The Relationship between Dietary Calcium and Age-Related Macular Degeneration
Abstract
1. Introduction
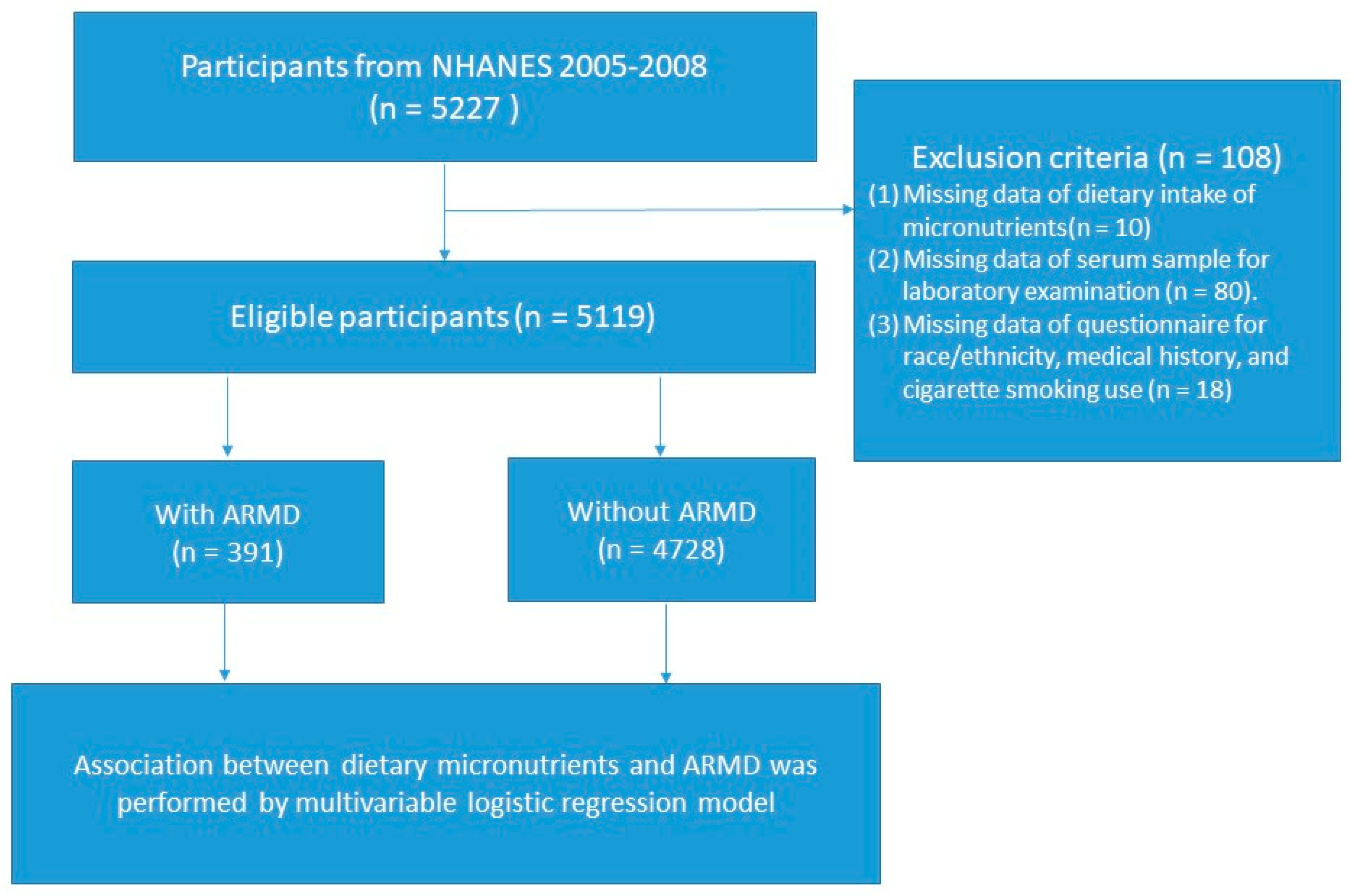
2. Methods
2.1. Study Design and Participant Recruitment
2.2. Definition of ARMD and Ophthalmic Examination
2.3. Assessment of Dietary Mineral Elements
2.4. Study Variables
2.5. Statistical Analyses
3. Results
3.1. Study Population
3.2. Association between Micronutrients and ARMD
3.3. Comparison of the Relationship between Dietary, Serum Concentration of Calcium, and ARMD
3.4. Association between Tertiles of Dietary Calcium and The Presence of ARMD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fleckenstein, M.; Keenan, T.D.L.; Guymer, R.H.; Chakravarthy, U.; Schmitz-Valckenberg, S.; Klaver, C.C.; Wong, W.T.; Chew, E.Y. Age-related macular degeneration. Nat. Rev. Dis. Prim. 2021, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Bressler, N.M. Age-related macular degeneration is the leading cause of blindness. JAMA 2004, 291, 1900–1901. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Klein, R.; Chou, C.F.; Klein, B.E.; Zhang, X.; Meuer, S.M.; Saaddine, J.B. Prevalence of age-related macular degeneration in the US population. Arch. Ophthalmol. 2011, 129, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Wittenborn, J.S.; Zhang, X.; Feagan, C.W.; Crouse, W.L.; Shrestha, S.; Kemper, A.R.; Hoerger, T.J.; Saaddine, J.B. The economic burden of vision loss and eye disorders among the United States population younger than 40 years. Ophthalmology 2013, 120, 1728–1735. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Zimbrón, L.F.; Zamora-Alvarado, R.; Ochoa-De la Paz, L.; Velez-Montoya, R.; Zenteno, E.; Gulias-Cañizo, R.; Quiroz-Mercado, H.; Gonzalez-Salinas, R. Age-Related Macular Degeneration: New Paradigms for Treatment and Management of AMD. Oxidative Med. Cell. Longev. 2018, 2018, 8374647. [Google Scholar] [CrossRef] [PubMed]
- Keenan, T.D.; Agrón, E.; Domalpally, A.; Clemons, T.E.; van Asten, F.; Wong, W.T.; Danis, R.G.; Sadda, S.; Rosenfeld, P.J.; Klein, M.L.; et al. Progression of Geographic Atrophy in Age-related Macular Degeneration: AREDS2 Report Number 16. Ophthalmology 2018, 125, 1913–1928. [Google Scholar] [CrossRef]
- Bowes Rickman, C.; Farsiu, S.; Toth, C.A.; Klingeborn, M. Dry age-related macular degeneration: Mechanisms, therapeutic targets, and imaging. Investig. Ophthalmol. Vis. Sci. 2013, 54, ORSF68–ORSF80. [Google Scholar] [CrossRef]
- Moutray, T.; Chakravarthy, U. Age-related macular degeneration: Current treatment and future options. Ther. Adv. Chronic Dis. 2011, 2, 325–331. [Google Scholar] [CrossRef]
- Thornton, J.; Edwards, R.; Mitchell, P.; Harrison, R.A.; Buchan, I.; Kelly, S.P. Smoking and age-related macular degeneration: A review of association. Eye 2005, 19, 935–944. [Google Scholar] [CrossRef]
