Sun Exposure Score and Vitamin D Levels in Moroccan Women of Childbearing Age
Abstract
1. Introduction
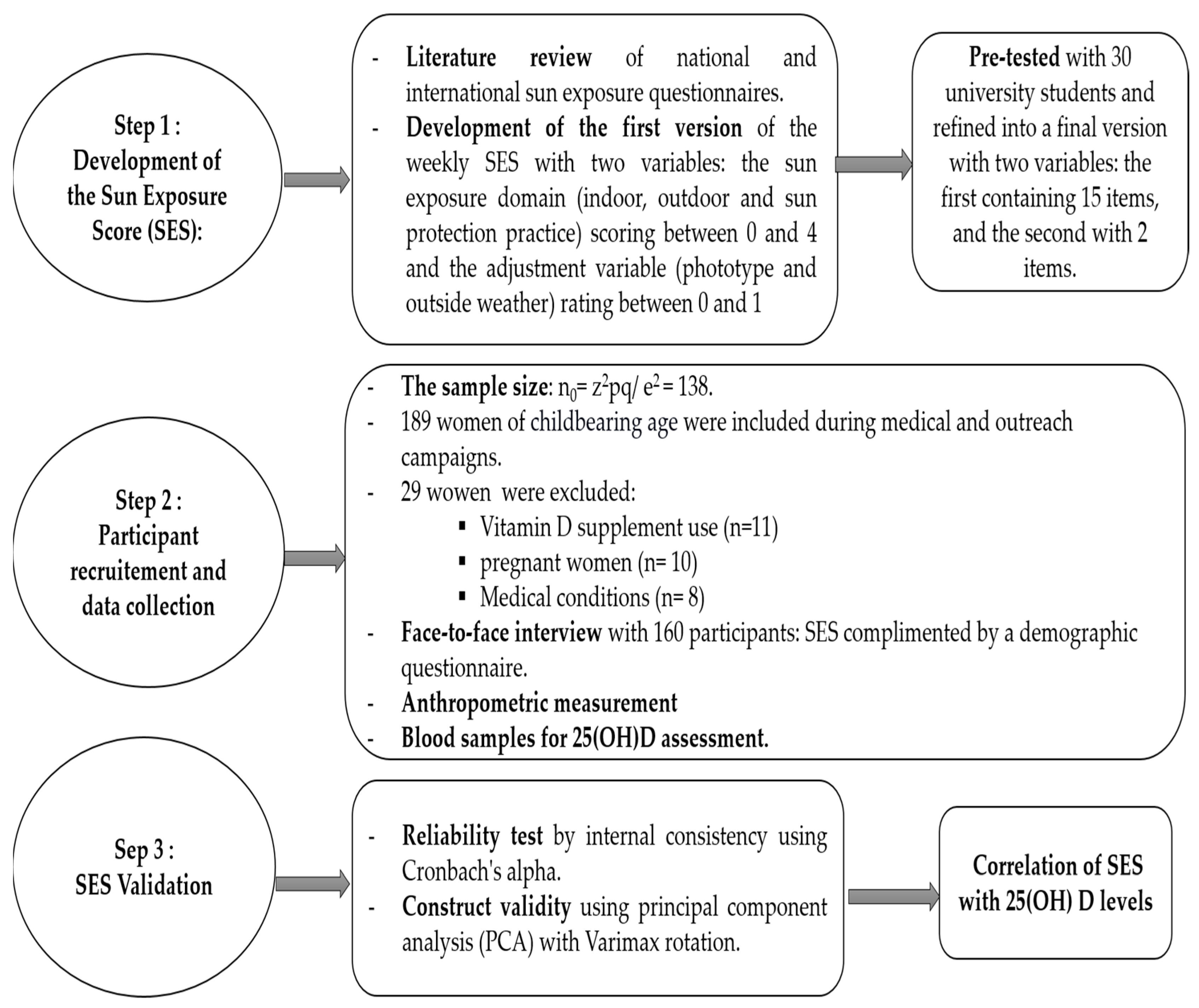
2. Methods
2.1. Step 1: Development of the Sun Exposure Score (SES)
2.1.1. Literature Review and Sun Exposure Score (SES) Development
2.1.2. Pretest of SES
2.2. Step 2: Participant Recruitment and Data Collection
2.2.1. Study Population
2.2.2. Data Collection
2.3. Step 3: SES Validation
Test of Construct Validity and Reliability of SES
2.4. Statistical Analyses
3. Results
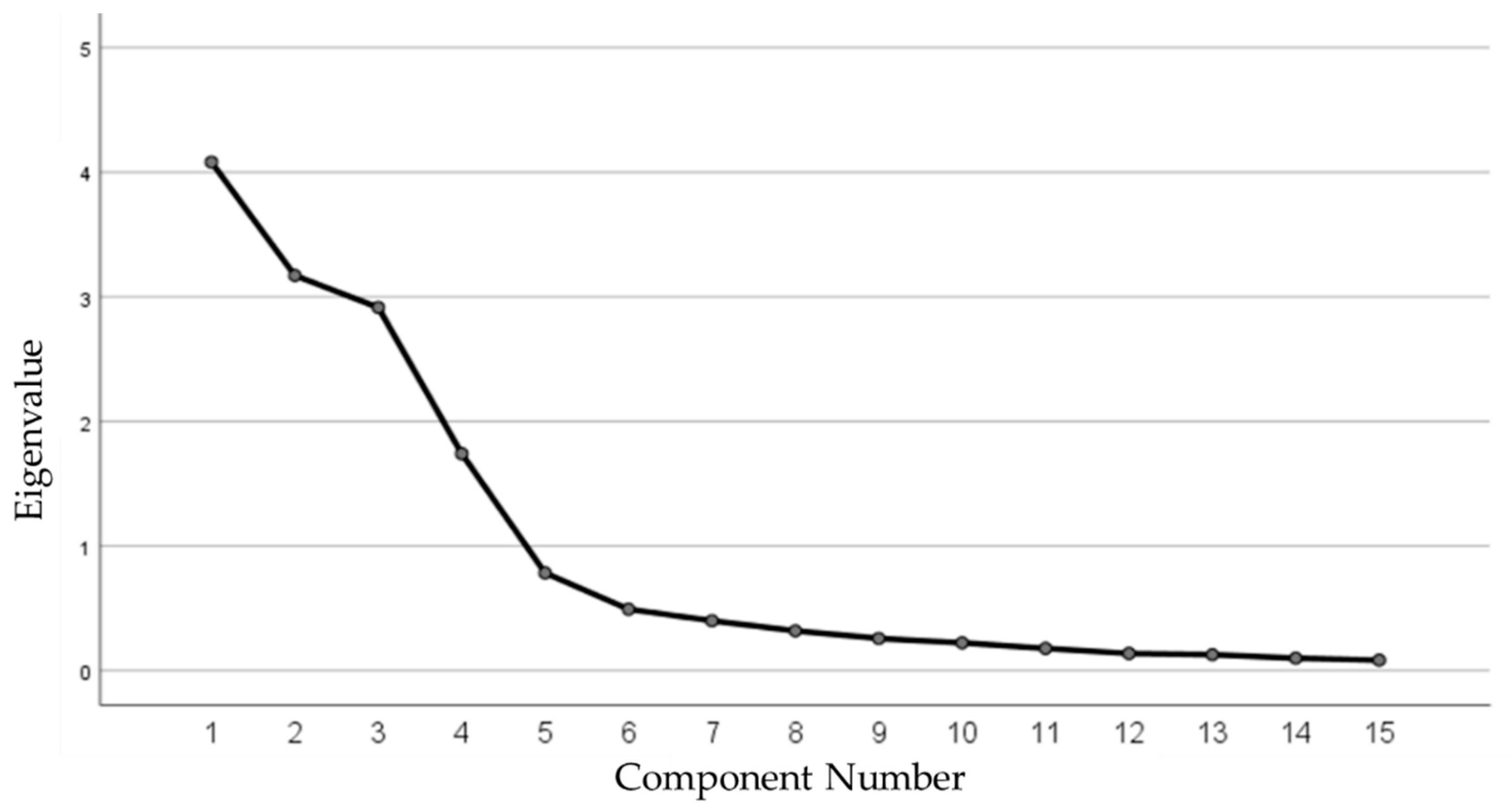
3.1. Validation of the Sun Exposure Score
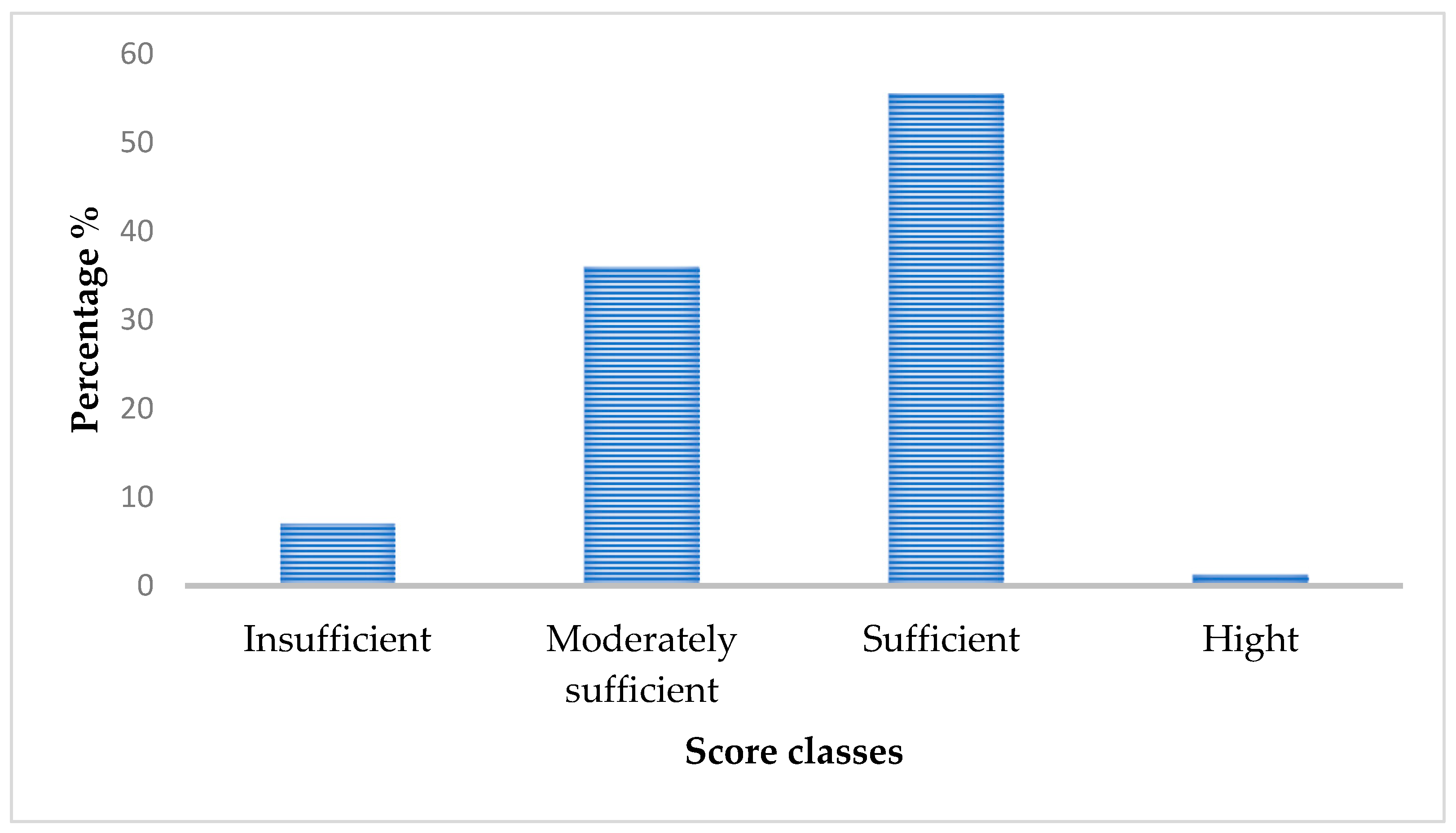
3.2. Participants’ Sun Exposure Score and Vitamin D Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Sun Exposure Score (SES) | ||||||
| Variable Sun Exposure Domains: | ||||||
| Items: | 0 | 1 | 2 | 3 | 4 | |
| Indoor sun exposure | Frequency of indoor sun exposure (in terrace, balcony, courtyard) | Never | 1 time/week | 2 to 3 times/week | 4 to 5 times/week | More than 5 times/week |
| Indoor exposed body part | Face | Face, hand | Face, arm | Face, arm, leg | ||
| Indoor duration sun exposure | Less than 5 min | 5 to 15 min | 15 to 30 min | 30 to 60 min | More than 60 min | |
| Indoor sun exposure time slot | Before 7 a.m. | 7 a.m. to 9 a.m. | 9 a.m. to 11 a.m./5 p.m. to 19 p.m. | 11 a.m. to 1 p.m. | 1 p.m. to 5 p.m. | |
| Outdoor sun exposure | Frequency of professional or routine outdoor activities | Never | 1 time/week | 2 to 3 times/week | 4 to 5 times/week | More than 5 times/week |
| A time slot for professional or routine outdoor activities | Before 7 a.m. | 7 a.m. to 9 a.m. | 9 a.m. to 11 a.m./5 p.m. to 19 p.m. | 11 a.m. to 1 p.m. | 1 p.m. to 5 p.m. | |
| Duration of professional or routine outdoor activities | Less than 5 min | 5 to 15 min | 15 to 30 min | 30 to 60 min | More than 60 min | |
| Outdoor exposed body part | Face | Face, hand | Face, arm | Face, arm, leg | ||
| Practice outdoor activities (sports, hiking, walking, camping, swimming, etc.) | Never | 1 time/week | 2 to 3 times/week | 4 to 5 times week | More than 5 times/week | |
| Duration outdoor activities | Less than 5 min | 5 to 15 min | 15 to 30 min | 30 to 60 min | More than 60 min | |
| Time slot for sun exposure in the open air | Before 7 a.m. | 7 a.m. to 9 a.m. | 9 a.m. to 11 a.m./5 p.m. to 19 p.m. | 11 a.m. to 1 p.m. | 1 p.m. to 5 p.m. | |
| Part of the body exposed in the open air | Face | Face, hand | Face, arm | Face, arm, leg | ||
| Sun protection practices | Staying out of the sun | Used shade of tree/building/ parasol etc. | Market in the shade | Car/bus/van smoked window/of | Car/bus/van glass windows up | No protection |
| Type of sunscreen used | SPF sunscreen of 50 and + | SPF sunscreen from 30 to 50 | Sunscreen SPF from 15 to 30 | Other creams and lotions without knowledge of the FPS or SPF 6 to 10 | No sunscreen | |
| Type of clothing usually used | Usual clothes with long sleeves/long pants/heavy/tight clothes with dark colorants | Usual clothes with long sleeves/long pants/loose with dark colorants | Long loose clothing with light coloring. | Short sleeve/pants/shorts | Short no shirt /bikini | |
| Adjustment variable | ||||||
| Items: | 0 | 0.25 | 0.5 | 0.75 | 1 | |
| Phototype | Type V & VI | Type III & IV | Type II | Type I | ||
