Arthritis Rheum. 2008, 37, 243–250. [Google Scholar] [CrossRef]
- Danve, A.; Sehra, S.T.; Neogi, T. Role of diet in hyperuricemia and gout. Best Pr. Res. Clin. Rheumatol. 2021, 35, 101723. [Google Scholar] [CrossRef]
- Li, R.; Yu, K.; Li, C. Dietary factors and risk of gout and hyperuricemia: A meta-analysis and systematic review. Asia Pac. J. Clin. Nutr. 2018, 27, 1344–1356. [Google Scholar] [CrossRef]
- Yanai, H.; Adachi, H.; Hakoshima, M.; Katsuyama, H. Molecular Biological and Clinical Understanding of the Pathophysiology and Treatments of Hyperuricemia and Its Association with Metabolic Syndrome, Cardiovascular Diseases and Chronic Kidney Disease. Int. J. Mol. Sci. 2021, 22, 9221. [Google Scholar] [CrossRef]
- Tack, I. Effects of Water Consumption on Kidney Function and Excretion. Nutr. Today 2010, 45, S37–S40. [Google Scholar] [CrossRef]
- Kakutani-Hatayama, M.; Kadoya, M.; Okazaki, H.; Kurajoh, M.; Shoji, T.; Koyama, H.; Tsutsumi, Z.; Moriwaki, Y.; Namba, M.; Yamamoto, T. Nonpharmacological Management of Gout and Hyperuricemia: Hints for Better Lifestyle. Am. J. Lifestyle Med. 2017, 11, 321–329. [Google Scholar] [CrossRef]
- Masot, O.; Miranda, J.; Santamaría, A.; Pueyo, E.P.; Pascual, A.; Botigué, T. Fluid Intake Recommendation Considering the Physiological Adaptations of Adults over 65 Years: A Critical Review. Nutrients 2020, 12, 3383. [Google Scholar] [CrossRef]
- Volkert, D.; Beck, A.M.; Cederholm, T.; Cruz-Jentoft, A.; Goisser, S.; Hooper, L.; Kiesswetter, E.; Maggio, M.; Raynaud-Simon, A.; Sieber, C.C.; et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin. Nutr. 2019, 38, 10–47. [Google Scholar] [CrossRef]
- Zizza, C.A.; Ellison, K.J.; Wernette, C.M. Total Water Intakes of Community-Living Middle-Old and Oldest-Old Adults. J. Gerontol. Ser. A 2009, 64A, 481–486. [Google Scholar] [CrossRef]