- Klein, B.E.; Klein, R. Lifestyle exposures and eye diseases in adults. Am. J. Ophthalmol. 2007, 144, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Karimi, S.; Arabi, A.; Shahraki, T. Alcohol and the Eye. J. Ophthalmic Vis. Res. 2021, 16, 260–270. [Google Scholar] [CrossRef]
- Wang, S.Y.; Singh, K.; Lin, S.C. Glaucoma Prevalence and the Intake of Iron and Calcium in a Population-based Study. Curr. Eye Res. 2013, 38, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Harb, E.N.; Wildsoet, C.F. Nutritional Factors and Myopia: An Analysis of National Health and Nutrition Examination Survey Data. Optom. Vis. Sci. 2021, 98, 458–468. [Google Scholar] [CrossRef]
- Smith, W.; Assink, J.; Klein, R.; Mitchell, P.; Klaver, C.C.; Klein, B.E.; Hofman, A.; Jensen, S.; Wang, J.J.; de Jong, P.T. Risk factors for age-related mac-ular degeneration: Pooled findings from three continents. Ophthalmology 2001, 108, 697–704. [Google Scholar] [CrossRef]
- Lim, L.S.; Mitchell, P.; Seddon, J.M.; Holz, F.G.; Wong, T.Y. Age-related macular degeneration. Lancet 2012, 379, 1728–1738. [Google Scholar] [CrossRef]
- Velilla, S.; García-Medina, J.J.; García-Layana, A.; Dolz-Marco, R.; Pons-Vázquez, S.; Pinazo-Durán, M.D.; Gómez-Ulla, F.; Arévalo, J.F.; Díaz-Llopis, M.; Gallego-Pinazo, R. Smoking and age-related macular degeneration: Review and update. J. Ophthalmol. 2013, 2013, 895147. [Google Scholar] [CrossRef]
- Zhang, Q.-Y.; Tie, L.-J.; Wu, S.-S.; Lv, P.-L.; Huang, H.-W.; Wang, W.-Q.; Wang, H.; Ma, L. Overweight, Obesity, and Risk of Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Haas, P.; Kubista, K.E.; Krugluger, W.; Huber, J.; Binder, S. Impact of visceral fat and pro-inflammatory factors on the pathogenesis of age-related macular degeneration. Acta Ophthalmol. 2015, 93, 533–538. [Google Scholar] [CrossRef]
- DeAngelis, M.M.; Owen, L.A.; Morrison, M.A.; Morgan, D.J.; Li, M.; Shakoor, A.; Vitale, A.; Iyengar, S.; Stambolian, D.; Kim, I.K.; et al. Genetics of age-related macular degeneration (AMD). Hum. Mol. Genet. 2017, 26, R45–R50. [Google Scholar] [CrossRef]
- Clark, S.J.; Bishop, P.N.; Day, A.J. Complement factor H and age-related macular degeneration: The role of glycosaminoglycan recognition in disease pathology. Biochem. Soc. Trans. 2010, 38, 1342–1348. [Google Scholar] [CrossRef]