| Weather outside | rainy | cloudy/light rainy | cloudy | sunny/light cloudy | sunny | |
| Total | Sun exposure score = the sum of the domain scores x phototype score x outdoor weather score. | |||||
| The score is considered insufficient if the score is below 7.5, moderate if the score is between 7.5–15, sufficient if the score is between 15–30, and as very sufficient or high if the score is above 30. Indoor sun exposure is considered insufficient if the score is below 4, moderately sufficient if the score is between 4 and 8, sufficient if the score is between 8 and 12, and high for a total above 12. For outdoor sun exposure, it is considered insufficient if the total is less than 8, moderately sufficient if the total is between 8 and 16, sufficient if the score is between 16 and 24, and high for a total greater than 24. Finally, for sun protection practices, this subscale has an inverse relationship with sun exposure: a score below 3 is considered high and considered sufficient from 3 to 6, moderately sufficient if the score is between 6 and 9, and insufficient sun protection practices for a score between 9 and 12. | ||||||
References
- Chen, P.; Hu, P.; Xie, D.; Qin, Y.; Wang, F.; Wang, H. Meta-Analysis of Vitamin D, Calcium and the Prevention of Breast Cancer. Breast Cancer Res. Treat. 2010, 121, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Szabó, A. Skeletal and Extra-Skeletal Consequences of Vitamin D Defi Ciency. Orv. Hetil. 2011, 152, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Chen, T.C. Vitamin D Deficiency: A Worldwide Problem with Health Consequences. Am. J. Clin. Nutr. 2008, 87, 1080–1086. [Google Scholar] [CrossRef]
- Hossein-Nezhad, A.; Holick, M.F. Vitamin D for Health: A Global Perspective. Mayo Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef]
- Garland, C.F.; Kim, J.J.; Mohr, S.B.; Gorham, E.D.; Grant, W.B.; Giovannucci, E.L.; Baggerly, L.; Hofflich, H.; Ramsdell, J.W.; Zeng, K.; et al. Meta-Analysis of All-Cause Mortality According to Serum 25-Hydroxyvitamin D. Am. J. Public Health 2014, 104, 43–50. [Google Scholar] [CrossRef]
- Grant, W.B. 25-Hydroxyvitamin D and Breast Cancer, Colorectal Cancer, and Colorectal Adenomas: Case-Control versus Nested Case-Control Studies. Anticancer Res. 2015, 35, 1153–1160. [Google Scholar]
- Infante, M.; Ricordi, C.; Sanchez, J.; Clare-Salzler, M.J.; Padilla, N.; Fuenmayor, V.; Chavez, C.; Alvarez, A.; Baidal, D.; Alejandro, R.; et al. Influence of Vitamin D on Islet Autoimmunity and Beta-Cell Function in Type 1 Diabetes. Nutrients 2019, 11, 2185. [Google Scholar] [CrossRef] [PubMed]
- Manousaki, D.; Harroud, A.; Mitchell, R.E.; Ross, S.; Forgetta, V.; Timpson, N.J.; Smith, G.D.; Polychronakos, C.; Richards, J.B. Vitamin D Levels and Risk of Type 1 Diabetes: A Mendelian Randomization Study. PLoS Med. 2021, 18, 1–16. [Google Scholar] [CrossRef]
- McDonnell, S.L.; Baggerly, C.; French, C.B.; Baggerly, L.L.; Garland, C.F.; Gorham, E.D.; Lappe, J.M.; Heaney, R.P. Serum 25-Hydroxyvitamin d Concentrations ≥40 Ng/Ml Are Associated with >65% Lower Cancer Risk: Pooled Analysis of Randomized Trial and Prospective Cohort Study. PLoS ONE 2016, 11, e0152441. [Google Scholar] [CrossRef]