- Ribeiro, A.G.; Mill, J.G.; Cade, N.V.; Velasquez-Melendez, G.; Matos, S.M.A.; Molina, M.D.C.B. Associations of Dairy Intake with Arterial Stiffness in Brazilian Adults: The Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Nutrients 2018, 10, 701. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Choi, H.Y.; Kim, S.-H.; Choi, A.R.; Kim, S.G.; Kim, H.; Lee, J.E.; Kim, H.J.; Park, H.C. Hyperuricemia and risk of increased arterial stiffness in healthy women based on health screening in Korean population. PLoS ONE 2017, 12, e0180406. [Google Scholar] [CrossRef]
- Laurent, S.; Boutouyrie, P. Arterial Stiffness and Hypertension in the Elderly. Front. Cardiovasc. Med. 2020, 7, 544302. [Google Scholar] [CrossRef] [PubMed]
- El Feghali, R.; Topouchian, J.; Pannier, B.; Asmar, R. Ageing and blood pressure modulate the relationship between metabolic syndrome and aortic stiffness in never-treated essential hypertensive patients. A comparative study. Diabetes Metab. 2007, 33, 183–188. [Google Scholar] [CrossRef] [PubMed]
- AlGhatrif, M.; Strait, J.B.; Morrell, C.H.; Canepa, M.; Wright, J.; Elango, P.; Scuteri, A.; Najjar, S.S.; Ferrucci, L.; Lakatta, E. Longitudinal Trajectories of Arterial Stiffness and the Role of Blood Pressure. Hypertension 2013, 62, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Pucci, G.; Alcidi, R.; Tap, L.; Battista, F.; Mattace-Raso, F.; Schillaci, G. Sex- and gender-related prevalence, cardiovascular risk and therapeutic approach in metabolic syndrome: A review of the literature. Pharmacol. Res. 2017, 120, 34–42. [Google Scholar] [CrossRef]