- de Koning-Backus, A.P.M.; Buitendijk, G.H.S.; Kiefte-de Jong, J.C.; Colijn, J.M.; Hofman, A.; Vingerling, J.R.; Haverkort, E.B.; Franco, O.H.; Klaver, C.C.W. Intake of Vegetables, Fruit, and Fish is Beneficial for Age-Related Macular Degeneration. Am. J. Ophthalmol. 2019, 198, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Meyers, K.J.; Mares, J.A.; Igo, R.P., Jr.; Truitt, B.; Liu, Z.; Millen, A.E.; Klein, M.; Johnson, E.J.; Engelman, C.D.; Karki, C.K.; et al. Genetic Evidence for Role of Carotenoids in Age-Related Macular Degeneration in the Carotenoids in Age-Related Eye Disease Study (CAREDS). Investig. Ophthalmol. Vis. Sci. 2014, 55, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Weikel, K.A.; Chiu, C.J.; Taylor, A. Nutritional modulation of age-related macular degeneration. Mol. Asp. Med. 2012, 33, 318–375. [Google Scholar] [CrossRef]
- Jiang, H.; Yin, Y.; Wu, C.R.; Liu, Y.; Guo, F.; Li, M.; Ma, L. Dietary vitamin and carotenoid intake and risk of age-related cataract. Am. J. Clin. Nutr. 2019, 109, 43–54. [Google Scholar] [CrossRef]
- Gorusupudi, A.; Nelson, K.; Bernstein, P.S. The Age-Related Eye Disease 2 Study: Micronutrients in the Treatment of Macular Degeneration. Adv. Nutr. 2017, 8, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Shinoda, H.; Koto, T.; Uchida, A.; Tsubota, K.; Ozawa, Y. Use of Micronutrient Supplement for Preventing Advanced Age-Related Macular Degeneration in Japan. Arch. Ophthalmol. 2012, 130, 254–255. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Davis, M.D.; Magli, Y.L.; Segal, P.; Klein, B.E.; Hubbard, L. The Wisconsin age-related maculopathy grading system. Ophthalmology 1991, 98, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Klein, B.E.; Jensen, S.C.; Mares-Perlman, J.A.; Cruickshanks, K.J.; Palta, M. Age-related maculopathy in a multiracial United States population: The National Health and Nutrition Examination Survey III. Ophthalmology 1999, 106, 1056–1065. [Google Scholar] [CrossRef]
- Wright, J.D.; Borrud, L.G.; McDowell, M.A.; Wang, C.Y.; Radimer, K.; Johnson, C.L. Nutrition assessment in the National Health And Nutrition Examination Survey 1999–2002. J. Am. Diet. Assoc. 2007, 107, 822–829. [Google Scholar] [CrossRef]
- Dodd, K.W.; Guenther, P.M.; Freedman, L.S.; Subar, A.F.; Kipnis, V.; Midthune, D.; Tooze, J.A.; Krebs-Smith, S.M. Statistical methods for estimating usual intake of nutrients and foods: A review of the theory. J. Am. Diet. Assoc. 2006, 106, 1640–1650. [Google Scholar] [CrossRef]
- Moshfegh, A.J.; Rhodes, D.G.; Baer, D.J.; Murayi, T.; Clemens, J.C.; Rumpler, W.V.; Paul, D.R.; Sebastian, R.S.; Kuczynski, K.J.; Ingwersen, L.A.; et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am. J. Clin. Nutr. 2008, 88, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Briefel, R.R.; Sempos, C.T. Dietary methodology workshop for the third National Health and Nutrition Examination Survey. March 1986. Vital Health Stat. Ser. 4 Doc. Comm. Rep. 1992, 1–108. [Google Scholar]