- Pludowski, P.; Holick, M.F.; Pilz, S.; Wagner, C.L.; Hollis, B.W.; Grant, W.B.; Shoenfeld, Y.; Lerchbaum, E.; Llewellyn, D.J.; Kienreich, K.; et al. Vitamin D Effects on Musculoskeletal Health, Immunity, Autoimmunity, Cardiovascular Disease, Cancer, Fertility, Pregnancy, Dementia and Mortality-A Review of Recent Evidence. Autoimmun. Rev. 2013, 12, 976–989. [Google Scholar] [CrossRef]
- Prietl, B.; Treiber, G.; Pieber, T.R.; Amrein, K. Vitamin D and Immune Function. Nutrients 2013, 5, 2502–2521. [Google Scholar] [CrossRef] [PubMed]
- Fernando, M.; Ellery, S.J.; Marquina, C.; Lim, S.; Naderpoor, N.; Mousa, A. Vitamin D-Binding Protein in Pregnancy and Reproductive Health. Nutrients 2020, 12, 1489. [Google Scholar] [CrossRef] [PubMed]
- Mousa, A.; Abell, S.; Scragg, R.; Courten, B. Vitamin D in Reproductive Health and Pregnancy. Semin. Reprod. Med. 2016, 34, e1–e13. [Google Scholar] [CrossRef]
- Irani, M.; Merhi, Z. Role of Vitamin D in Ovarian Physiology and Its Implication in Reproduction: A Systematic Review. Fertil Steril. 2014, 102, 460–468.e3. [Google Scholar] [CrossRef] [PubMed]
- Moridi, I.; Chen, A.; Tal, O.; Tal, R. The Association between Vitamin D and Anti-Müllerian Hormone: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1567. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, M.; Shah, B.C. Role of Vitamin D Levels on the Ovarian Function and Androgen Profile in Adolescents with Polycystic Ovarian Syndrome. Gynecol. Pelvic Med. 2019, 2, 22. [Google Scholar] [CrossRef]
- Simpson, S.; Seifer, D.B.; Shabanova, V.; Lynn, A.Y.; Howe, C.; Rowe, E.; Caprio, S.; Vash-Margita, A. The Association between Anti-Müllerian Hormone and Vitamin 25(OH)D Serum Levels and Polycystic Ovarian Syndrome in Adolescent Females. Reprod. Biol. Endocrinol. 2020, 18, 118. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Altieri, B.; de Angelis, C.; Palomba, S.; Pivonello, R.; Colao, A.; Orio, F. Shedding New Light on Female Fertility: The Role of Vitamin D. Rev. Endocr. Metab. Disord. 2017, 18, 273–283. [Google Scholar] [CrossRef]
- Voulgaris, N.; Papanastasiou, L.; Piaditis, G.; Angelousi, A.; Kaltsas, G.; Mastorakos, G.; Kassi, E. Vitamin D and Aspects of Female Fertility. Hormones 2017, 16, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Almassinokiani, F.; Khodaverdi, S.; Solaymani-dodaran, M.; Akbari, P.; Pazouki, A. Effects of Vitamin D on Endometriosis-Related Pain: A Double-Blind Clinical Trial. Med. Sci. Monit. 2016, 22, 4960–4966. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, S.; Wang, Y.; Wang, P.; Qu, D.; Liu, M.; Ma, W.; Li, Y. Vitamin D Improves In-Vitro Fertilization Outcomes in Infertile Women with Polycystic Ovary Syndrome and Insulin Resistance. Minerva. Med. 2019, 110, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Cheng, D.; Yin, T.; Yang, J. Vitamin D and Polycystic Ovary Syndrome: A Narrative Review. Reprod. Sci. 2021, 28, 2110–2117. [Google Scholar] [CrossRef] [PubMed]
- Kasim, S.F. The Relationship between Vitamin D and Spontaneous Abortion among Iraqi Women. J. Med. Life 2022, 15, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Azzam, E.Z.; El-Aghoury, A.A.; Abd El-naby, E.S.E.; El-Maadawy, S.A. Studying the Relation between Vitamin D Deficiency and Glycemic State among Pregnant Women with Gestational Diabetes. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1505–1509. [Google Scholar] [CrossRef] [PubMed]