- Ogola, B.O.; Zimmerman, M.A.; Clark, G.L.; Abshire, C.M.; Gentry, K.M.; Miller, K.S.; Lindsey, S.H. New insights into arterial stiffening: Does sex matter? Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1073–H1087. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Morton, J.S.; Davidge, S.T. Mechanisms of Estrogen Effects on the Endothelium: An Overview. Can. J. Cardiol. 2014, 30, 705–712. [Google Scholar] [CrossRef]
- Kampe, R.T.; Janssen, M.; van Durme, C.; Jansen, T.L.; Boonen, A. Sex Differences in the Clinical Profile among Patients with Gout: Cross-sectional Analyses of an Observational Study. J. Rheumatol. 2021, 48, 286–292. [Google Scholar] [CrossRef]
- DuPont, J.J.; Kenney, R.M.; Patel, A.R.; Jaffe, I.Z. Sex differences in mechanisms of arterial stiffness. Br. J. Pharmacol. 2019, 176, 4208–4225. [Google Scholar] [CrossRef]
- Hougaku, H.; Fleg, J.L.; Najjar, S.S.; Lakatta, E.G.; Harman, S.M.; Blackman, M.R.; Metter, E.J. Relationship between androgenic hormones and arterial stiffness, based on longitudinal hormone measurements. Am. J. Physiol. Metab. 2006, 290, E234–E242. [Google Scholar] [CrossRef]
- Park, K.-H.; Park, W.J. Endothelial Dysfunction: Clinical Implications in Cardiovascular Disease and Therapeutic Approaches. J. Korean Med. Sci. 2015, 30, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Davignon, J.; Ganz, P. Role of endothelial dysfunction in atherosclerosis. Circulation 2004, 109, III27–III32. [Google Scholar] [CrossRef] [PubMed]
- Maruhashi, T.; Hisatome, I.; Kihara, Y.; Higashi, Y. Hyperuricemia and endothelial function: From molecular background to clinical perspectives. Atherosclerosis 2018, 278, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Yoon, Y.; Lee, K.; Hien, T.T.; Kang, K.W.; Kim, K.; Lee, J.; Lee, M.; Lee, S.M.; Kang, D.; et al. Uric acid induces endothelial dysfunction by vascular insulin resistance associated with the impairment of nitric oxide synthesis. FASEB J. 2014, 28, 3197–3204. [Google Scholar] [CrossRef]