- Zeece, M. Introduction to the Chemistry of Food; Academic Press: Cambridge, MA, USA, 2020; pp. 163–212. [Google Scholar]
- Gopinath, B.; Flood, V.M.; Louie, J.C.; Wang, J.J.; Burlutsky, G.; Rochtchina, E.; Mitchell, P. Consumption of dairy products and the 15-year incidence of age-related macular degeneration. Br. J. Nutr. 2014, 111, 1673–1679. [Google Scholar] [CrossRef]
- Tisdale, A.K.; Agrón, E.; Sunshine, S.B.; Clemons, T.E.; Ferris, F.L., 3rd; Chew, E.Y. Association of Dietary and Supplementary Calcium Intake with Age-Related Macular Degeneration: Age-Related Eye Disease Study Report 39. JAMA Ophthalmol. 2019, 137, 543–550. [Google Scholar] [CrossRef]
- Cormick, G.; Belizán, J.M. Calcium Intake and Health. Nutrients 2019, 11, 1606. [Google Scholar] [CrossRef] [PubMed]
- Villa-Etchegoyen, C.; Lombarte, M.; Matamoros, N.; Belizán, J.M.; Cormick, G. Mechanisms Involved in the Relationship between Low Calcium Intake and High Blood Pressure. Nutrients 2019, 11, 1112. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Ouyang, Y.; Liu, J.; Zhao, G.; Bao, W.; Yan, M. Dietary calcium intake and mortality risk from cardiovascular disease and all causes: A meta-analysis of prospective cohort studies. BMC Med. 2014, 12, 158. [Google Scholar] [CrossRef] [PubMed]
- Bonovas, S.; Fiorino, G.; Lytras, T.; Malesci, A.; Danese, S. Calcium supplementation for the prevention of colorectal adenomas: A systematic review and meta-analysis of randomized controlled trials. World J. Gastroenterol. 2016, 22, 4594–4603. [Google Scholar] [CrossRef]
- Flood, A.; Peters, U.; Chatterjee, N.; Lacey, J.V., Jr.; Schairer, C.; Schatzkin, A. Calcium from Diet and Supplements is Associated With Reduced Risk of Colorectal Cancer in a Prospective Cohort of Women. Cancer Epidemiol. Biomark. Prev. 2005, 14, 126–132. [Google Scholar] [CrossRef]
- Baloch, Z.; LiVolsi, V.A. Fifty years of thyroid pathology: Concepts and developments. Hum. Pathol. 2020, 95, 46–54. [Google Scholar] [CrossRef]
- Gleichmann, M.; Mattson, M.P. Neuronal calcium homeostasis and dysregulation. Antioxid. Redox Signal. 2011, 14, 1261–1273. [Google Scholar] [CrossRef] [PubMed]
- Brini, M.; Calì, T.; Ottolini, D.; Carafoli, E. Neuronal calcium signaling: Function and dysfunction. Cell. Mol. Life Sci. 2014, 71, 2787–2814. [Google Scholar] [CrossRef] [PubMed]
- Wojda, U.; Salinska, E.; Kuznicki, J. Calcium ions in neuronal degeneration. IUBMB Life 2008, 60, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Bezprozvanny, I. Calcium signaling and neurodegenerative diseases. Trends Mol. Med. 2009, 15, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Crish, S.D.; Calkins, D.J. Neurodegeneration in glaucoma: Progression and calcium-dependent intracellular mechanisms. Neuroscience 2011, 176, 1–11. [Google Scholar] [CrossRef]