- Lithy, A.E.; Abdella, R.M.; El-Faissal, Y.M.; Sayed, A.M.; Abdel Samie, R.M. The Relationship between Low Maternal Serum Vitamin D Levels and Glycemic Control in Gestational Diabetes Assessed by HbA1c Levels: An Observational Cross-Sectional Study. BMC Pregnancy Childbirth 2014, 14, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhang, Z.; Li, L.; Zhang, L.; Lin, Z.; Qin, H. Vitamin D Deficiency Increases the Risk of Bacterial Vaginosis during Pregnancy: Evidence from a Meta-Analysis Based on Observational Studies. Front. Nutr. 2022, 9, 1016592. [Google Scholar] [CrossRef] [PubMed]
- Bakacak, M.; Serin, S.; Ercan, O.; Köstü, B.; Avci, F.; Kılınç, M.; Kıran, H.; Kiran, G. Comparison of Vitamin D Levels in Cases with Preeclampsia, Eclampsia and Healthy Pregnant Women. Int. J. Clin. Exp. Med. 2015, 8, 16280–16286. [Google Scholar] [PubMed]
- Benachi, A.; Baptiste, A.; Taieb, J.; Tsatsaris, V.; Guibourdenche, J.; Senat, M.V.; Haidar, H.; Jani, J.; Guizani, M.; Jouannic, J.M.; et al. Relationship between Vitamin D Status in Pregnancy and the Risk for Preeclampsia: A Nested Case-Control Study. Clin. Nutr. 2020, 39, 440–446. [Google Scholar] [CrossRef]
- Adán Lanceta, V.; Martín Ruiz, N.; Benito Costey, S.; Alijarde Lorente, R.; Martínez de Zabarte Fernández, J.M. A Neonatal Hypocalcemia Due to Maternal Vitamin D Deficiency. Reviewing Supplementation. Pediatr 2022, 96, 153–154. [Google Scholar] [CrossRef]
- Khalessi, N.; Kalani, M.; Araghi, M.; Farahani, Z. The Relationship between Maternal Vitamin D Deficiency and Low Birth Weight Neonates. J. Fam. Reprod. Health 2015, 9, 113–117. [Google Scholar]
- Cannell, J. Vitamin D and Autism, What’s New? Rev. Endocr. Metab. Disord. 2017, 18, 183–193. [Google Scholar] [CrossRef] [PubMed]
- The World Medical Association Wma Statement on Vitamin D Insufficiency. 2015. Available online: https://www.wma.net/policies-post/wma-statement-on-vitamin-dinsufficiency/ (accessed on 20 May 2019).
- Allali, F.; El Aichaoui, S.; Khazani, H.; Benyahia, B.; Saoud, B.; El Kabbaj, S.; Bahiri, R.; Abouqal, R.; Hajjaj-Hassouni, N. High Prevalence of Hypovitaminosis D in Morocco: Relationship to Lifestyle, Physical Performance, Bone Markers, and Bone Mineral Density. Semin. Arthritis Rheum. 2009, 38, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Bour, A.; Nejjar, B. Connaissance Sur La Vitamine D: État Des Lieux de La Prévalence de l’Hypovitaminose D Chez La Population Marocaine. Rev. Annales des Sciences de la Santé. 2017, 1, 24–31. [Google Scholar]
- El Maataoui, A.; Biaz, A.; El Machtani, S.; Bouhsain, S.; Dami, A.; El Maghraoui, A.; Ouzzif, Z. Vitamin D Status in Healthy Moroccan Men and Women Aged 50 Years and Older: A Cross-Sectional Study. Arch. Osteoporos. 2016, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Baki, S.; El Mghari, G.; El Ansari, N.; Harkati, I.; Tali, A.; Chabaa, L. Statut de La Vitamine de La Vitamine D Chez Les Femmes Marocaines Vivant a Marrakech. Ann. Endocrinol. 2015, 76, 490. [Google Scholar] [CrossRef]
- Ministère de la Santé. ENQUETE NATIONALE SUR LA NUTRITION (ENN 2019–2020). 2022. pp. 1–144. Available online: https://www.sante.gov.ma/Documents/2022/07/rapport%20ENN%202019-2020%20ajout%20preface%20(1).pdf (accessed on 15 September 2022).