- Khosla, U.M.; Zharikov, S.; Finch, J.L.; Nakagawa, T.; Roncal, C.; Mu, W.; Krotova, K.; Block, E.R.; Prabhakar, S.; Johnson, R.J. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005, 67, 1739–1742. [Google Scholar] [CrossRef]
- Lang, F.; Guelinckx, I.; Lemetais, G.; Melander, O. Two Liters a Day Keep the Doctor away? Considerations on the Pathophysiology of Suboptimal Fluid Intake in the Common Population. Kidney Blood Press. Res. 2017, 42, 483–494. [Google Scholar] [CrossRef]
- Drewnowski, A.; Rehm, C.D.; Constant, F. Water and beverage consumption among adults in the United States: Cross-sectional study using data from NHANES 2005–2010. BMC Public Health 2013, 13, 1068. [Google Scholar] [CrossRef]
- Ferreira-Pêgo, C.; Guelinckx, I.; Moreno, L.A.; Kavouras, S.A.; Gandy, J.; Martinez, H.; Bardosono, S.; Abdollahi, M.; Nasseri, E.; Jarosz, A.; et al. Total fluid intake and its determinants: Cross-sectional surveys among adults in 13 countries worldwide. Eur. J. Nutr. 2015, 54, 35–43. [Google Scholar] [CrossRef]
- Dore, M.P.; Parodi, G.; Portoghese, M.; Errigo, A.; Pes, G.M. Water Quality and Mortality from Coronary Artery Disease in Sardinia: A Geospatial Analysis. Nutrients 2021, 13, 2858. [Google Scholar] [CrossRef]
- Kanbara, A.; Miura, Y.; Hyogo, H.; Chayama, K.; Seyama, I. Effect of urine pH changed by dietary intervention on uric acid clearance mechanism of pH-dependent excretion of urinary uric acid. Nutr. J. 2012, 11, 39. [Google Scholar] [CrossRef]
- Paz-Graniel, I.; Becerra-Tomás, N.; Babio, N.; Serra-Majem, L.; Vioque, J.; Zomeño, M.D.; Corella, D.; Pintó, X.; Bueno-Cavanillas, A.; Tur, J.A.; et al. Baseline drinking water consumption and changes in body weight and waist circumference at 2-years of follow-up in a senior Mediterranean population. Clin. Nutr. 2021, 40, 3982–3991. [Google Scholar] [CrossRef]