- Kakigi, C.L.M.; Singh, K.; Wang, S.Y.; Enanoria, W.T.; Lin, S.C. Self-reported Calcium Supplementation and Age-Related Macular Degeneration. JAMA Ophthalmol. 2015, 133, 746–754. [Google Scholar] [CrossRef]
- Irnaten, M.; Duff, A.; Clark, A.; O’Brien, C. Intra-Cellular Calcium Signaling Pathways (PKC, RAS/RAF/MAPK, PI3K) in Lamina Cribrosa Cells in Glaucoma. J. Clin. Med. 2020, 10, 62. [Google Scholar] [CrossRef]
- Zhang, L.; Hui, Y.N.; Wang, Y.S.; Ma, J.X.; Wang, J.B.; Ma, L.N. Calcium overload is associated with lipofuscin formation in human retinal pigment epithelial cells fed with photoreceptor outer segments. Eye 2011, 25, 519–527. [Google Scholar] [CrossRef]
- Aslam, T.; Delcourt, C.; Silva, R.; Holz, F.G.; Leys, A.; Garcià Layana, A.; Souied, E. Micronutrients in Age-Related Macular Degeneration. Ophthalmologica 2013, 229, 75–79. [Google Scholar] [CrossRef]
- Evans, J.R.; Lawrenson, J.G. Antioxidant vitamin and mineral supplements for preventing age-related macular degeneration. Cochrane Database Syst. Rev. 2017, 7, CD000253. [Google Scholar] [CrossRef]
- Itoh, M.; Oh-Ishi, S.; Hatao, H.; Leeuwenburgh, C.; Selman, C.; Ohno, H.; Kizaki, T.; Nakamura, H.; Matsuoka, T. Effects of dietary calcium restriction and acute exercise on the antioxidant enzyme system and oxidative stress in rat diaphragm. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R33–R38. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.; Ahn, J.; Kim, M.; Hwang, S.Y.; Kim, S.W.; Oh, J. Ocular Perfusion Pressure and Choroidal Thickness in Early Age-Related Macular Degeneration Patients With Reticular Pseudodrusen. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6604–6609. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Tsujiguchi, H.; Hara, A.; Kambayashi, Y.; Miyagi, S.; Thu Nguyen, T.T.; Suzuki, K.; Tao, Y.; Sakamoto, Y.; Shimizu, Y.; et al. Dietary Calcium Intake and Hypertension: Importance of Serum Concentrations of 25-Hydroxyvitamin D. Nutrients 2019, 11, 911. [Google Scholar] [CrossRef]
- Lee, J.; Kim, M.; Lee, C.S.; Kim, S.S.; Koh, H.J.; Lee, S.C.; Byeon, S.H. Drusen Subtypes and Choroidal Characteristics in Asian Eyes with Typical Neovascular Age-Related Macular Degeneration. Retina 2020, 40, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Kankanahalli, S.; Burlina, P.M.; Wolfson, Y.; Freund, D.E.; Bressler, N.M. Automated Classification of Severity of Age-Related Macular Degeneration from Fundus Photographs. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1789–1796. [Google Scholar] [CrossRef]
- Abdelsalam, A.; Del Priore, L.; Zarbin, M.A. Drusen in age-related macular degeneration: Pathogenesis, natural course, and laser photocoagula-tion-induced regression. Surv. Ophthalmol. 1999, 44, 1–29. [Google Scholar] [CrossRef]