- Zouiri, H.; Elmessaoudi, H. Energies Renouvelables et Développment Durable Au Maroc. Rev. Estud. Front. Estrecho Gibraltar. 2018, 6, 1–29. [Google Scholar]
- Dadda, S.; Azekour, K.; Sebbari, F.; El Houate, B.; El Bouhali, B. Sun Exposure, Dressing Habits, and Vitamin D Status in Morocco. E3S Web Conf. 2021, 319, 01097. [Google Scholar] [CrossRef]
- Barrea, L.; Savastano, S.; Di Somma, C.; Savanelli, M.C.; Nappi, F.; Albanese, L.; Orio, F.; Colao, A. Low Serum Vitamin D-Status, Air Pollution and Obesity: A Dangerous Liaison. Rev. Endocr. Metab. Disord. 2017, 18, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Mendes, M.M.; Hart, K.H.; Botelho, P.B.; Lanham-New, S.A. Vitamin D Status in the Tropics: Is Sunlight Exposure the Main Determinant? Nutr. Bull. 2018, 43, 428–434. [Google Scholar] [CrossRef]
- Aguilar-Shea, A. Vitamin D, the Natural Way. Clin. Nutr. ESPEN 2021, 41, 10–12. [Google Scholar] [CrossRef]
- Schmid, A.; Walther, B. Natural Vitamin D Content in Animal Products. Adv. Nutr. 2013, 4, 453–462. [Google Scholar] [CrossRef] [PubMed]
- De Souza de Santana, K.V.; Oliver, S.L.; Mendes, M.M.; Lanham-New, S.; Charlton, K.E.; Ribeiro, H. Association between Vitamin D Status and Lifestyle Factors in Brazilian Women: Implications of Sun Exposure Levels, Diet, and Health. eClinicalMedicine 2022, 47, 101400. [Google Scholar] [CrossRef]
- Chen, T.C.; Chimeh, F.; Lu, Z.; Mathieu, J.; Person, K.S.; Zhang, A.; Kohn, N.; Martinello, S.; Berkowitz, R.; Holick, M.F. Factors That Influence the Cutaneous Synthesis and Dietary Sources of Vitamin D. Arch. Biochem. Biophys. 2007, 460, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Norval, M.; Björn, L.O.; de Gruijl, F.R. Is the Action Spectrum for the UV-Induced Production of Previtamin D3 in Human Skin Correct? Photochem. Photobiol. Sci. Off. J. Eur. Photochem. Assoc. Eur. Soc. Photobiol. 2010, 9, 11–17. [Google Scholar] [CrossRef]
- Holick, M.F. Sunlight and Vitamin D for Bone Health and Prevention of Autoimmune Diseases, Cancers, and Cardiovascular Disease. Am. J. Clin. Nutr. 2004, 80, 1678–1688. [Google Scholar] [CrossRef]
- Holick, M.F.; Chen, T.C.; Lu, Z.; Sauter, E. Vitamin D and Skin Physiology: A D-Lightful Story. J. Bone Miner. Res. 2007, 22, 28–33. [Google Scholar] [CrossRef]
- Matthias, W.; Michaela, F. Holick Sunlight and Vitamin D—A Global Perspective for Health. Dermato-Endocrinology 2013, 5, 51–108. [Google Scholar] [CrossRef]
- Webb, A.R. Who, What, Where and When—Influences on Cutaneous Vitamin D Synthesis. Prog. Biophys. Mol. Biol. 2006, 92, 17–25. [Google Scholar] [CrossRef]
- Webb, A.R.; Engelsen, O. Ultraviolet Exposure Scenarios: Risks of Erythema from Recommendations on Cutaneous Vitamin D Synthesis. Adv. Exp. Med. Biol. 2008, 624, 72–85. [Google Scholar] [CrossRef]
- Moan, J.; Dahlback, A. PAC At What Time Should One Go Out in the Sun? Adv. Exp. Med. Biol. 2008, 624, 86–88. [Google Scholar] [CrossRef]
- Tsiaras, W.G.; Weinstock, M.A. Factors Influencing Vitamin d Status. Acta Derm. Venereol. 2011, 91, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, J.C. Vitamin D and Aging. Endocrinol. Metab. Clin. N. Am. 2013, 42, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.R.; Kazantzidis, A.; Kift, R.C.; Farrar, M.D.; Wilkinson, J.; Rhodes, L.E. Meeting Vitamin D Requirements in White Caucasians at UK Latitudes: Providing a Choice. Nutrients 2018, 10, 497. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.R.; Kazantzidis, A.; Kift, R.C.; Farrar, M.D.; Wilkinson, J.; Rhodes, L.E. Colour Counts: Sunlight and Skin Type as Drivers of Vitamin D Deficiency at UK Latitudes. Nutrients 2018, 10, 457. [Google Scholar] [CrossRef]
- Farrar, M.D.; Webb, A.R.; Kift, R.; Durkin, M.T.; Allan, D.; Herbert, A.; Berry, J.L.; Rhodes, L.E. Efficacy of a dose range of simulated sunlight exposures in raising vitamin D status in South Asian adults: Implications for targeted guidance on sun exposure. Am. J. Clin. Nutr. 2013, 97, 1210–1216. [Google Scholar] [CrossRef]
- Fernandes, M.R.; Barreto, W.D.R., Jr. Association between Physical Activity and Vitamin D: A Narrative Literature Review. Rev. Assoc. Médica Bras. 2017, 63, 550–556. [Google Scholar] [CrossRef]
- Sarkar, A.K. An Evaluation of UV Protection Imparted by Cotton Fabrics Dyed with Natural Colorants. BMC Dermatol. 2004, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kocić, A.; Bizjak, M.; Popović, D.; Poparić, G.B.; Stanković, S.B. UV Protection Afforded by Textile Fabrics Made of Natural and Regenerated Cellulose Fibres. J. Clean. Prod. 2019, 228, 1229–1237. [Google Scholar] [CrossRef]
- Gies, P. Photoprotection by Clothing. Photodermatol. Photoimmunol. Photomed. 2007, 23, 264–274. [Google Scholar] [CrossRef]
- Aguilera, J.; De Gálvez, M.V.; Sánchez-Roldán, C.; Herrera-Ceballos, E. New Advances in Protection against Solar Ultraviolet Radiation in Textiles for Summer Clothing. Photochem. Photobiol. 2014, 90, 1199–1206. [Google Scholar] [CrossRef]
- Humayun, Q.; Iqbal, R.; Azam, I.; Khan, A.H.; Siddiqui, A.R.; Baig-Ansari, N. Development and Validation of Sunlight Exposure Measurement Questionnaire (SEM-Q) for Use in Adult Population Residing in Pakistan. BMC Public Health 2012, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R.; Gallagher, D.J.A.; Bosworth, J. Prophylaxis Against Vitamin D Deficiency in The Elderly by Regular Sunlight Exposure. Age Ageing 1986, 15, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Okan, F.; Zincir, H.; Deveci, K. The Effect of Sun Light Exposure to the Level of Vitamin D in Elderly People Living in Nursing Home. J. Clin. Densitom. 2022, 25, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Fédération des Agences Urbaines du Maroc (MAJAL). Sur Les Traces Des Pratiques et de Savoir-Faire éco-Responsables Architecture et Urbanisme Traditionnels Au Maroc. 2016. pp. 1–126. Available online: https://www.federation-majal.ma/fr/Actualite/sur-les-traces-des-pratiques-et-de-savoir-faire-éco-responsables-architecture-et-urbanisme (accessed on 24 May 2022).
- Brunzell, T.; Duric, S. Moroccan Architecture, Traditional and Modern. Ph.D. Thesis, LTH School of Engineering at Campus Helsingborg Housing Development & Management, Lund University, Lund, Sweden, 2012. [Google Scholar]
- Daemei, A.B.; Eghbali, S.R.; Khotbehsara, E.M. Bioclimatic Design Strategies: A Guideline to Enhance Human Thermal Comfort in Cfa Climate Zones. J. Build. Eng. 2019, 25, 100758. [Google Scholar] [CrossRef]
- Ministère de l’Habitat et de la Politique de la Ville; Direction de la Qualité et des Affaires Techniques. Guide Des Bonnes Pratiques Pour La Maîtrise de l’énergie à l’échelle de La Ville et de l’habitat. 2014. pp. 1–124. Available online: https://www.mhpv.gov.ma/wp-content/uploads/2021/11/Guide-de-bonnes-pratiques-pour-la-maitrise-de-l-energie.pdf (accessed on 24 May 2022).