- Stookey, J.D.; Constant, F.; Popkin, B.; Gardner, C.D. Drinking Water Is Associated with Weight Loss in Overweight Dieting Women Independent of Diet and Activity. Obesity 2008, 16, 2481–2488. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Luo, K.-L.; Lin, X.-X.; Gao, X.; Ni, R.-X.; Wang, S.-B.; Tian, X.-L. Regional distribution of longevity population and chemical characteristics of natural water in Xinjiang, China. Sci. Total. Environ. 2014, 473–474, 54–62. [Google Scholar] [CrossRef]
- Cotruvo, J.; Bartram, J. (Eds.) Calcium and Magnesium in Drinking-Water: Public Health Significance; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Fang, C.-H.; Tsai, C.-C.; Shyong, Y.-J.; Yang, C.-T.; Li, K.-Y.; Lin, Y.-W.; Chang, K.-C.; Wang, M.-H.; Wu, T.-M.; Lin, F.-H. Effects of Highly Oxygenated Water in a Hyperuricemia Rat Model. J. Health Eng. 2020, 2020, 1323270. [Google Scholar] [CrossRef]
- Wei, Y.; Zhu, J.; Wetzstein, S.A. Plasma and water fluoride levels and hyperuricemia among adolescents: A cross-sectional study of a nationally representative sample of the United States for 2013–2016. Ecotoxicol. Environ. Saf. 2020, 208, 111670. [Google Scholar] [CrossRef]
- Schorr, U.; Distler, A.; Sharma, A.M. Effect of sodium chloride- and sodium bicarbonate-rich mineral water on blood pressure and metabolic parameters in elderly normotensive individuals: A randomized double-blind crossover trial. J. Hypertens. 1996, 14, 131–135. [Google Scholar]
- Del Campo, L.; Sánchez-López, A.; Salaices, M.; von Kleeck, R.A.; Expósito, E.; González-Gómez, C.; Cussó, L.; Guzmán-Martínez, G.; Ruiz-Cabello, J.; Desco, M.; et al. Vascular smooth muscle cell-specific progerin expression in a mouse model of Hutchinson–Gilford progeria syndrome promotes arterial stiffness: Therapeutic effect of dietary nitrite. Aging Cell 2019, 18, e12936. [Google Scholar] [CrossRef]