- Thompson, R.B.; Reffatto, V.; Bundy, J.G.; Kortvely, E.; Flinn, J.M.; Lanzirotti, A.; Jones, E.A.; McPhail, D.S.; Fearn, S.; Boldt, K.; et al. Identification of hydroxyapatite spherules provides new insight into subretinal pigment epithelial deposit formation in the aging eye. Proc. Natl. Acad. Sci. USA 2015, 112, 1565–1570. [Google Scholar] [CrossRef]
- Pilgrim, M.G.; Lengyel, I.; Lanzirotti, A.; Newville, M.; Fearn, S.; Emri, E.; Knowles, J.C.; Messinger, J.D.; Read, R.W.; Guidry, C.; et al. Subretinal Pigment Epithelial Deposition of Drusen Components Including Hydroxyapatite in a Primary Cell Culture Model. Investig. Ophthalmol. Vis. Sci. 2017, 58, 708–719. [Google Scholar] [CrossRef]
- Chew, E.Y.; Clemons, T.E.; Sangiovanni, J.P.; Danis, R.P.; Ferris, F.L., 3rd; Elman, M.J.; Antoszyk, A.N.; Ruby, A.J.; Orth, D.; Bressler, S.B.; et al. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report No. 3. JAMA Ophthalmol. 2014, 132, 142–149. [Google Scholar] [CrossRef]
- Goltzman, D.; Mannstadt, M.; Marcocci, C. Physiology of the Calcium-Parathyroid Hormone-Vitamin D Axis. Front. Horm. Res. 2018, 50, 1–13. [Google Scholar] [CrossRef]
- Parekh, N.; Chappell, R.J.; Millen, A.E.; Albert, D.M.; Mares, J.A. Association between vitamin D and age-related macular degeneration in the Third National Health and Nutrition Examination Survey, 1988 through 1994. Arch. Ophthalmol. 2007, 125, 661–669. [Google Scholar] [CrossRef]
- Layana, A.G.; Minnella, A.M.; Garhöfer, G.; Aslam, T.; Holz, F.G.; Leys, A.; Silva, R.; Delcourt, C.; Souied, E.; Seddon, J.M. Vitamin D and Age-Related Macular Degeneration. Nutrients 2017, 9, 1120. [Google Scholar] [CrossRef] [PubMed]
- Millen, A.E.; Voland, R.; Sondel, S.A.; Parekh, N.; Horst, R.L.; Wallace, R.B.; Hageman, G.S.; Chappell, R.; Blodi, B.A.; Klein, M.L.; et al. Vitamin D status and early age-related macular degeneration in postmenopausal women. Arch. Ophthalmol. 2011, 129, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Seddon, J.M.; Reynolds, R.; Shah, H.R.; Rosner, B. Smoking, dietary betaine, methionine, and vitamin D in monozygotic twins with discordant macular degeneration: Epigenetic implications. Ophthalmology 2011, 118, 1386–1394. [Google Scholar] [CrossRef]
- Aoki, A.; Inoue, M.; Nguyen, E.; Obata, R.; Kadonosono, K.; Shinkai, S.; Hashimoto, H.; Sasaki, S.; Yanagi, Y. Dietary n-3 Fatty Acid, α-Tocopherol, Zinc, vitamin D, vitamin C, and β-carotene are Associated with Age-Related Macular Degeneration in Japan. Sci. Rep. 2016, 6, 20723. [Google Scholar] [CrossRef] [PubMed]