- Cargill, J.; Lucas, R.M.; Gies, P.; King, K.; Swaminathan, A.; Allen, M.W.; Banks, E. Validation of Brief Questionnaire Measures of Sun Exposure and Skin Pigmentation against Detailed and Objective Measures Including Vitamin D Status. Photochem. Photobiol. 2013, 89, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Detert, H.; Hedlund, S.; Anderson, C.D.; Rodvall, Y.; Festin, K.; Whiteman, D.C.; Falk, M. Validation of Sun Exposure and Protection Index (SEPI) for Estimation of Sun Habits. Cancer Epidemiol. 2015, 39, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Hanwell, H.E.C.; Vieth, R.; Cole, D.E.C.; Scillitani, A.; Modoni, S.; Frusciante, V.; Ritrovato, G.; Chiodini, I.; Minisola, S.; Carnevale, V. Sun Exposure Questionnaire Predicts Circulating 25-Hydroxyvitamin D Concentrations in Caucasian Hospital Workers in Southern Italy. J. Steroid Biochem. Mol. Biol. 2010, 121, 334–337. [Google Scholar] [CrossRef]
- Køster, B.; Søndergaard, J.; Nielsen, J.B.; Allen, M.; Olsen, A.; Bentzen, J. The Validated Sun Exposure Questionnaire: Association of Objective and Subjective Measures of Sun Exposure in a Danish Population-Based Sample. Br. J. Dermatol. 2017, 176, 446–456. [Google Scholar] [CrossRef]
- Køster, B.; Søndergaard, J.; Nielsen, J.B.; Olsen, A.; Bentzen, J. Reliability and Consistency of a Validated Sun Exposure Questionnaire in a Population-Based Danish Sample. Prev. Med. Rep. 2018, 10, 43–48. [Google Scholar] [CrossRef]
- Vignali, E.; Macchia, E.; Cetani, F.; Reggiardo, G.; Cianferotti, L.; Saponaro, F.; Marcocci, C. Development of an Algorithm to Predict Serum Vitamin D Levels Using a Simple Questionnaire Based on Sunlight Exposure. Endocrine 2017, 55, 85–92. [Google Scholar] [CrossRef]
- Wu, S.; Ho, S.C.; Lam, T.P.; Woo, J.; Yuen, P.Y.; Qin, L.; Ku, S. Development and Validation of a Lifetime Exposure Questionnaire for Use among Chinese Populations. Sci. Rep. 2013, 3, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.G.; Castillo-Carandang, N.; Sison, M.E.G.; Uy, A.B.; Villarante, K.L.; Maningat, P.; Paz-Pacheco, E.; Abesamis-Cubillan, E. Development and Validation of a Sunlight Exposure Questionnaire for Urban Adult Filipinos. Epidemiol. Health 2018, 40, e2018050. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, T.B. The Validity and Practicality of Sun-Reactive Skin Types I Through VI. Arch. Dermatol. 1988, 124, 869–871. [Google Scholar] [CrossRef] [PubMed]
- Glenn, D. Israel. Determining Sample Size 1. University of Florida IFAS Extension. 2012. pp. 1–5. Available online: Http://Edis.Ifas.Ufl.Edu (accessed on 10 February 2019).
- Benhamou, C.; Souberbielle, J.; Cortet, B. La Vitamine D Chez l’adulte: Recommandations Du GRIO. Presse Med. 2011, 40, 673–682. [Google Scholar] [CrossRef]
- Abourazzak, F.E.; Khazzani, H.; Mansouri, S.; Ali, S.; Alla, O.; Allali, F.; Maghraoui, A.E.; Larhrissi, S.; Rachidi, W.; Ichchou, L. Recommandations de la Société Marocaine de Rhumatologie sur la vitamine D chez l’Adulte. Rev. Mar. Rhum. 2016, 35, 3–15. [Google Scholar] [CrossRef]
- Wadkar, S.K.; Singh, K.; Chakravarty, R.T.; Argade, S.D. Assessing the Reliability of Attitude Scale by Cronbach’s Alpha. J. Glob. Commun. 2016, 9, 113–117. [Google Scholar] [CrossRef]
- George, D.; Mallery, P. SPSS for Windows Step by Step: A Simple Guide and Reference, 11.0 Update; Allyn and Bacon: Boston, MA, USA, 2003; ISBN 978-0-205-37552-3. [Google Scholar]
- Kaiser, H.F. An Index of Factorial Simplicity. Psychometrika 1974, 39, 31–36. [Google Scholar] [CrossRef]
- Liau, A.; Tan, T.K.; Angeline, K. Scale Measurement: Comparing Factor Analysis and Variable Clustering. SAS Glob. Forum 2011, 1–19. Available online: https://support.sas.com/resources/papers/proceedings11/352-2011.pdf (accessed on 5 December 2021).