- Gebremichael, S.G.; Yismaw, E.; Tsegaw, B.D.; Shibeshi, A.D. Determinants of water source use, quality of water, sanitation and hygiene perceptions among urban households in North-West Ethiopia: A cross-sectional study. PLoS ONE 2021, 16, e0239502. [Google Scholar] [CrossRef]
- May, M.; Jordan, J. The osmopressor response to water drinking. Am. J. Physiol. Integr. Comp. Physiol. 2011, 300, R40–R46. [Google Scholar] [CrossRef]
| Variable | Men (N = 241) | Women (N = 420) | Overall | ||||
|---|---|---|---|---|---|---|---|
| sUA Increased (N = 70) | sUA Normal (N = 171) | p-Value | sUA Increased (N = 144) | sUA Normal (N = 276) | p-Value | ||
| Age, years | <0.05 | 0.51 | |||||
| Median (Q1, Q3) | 46.0 (43.0, 48.0) | 48.0 (44.0, 51.0) | 58.0 (54.0, 61.0) | 58.0 (54.0, 61.0) | 54.0 (49.0, 59.0) | ||
| BMI, kg/m2 | <0.05 | <0.05 | |||||
| Median (Q1, Q3) | 31.7 (30.1, 35.1) | 30.5 (28.1, 32.8) | 33.6 (30.1, 37.5) | 30.5 (27.4, 33.6) | 31.2 (28.4, 34.3) | ||
| Circumference of waist, cm | <0.001 | 0.06 | |||||
| Median (Q1, Q3) | 109 (104, 113) | 105 (102, 110) | 105 (97.0, 112) | 97.0 (91.0, 105) | 103 (95.0, 109) | ||
| SBP, mmHg | 0.05 | 0.04 | |||||
| Median (Q1, Q3) | 139 (128, 148) | 133 (126, 142) | 137 (130, 146) | 135 (123, 145) | 135 (126, 145) | ||
| sUA, µmol/L | <0.05 | <0.05 | |||||
| Median (Q1, Q3) | 481 (457, 520) | 366 (328, 393) | 397 (378, 447) | 291 (259, 324) | 352 (298, 404) | ||
| Median (Q1, Q3) | 1.04 (0.913, 1.19) | 1.05 (0.910, 1.25) | 1.25 (1.09, 1.46) | 1.35 (1.19, 1.56) | 1.23 (1.04, 1.44) | ||
| LDL cholesterol, mmol/L | 0.37 | 0.36 | |||||
| Median (Q1, Q3) | 3.81 (3.14, 4.28) | 3.60 (2.92, 4.25) | 3.67 (3.06, 4.36) | 3.89 (3.01, 4.83) | 3.74 (2.98, 4.49) | ||
| TG, mmol/L | 0.44 | <0.001 | |||||