- Serrano, M.A.; Cañada, J.; Moreno, J.C.; Gurrea, G. Solar Ultraviolet Doses and Vitamin D in a Northern Mid-Latitude. Sci. Total Environ. 2017, 574, 744–750. [Google Scholar] [CrossRef]
- Blarduni Cardón, E.; Cardón, B.E.; Ugarte, A.H.; Etxebarria, U.I.; González, C.L.; Goivide, G. La Dieta Como Factor de Riesgo de Hipovitaminosis D En La Población Pediátrica Española. Rev. Osteoporos. Metab. Min. 2021, 13, 122–129. [Google Scholar] [CrossRef]
- Castano, L.; Madariaga, L.; Grau, G.; García-Castaño, A. 25(OH)Vitamin D Deficiency and Calcifediol Treatment in Pediatrics. Nutrients 2022, 14, 1854. [Google Scholar] [CrossRef]
- van der Mei, I.A.F.; Blizzard, L.; Ponsonby, A.-L.; Dwyer, T. Validity and Reliability of Adult Recall of Past Sun Exposure in a Case-Control Study of Multiple Sclerosis. Cancer Epidemiol. Biomarkers Prev. 2006, 15, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.E.; Kimlin, M.G. The Dislike of Hot Thermal Conditions and Its Relationship with Sun (Ultraviolet Radiation) Exposure in the Southeastern United States. Int. J. Environ. Res. Public. Health 2018, 15, 2161. [Google Scholar] [CrossRef] [PubMed]
- Diffey, B.L. Time and Place as Modifiers of Personal UV Exposure. Int. J. Environ. Res. Public. Health 2018, 15, 1112. [Google Scholar] [CrossRef] [PubMed]
- Tavakol, M.; Dennick, R. Making Sense of Cronbach’s Alpha. Int. J. Med. Educ. 2011, 2, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Blázquez-Sánchez, N.; Rivas-Ruiz, F.; Bueno-Fernández, S.; Arias-Santiago, S.; Fernández-Morano, M.T.; deTroya-Martín, M. Validation of a Questionnaire Designed to Study Knowledge, Attitudes, and Habits Related to Sun Exposure Among Young Adults: The CHACES Questionnaire. Actas Dermo-Sifiliográficas Engl. Ed. 2020, 111, 579–589. [Google Scholar] [CrossRef]
- Mansibang, N.M.M.; Yu, M.G.Y.; Jimeno, C.A.; Lantion-Ang, F.L. Association of Sunlight Exposure with 25-Hydroxyvitamin D Levels among Working Urban Adult Filipinos. Osteoporos. Sarcopenia 2020, 6, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Abda, N.; El Rhazi, K.; Obtel, M.; Bendahhou, K.; Zidouh, A.; Bennani, M.; Bekkali, R.; Nejjari, C. Determinants of Self-Reported Sun Protection Practices among Moroccan Population. Prev. Med. 2012, 54, 422–424. [Google Scholar] [CrossRef] [PubMed]
- Sham, L.; Yeh, E.A.; Magalhaes, S.; Parra, E.J.; Gozdzik, A.; Banwell, B.; Hanwell, H.E. Evaluation of Fall Sun Exposure Score in Predicting Vitamin D Status in Young Canadian Adults, and the Influence of Ancestry. J. Photochem. Photobiol. B 2015, 145, 25–29. [Google Scholar] [CrossRef]
| KMO Measure of Sampling Adequacy | 0.755 | |
| Bartlett’s Test of Sphericity | Approx. Chi-Square | 1882.232 |
| Df | 105 | |
| Sig. | 0.000 | |
| Item Code | Item Description | Component | Communalities | |||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| Indoor sun exposure | ||||||
| 1 | Frequency of indoor sun exposure (in terrace, balcony, courtyard) | 0.111 | 0.842 | −0.146 | 0.141 | 0.763 |
| 2 | Indoor exposed body part | 0.012 | 0.936 | −0.102 | −0.011 | 0.887 |
| 3 | Indoor duration sun exposure | 0.004 | 0.913 | 0.017 | 0.021 | 0.834 |
| 4 | Indoor sun exposure time slot | −0.050 | 0.907 | −0.034 | 0.074 | 0.832 |
| Outdoor professional or routine activities | ||||||
| 5 | Frequency of professional or routine outdoor activities, | −0.059 | −0.248 | 0.746 | 0.002 | 0.622 |
| 6 | A time slot for professional or routine outdoor activities | −0.002 | −0.044 | 0.856 | 0.039 | 0.736 |
| 7 | Duration of professional or routine outdoor activities | −0.013 | 0.068 | 0.895 | 0.131 | 0.824 |
| 8 | Outdoor exposed body part | −0.166 | −0.046 | 0.873 | −0.009 | 0.792 |
| Outdoor recreational activities | ||||||
| 9 | Practice outdoor activities (sports, hiking, walking, camping, swimming, etc.) | 0.896 | 0.012 | 0.024 | 0.008 | 0.803 |
| 10 | Duration outdoor activities | 0.922 | 0.025 | 0.020 | 0.087 | 0.858 |
| 11 | Time slot for sun exposure in the open air | 0.838 | 0.008 | −0.306 | 0.079 | 0.802 |
| 12 | Part of the body exposed in the open air | 0.948 | 0.026 | −0.057 | 0.041 | 0.904 |
| Sun protection practices | ||||||
| 13 | Type of clothing usually used | −0.176 | −0.041 | 0.004 | 0.699 | 0.522 |
| 14 | Type of sunscreen used | 0.211 | 0.126 | 0.041 | 0.911 | 0.892 |
| 15 | Staying out of the sun | 0.221 | 0.138 | 0.112 | 0.868 | 0.834 |
| Initial Eigenvalues | 4.081 | 3.170 | 2.914 | 1.741 | ||
| Explained variance %. | 27.207 | 21.136 | 19.427 | 11.604 | ||
| Cumulative variance %. | 27.207 | 48.343 | 67.770 | 79.374 | ||
| Cronbach’s alpha coefficient | 0.909 | 0.923 | 0.835 | 0.787 | ||
| Socio-Demographic Characteristics | Total (N = 160) | Sun Exposure Score | Sun Exposure Classes | |||||
|---|---|---|---|---|---|---|---|---|
| (N = 160) | p-Value | Insufficient (N = 11) | Moderately Sufficient (N = 58) | Sufficient e (N = 91) | p-Value | |||
| Mean ± SD | Frequency (%) | |||||||
| Age (yr) (Median [IQR]) | 25 [11] | 15.82 ± 5.64 | 0.211 a | 0.858 b | ||||
| 18–25 | 86 (53.7) | 15.23 ± 5.70 | 6 (54.5) | 34 (58.6) | 46 (50.5) | |||
| 26–35 | 54 (33.8) | 17.05 ± 5.62 | 3 (27.3) | 18 (31) | 33 (36.3) | |||
| 36–45 | 20 (12.5) | 14.97 ± 5.09 | 2 (18.2) | 6 (10.4) | 12 (13.2) | |||
| BMI (kg/m2) (Median [IQR]) | 25.70 [8.65] | 0.018a | 0.02 b | |||||
| Normal weight (<25 kg/m2) | 68 (42.5) | 16.31 ± 5.61 | 3 (27.3) | 24 (41.4) | 41 (45.1) | |||
| Overweight (25.0 to <30 kg/m2 | 44 (27.5) | 16.93 ± 6.07 | 4 (36.4) | 9 (15.5) | 31 (34.1) | |||
| Obese (>30 kg/m2) | 48 (30) | 14.09 ± 4.94 | 4 (36.4) | 25 (43.1) | 19 (20.9) | |||