| Median (Q1, Q3) | 2.39 (1.59, 2.83) | 1.82 (1.26, 2.53) | 1.79 (1.37, 2.43) | 1.52 (1.07, 2.03) | 1.73 (1.24, 2.39) | ||
| Fasting glucose, mmol/L | 0.04 | 0.39 | |||||
| Median (Q1, Q3) | 5.98 (5.61, 6.51) | 6.03 (5.63, 6.49) | 6.13 (5.79, 6.94) | 6.00 (5.66, 6.54) | 6.06 (5.66, 6.60) | ||
| hs-CRP, mg/L | 0.09 | <0.001 | |||||
| Median (Q1, Q3) | 1.99 (1.16, 3.91) | 1.46 (0.783, 2.35) | 2.61 (1.15, 4.62) | 1.46 (0.810, 2.76) | 1.60 (0.880, 3.10) | ||
| Creatinine, μmol/L | <0.001 | <0.001 | |||||
| Median (Q1, Q3) | 81.5 (77.0, 91.0) | 77.0 (71.5, 84.0) | 68.0 (62.0, 73.0) | 65.0 (60.0, 70.0) | 70.0 (63.0, 78.0) | ||
| Diabetes | 0.92 | 0.07 | |||||
| Yes | 11.0 (15.7%) | 26.0 (15.2%) | 38.0 (26.4%) | 52.0 (18.8%) | 127 (19.2%) | ||
| No | 59.0 (84.3%) | 145 (84.8%) | 106 (73.6%) | 224 (81.2%) | 534 (80.8%) | ||
| Variable | Men (N = 241) | Women (N = 420) | Overall | ||||
|---|---|---|---|---|---|---|---|
| sUA Increased (N = 70) | sUA Normal (N = 171) | p-Value (Fisher’s) | sUA Increased (N = 144) | sUA Normal (N = 276) | p-Value (Fisher’s) | ||
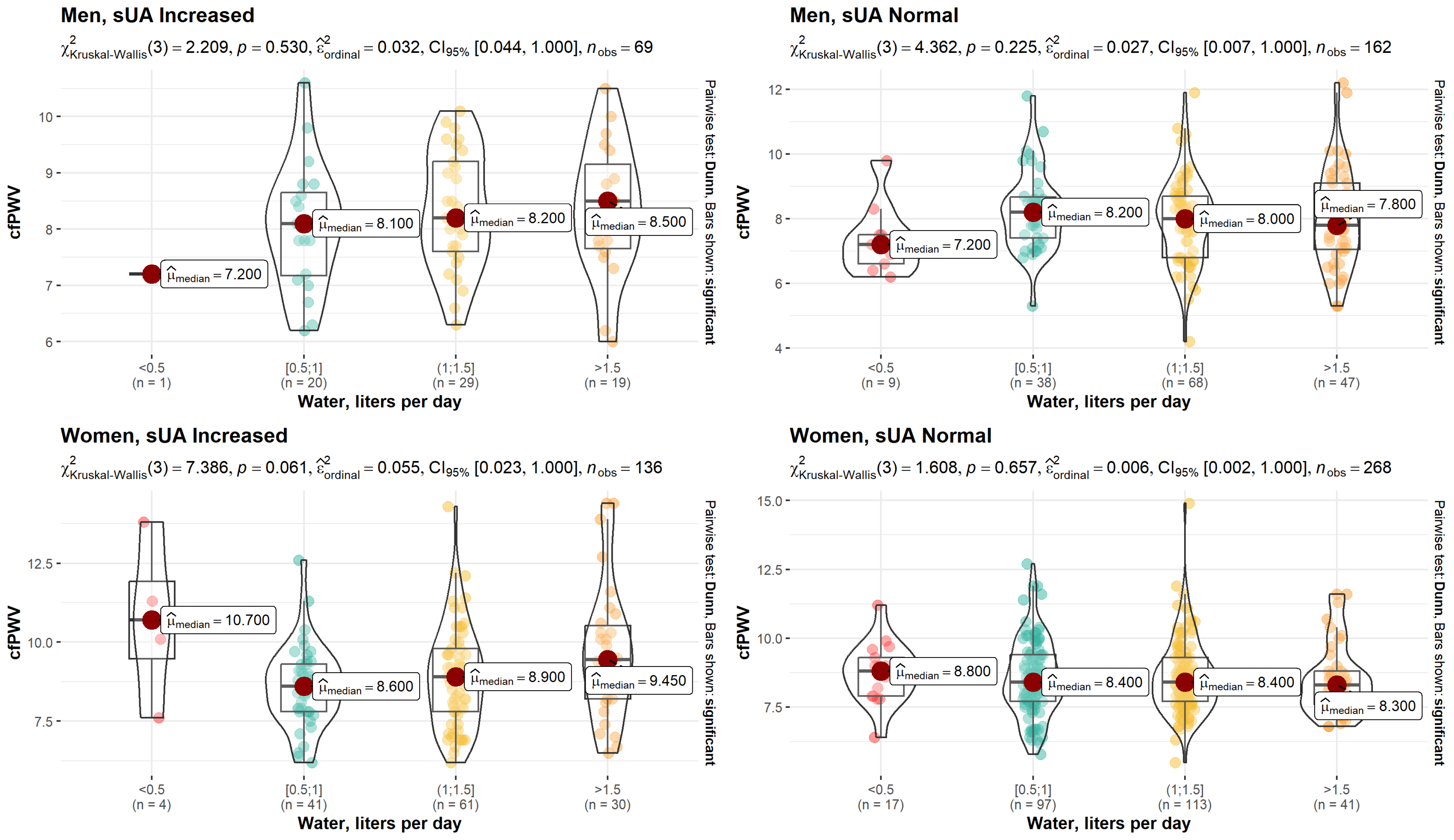
| cfPWV, m/s | 0.14 | 0.004 | |||||
| Median (Q1, Q3) | 8.15 (7.53, 8.98) | 8.00 (7.00, 8.75) | 8.90 (7.80, 9.90) | 8.40 (7.70, 9.33) | 8.40 (7.60, 9.30) | ||
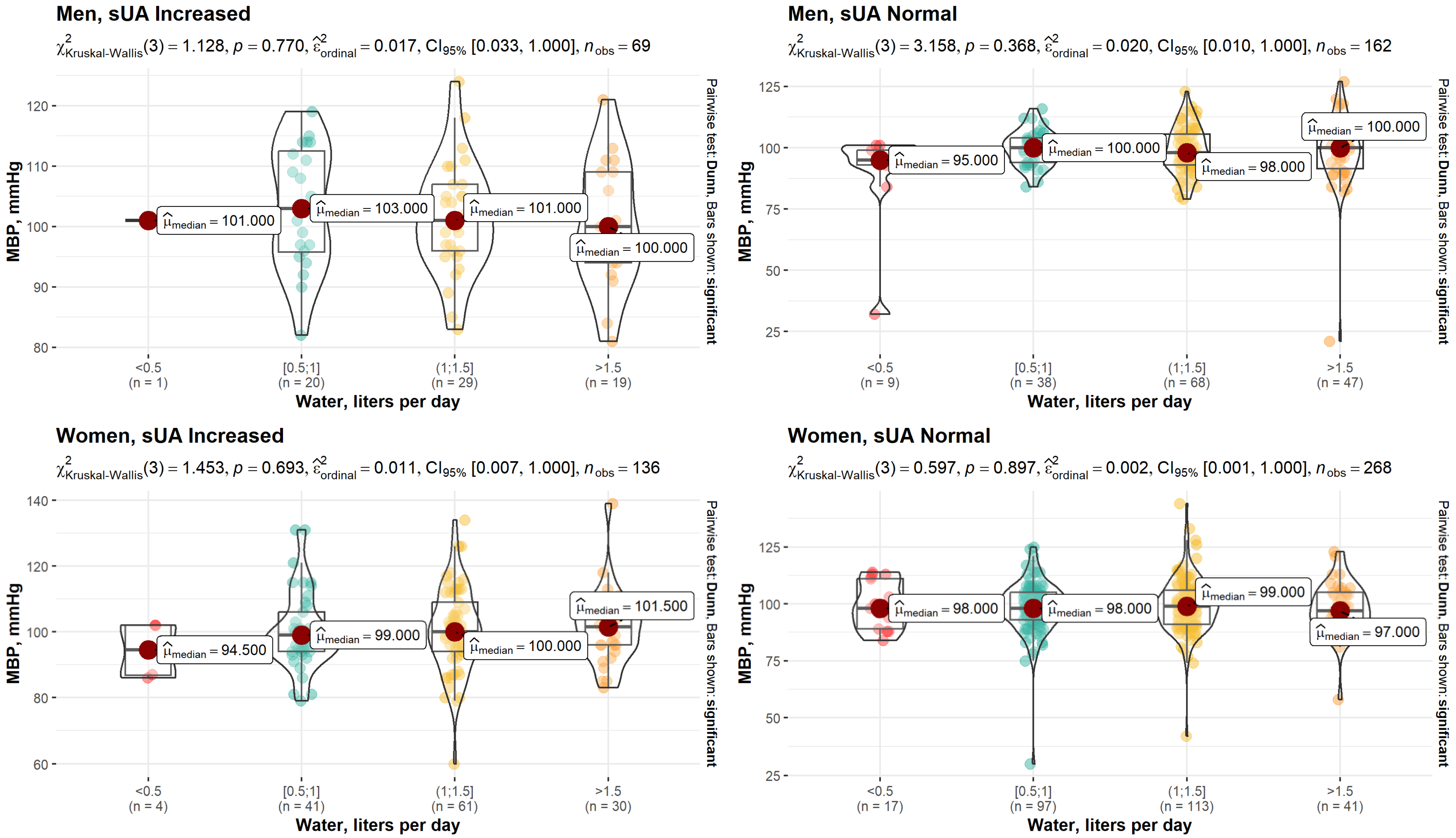
| MBP, mmHg | 0.05 | 0.519 | |||||
| Median (Q1, Q3) | 101 (95.0, 109) | 99.0 (93.0, 106) | 99.0 (94.0, 106) | 99.0 (91.8, 106) | 99.0 (93.0, 106) | ||
| Variable | Men (N = 241) | Women (N = 420) | Overall | ||||
|---|---|---|---|---|---|---|---|
| sUA Increased | sUA Normal | p-Value (Fisher’s) | sUA Increased | sUA Normal | p-Value (Fisher’s) | ||
| (N = 70) | (N = 171) | (N = 144) | (N = 276) | ||||
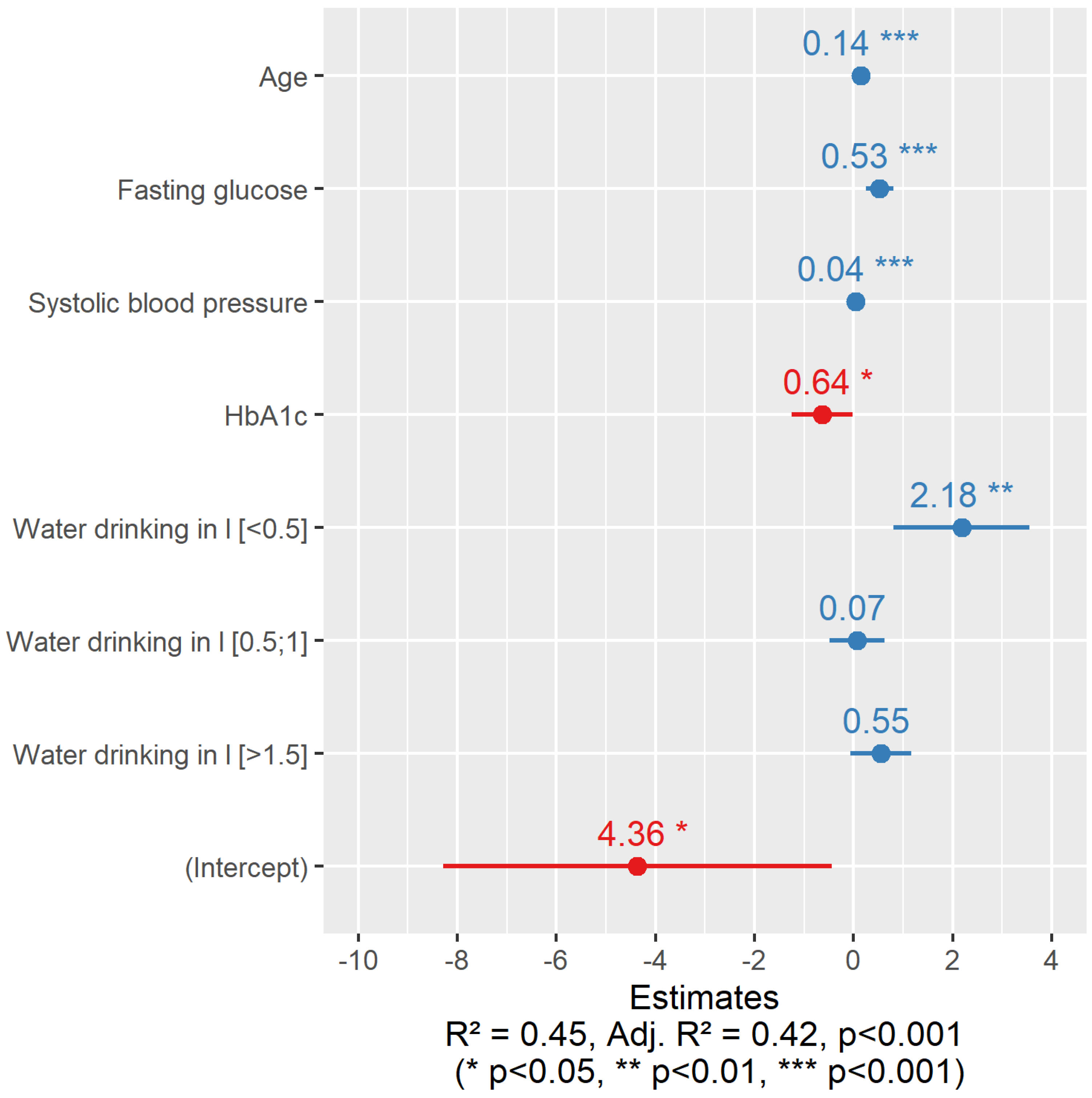
| Water, liters per day | 0.473 | 0.14 | |||||
| <0.5 | 1 (1.45%) | 9 (5.56%) | 4 (2.94%) | 17 (6.34%) | 31 (4.88%) | ||
| (0.5–1) | 20 (29.0%) | 38 (23.5%) | 41 (30.1%) | 97 (36.2%) | 196 (30.9%) | ||
| (1–1.5) | 29 (42.0%) | 68 (42.0%) | 61 (44.9%) | 113 (42.2%) | 271 (42.7%) | ||
| >1.5 | 19 (27.5%) | 47 (29.0%) | 30 (22.1%) | 41 (15.3%) | 137 (21.6%) | ||
| Missing | 1.00 (1.4%) | 9 (5.3%) | 8 (5.6%) | 8 (2.9%) | 26 (3.9%) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čypienė, A.; Gimžauskaitė, S.; Rinkūnienė, E.; Jasiūnas, E.; Rugienė, R.; Kazėnaitė, E.; Ryliškytė, L.; Badarienė, J. The Association between Water Consumption and Hyperuricemia and Its Relation with Early Arterial Aging in Middle-Aged Lithuanian Metabolic Patients. Nutrients 2023, 15, 723. https://doi.org/10.3390/nu15030723
Čypienė A, Gimžauskaitė S, Rinkūnienė E, Jasiūnas E, Rugienė R, Kazėnaitė E, Ryliškytė L, Badarienė J. The Association between Water Consumption and Hyperuricemia and Its Relation with Early Arterial Aging in Middle-Aged Lithuanian Metabolic Patients. Nutrients. 2023; 15(3):723. https://doi.org/10.3390/nu15030723
Chicago/Turabian StyleČypienė, Alma, Silvija Gimžauskaitė, Egidija Rinkūnienė, Eugenijus Jasiūnas, Rita Rugienė, Edita Kazėnaitė, Ligita Ryliškytė, and Jolita Badarienė. 2023. "The Association between Water Consumption and Hyperuricemia and Its Relation with Early Arterial Aging in Middle-Aged Lithuanian Metabolic Patients" Nutrients 15, no. 3: 723. https://doi.org/10.3390/nu15030723
APA StyleČypienė, A., Gimžauskaitė, S., Rinkūnienė, E., Jasiūnas, E., Rugienė, R., Kazėnaitė, E., Ryliškytė, L., & Badarienė, J. (2023). The Association between Water Consumption and Hyperuricemia and Its Relation with Early Arterial Aging in Middle-Aged Lithuanian Metabolic Patients. Nutrients, 15(3), 723. https://doi.org/10.3390/nu15030723