| Location living % | 0.001C | |||||||
| Urban | 102 (63.7) | 14.73 ± 5.47 | 9 (81.8) | 42 (72.4) | 51 (56) | 0.056 d | ||
| Rural | 58 (36.3) | 17.72 ± 5.38 | 2 (18.2) | 16 (27.6) | 40 (44) | |||
| Housing % | 0.001C | 0.003d | ||||||
| House | 93 (58.1) | 17.03 ± 5.65 | 6 (54.5) | 24 (41.4) | 63 (69.2) | |||
| Apartment | 67 (41.9) | 14.13 ± 5.21 | 5 (45.5) | 34 (58.6) | 28 (30.8) | |||
| Marital status % | <0.0001C | 0.002d | ||||||
| Married | 84 (52.5) | 14.73 ± 5.47 | 4 (36.4) | 21 (36.2) | 59 (64.8) | |||
| Single | 76 (47.5) | 14.73 ± 5.47 | 7 (63.6) | 37 (63.8) | 76 (47.5) | |||
| Parity % | <0.0001C | 0.006d | ||||||
| Nulliparous | 76 (47.5) | 14.05 ± 5.68 | 7 (36.6) | 37 (63.8) | 32 (35.2) | |||
| Primiparous | 18 (11.3) | 19.46 ± 5.78 | 1 (9.1) | 2 (3.4) | 15 (16.5) | |||
| Multiparous | 66 (41.2) | 16.84 ± 4.85 | 3 (27.3) | 19 (32.8) | 44 (48.4) | |||
| Profession % | <0.0001C | 0.033d | ||||||
| Housewife | 80 (50) | 16.73 ± 5.58 | 6 (54.5) | 22 (37.9) | 52(57.1) | |||
| Students | 56 (35) | 13.56 ± 5.04 | 5 (45.5) | 28 (48.3) | 23 (25.3) | |||
| Official/salaried | 24 (15) | 18.02 ± 5.67 | 0 | 8 (13.8) | 16 (17.6) | |||
| Socio-Demographic Characteristics | Indoor Sun Exposure | Outdoor Sun Exposure | Sun Protection Practices | ||||
|---|---|---|---|---|---|---|---|
| Median [IQR] | p-Value | Median [IQR] | p-Value | Median [IQR] | p-Value | ||
| Age (yr) | 0.16 a | 0.633 a | 0.266 a | ||||
| 18–25 | 4.50 [4.5] | 7.31 [6.19] | 3.38 [3.52] | ||||
| 26–35 | 5.06 [2.81] | 9.56 [6.75] | 3.38 [2.95] | ||||
| 36–45 | 4.5 [4.22] | 7.31 [6.47] | 3.38 [1.64] | ||||
| BMI (Kg/m2) | 0.152 a | <0.0001a | 0.673 a | ||||
| Normal weight (<25 kg/m2) | 4.50 [5.06] | 10.13 [6.19] | 3.38 [3.94] | ||||
| Overweight (25.0 to <30 kg/m2) | 5.16 [2.16] | 8.34 [5.91] | 3.38 [2.06] | ||||
| Obese(>30 kg/m2) | 4.78 [3.33] | 6.28 [3.38] | 3.38 [2.67] | ||||
| Location living | <0.0001b | 0.907 b | 0.001b | ||||
| Urban | 3.75 [5.06] | 7.41 [5.63] | 3.56 [2.95] | ||||
| Rural | 5.63 [1.69] | 8.91 [6.75] | 3.38 [2.06] | ||||
| Housing | <0.0001b | 0.356 b | 0.007b | ||||
| House | 5.06 [1.41] | 7.88 [6.75] | 3.38 [3.38] | ||||
| Apartment | 1.5 [4.5] | 7.88 [5.63] | 3.38 [2.06] | ||||
| Marital status | <0.0001b | 0.309 b | <0.0001b | ||||
| Married | 5.06 [1.13] | 9.56 [6.19] | 3.38 [3.38] | ||||
| Single | 3.38 [5.06] | 7.31 [5.90] | 3.38 [1.69] | ||||
| Parity | <0.0001b | 0.14 b | 0.001b | ||||
| Nulliparous | 3.38 [5.06] | 7.31 [5.90] | 3.38 [1.68] | ||||
| Primiparous | 5.63 [1.26] | 10.97 [5.06] | 3.38 [2.25] | ||||
| Multiparous | 5.06 [1.73] | 7.59 [6.75] | 3.38 [3.38] | ||||
| Profession | <0.0001b | 0.007b | 0.003b | ||||
| Housewife | 5.06 [1.5] | 7.41 [6.61] | 3.38 [3.38] | ||||
| Students | 3.38 [5.06] | 7.31 [5.34] | 3.09 [1.69] | ||||
| Official/salaried | 4.78 [5.48] | 10.69 [5.48] | 3.38 [1.36] | ||||
| SES Domains | Sun Exposure Classes | ||||||
|---|---|---|---|---|---|---|---|
| Total Median [IQR] | Spearman’s Rho | p-Value a | Insufficient | Moderately Sufficient | Sufficient | p-Value b | |
| Median [IQR] | |||||||
| Indoor sun exposure | 4.78 [3.00] | 0.65 | <0.0001 | 1.5 [2.06] | 6.80 [4.84] | 10.47 [6.66] | <0.0001 |
| Outdoor sun exposure | 7.88 [6.19] | 0.827 | <0.0001 | 2.48 [1.31] | 6.09 [2.25] | 11.25 [4.5] | <0.0001 |
| Sun protection practices | 3.38 [3.18] | 0.658 | <0.0001 | 1.13 [0.75] | 2.25 [1.69] | 3.75 [2.25] | <0.0001 |
| Pairwise comparisons of sun exposure classes | |||||||
| Insufficient-moderately sufficient | 0.103 | ||||||
| Insufficient-sufficient | <0.0001 | ||||||
| Moderately sufficient-sufficient | <0.0001 | ||||||
| SES Domains | Serum 25-OHD (ng/mL) | |
|---|---|---|
| Spearman’s Rho | p-Value | |
| Sun exposure score | 0.615 | <0.0001 |
| Indoor sun exposure | 0.307 | <0.0001 |
| Outdoor sun exposure | 0.605 | <0.0001 |
| Sun protection practices | 0.424 | <0.0001 |
| Sun Exposure Classes | Serum 25-OHD (ng/mL) | Vitamin D Status | |||
|---|---|---|---|---|---|
| N = 160 Median [IQR] | p-Value a | Deficiency | Insufficiency | p-Value | |
| N = 104 | N = 56 | ||||
| Frequency (%) | |||||
| Insufficient | 4.78 [4.65] | <0.0001 | 11 (10.6) | 0 | 0.001 b |
| Moderately sufficient | 6.80 [4.84] | <0.0001 | 49 (47.1) | 9 (16.1) | |
| Sufficient | 10.47 [6.66] | <0.0001 | 44 (42.3) | 47 (83.9) | |
| Sun exposure score (Mean ± SD) | 8.42 [6.41] | <0.0001 | 13.62 ± 4.81 | 19.88 ± 4.78 | <0.0001 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lhilali, I.; Zouine, N.; Menouni, A.; Godderis, L.; Kestemont, M.-P.; El Midaoui, A.; El Jaafari, S.; Filali-Zegzouti, Y. Sun Exposure Score and Vitamin D Levels in Moroccan Women of Childbearing Age. Nutrients 2023, 15, 688. https://doi.org/10.3390/nu15030688
Lhilali I, Zouine N, Menouni A, Godderis L, Kestemont M-P, El Midaoui A, El Jaafari S, Filali-Zegzouti Y. Sun Exposure Score and Vitamin D Levels in Moroccan Women of Childbearing Age. Nutrients. 2023; 15(3):688. https://doi.org/10.3390/nu15030688
Chicago/Turabian StyleLhilali, Ilham, Noura Zouine, Aziza Menouni, Lode Godderis, Marie-Paule Kestemont, Adil El Midaoui, Samir El Jaafari, and Younes Filali-Zegzouti. 2023. "Sun Exposure Score and Vitamin D Levels in Moroccan Women of Childbearing Age" Nutrients 15, no. 3: 688. https://doi.org/10.3390/nu15030688
APA StyleLhilali, I., Zouine, N., Menouni, A., Godderis, L., Kestemont, M.-P., El Midaoui, A., El Jaafari, S., & Filali-Zegzouti, Y. (2023). Sun Exposure Score and Vitamin D Levels in Moroccan Women of Childbearing Age. Nutrients, 15(3), 688. https://doi.org/10.3390/nu15030688