- Day, S.; Acquah, K.; Platt, A.; Lee, P.P.; Mruthyunjaya, P.; Sloan, F.A. Association of Vitamin D Deficiency and Age-Related Macular Degeneration in Medicare Beneficiaries. Arch. Ophthalmol. 2012, 130, 1070–1071. [Google Scholar] [CrossRef]
- Patel, P.; Mughal, M.Z.; Patel, P.; Yagnik, B.; Kajale, N.; Mandlik, R.; Khadilkar, V.; Chiplonkar, S.A.; Phanse, S.; Patwardhan, V.; et al. Dietary calcium intake influences the relationship between serum 25-hydroxyvitamin D3 (25OHD) concentration and parathyroid hormone (PTH) concentration. Arch. Dis. Child. 2016, 101, 316–319. [Google Scholar] [CrossRef]
- Pepe, J.; Cipriani, C.; Tedeschi, M.; Curione, M.; Parravano, M.; Varano, M.; Biamonte, F.; Colangelo, L.; Minisola, S. Retinal micro-vascular and aortic macro-vascular changes in postmenopausal women with primary hyperparathyroidism. Sci. Rep. 2018, 8, 16521. [Google Scholar] [CrossRef]
- Toulouie, S.; Yiu, G.; Pan, J.; Chang, S.; Snyder, K. Relationship of Retinal Vessel Diameters with Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2021, 62, 339. [Google Scholar]
- Trinh, M.; Kalloniatis, M.; Nivison-Smith, L. Vascular Changes in Intermediate Age-Related Macular Degeneration Quantified Using Optical Coherence Tomography Angiography. Transl. Vis. Sci. Technol. 2019, 8, 20. [Google Scholar] [CrossRef] [PubMed]
| Variables | ARMD (n = 391) | No ARMD (n = 4728) | p-Value |
|---|---|---|---|
| Continuous variables, mean (SD) | |||
| Age (years) | 70.20 (11.43) | 58.33 (11.99) | 0.008 |
| Body mass index (kg/m2) | 28.51 (5.55) | 29.28 (5.58) | 0.005 |
| Dietary micronutrients | |||
| Calcium (mg) | 801.65 (455.62) | 864.34 (535.11) | 0.013 |
| Magnesium (mg) | 261.18 (116.10) | 286.87 (144.44) | 0.002 |
| Iron (mg) | 14.35 (8.18) | 14.85 (8.32) | 0.540 |
| Zinc (mg) | 10.69 (9.30) | 11.64 (11.65) | 0.506 |
| Copper (mg) | 1.34 (1.09) | 1.32 (1.23) | 0.176 |
| Sodium (mg) | 2844.92 (1425.18) | 3209.50 (1713.98) | <0.001 |
| Selenium (mcg) | 92.25 (47.49) | 103.90 (58.44) | 0.003 |
| Laboratory data | |||
| Serum calcium (mg/dL) | 8.00 (4.21) | 8.60 (5.32) | 0.013 |
| Serum glucose (mg/dL) | 104.61 (31.33) | 106.58 (40.59) | 0.010 |
| ALT (U/L) | 23.91 (23.01) | 25.83 (20.12) | 0.351 |
| Platelet (103/uL) | 255.71 (72.89) | 268.51 (69.37) | 0.858 |
| Creatinine (mg/dL) | 1.01 (0.34) | 0.94 (0.45) | 0.208 |
| HDL-Cholesterol (mg/dL) | 55.04 (16.36) | 53.32 (16.31) | 0.493 |
| Cholesterol (mg/dL) | 200.45 (41.72) | 203.63 (43.08) | 0.799 |
| C-reactive protein (mg/dL) | 0.57 (1.19) | 0.46 (0.85) | <0.001 |
| Category Variables, (%) | |||
| Gender (male) | 209 (53.4) | 2395 (50.6) | 0.338 |
| Non-Hispanic White | 283 (72.3) | 2532 (53.5) | 0.144 |
| Coronary heart disease | 40 (10.2) | 256 (5.4) | 0.659 |
| Angina pectoris | 26 (6.6) | 184 (3.9) | 0.559 |
| Liver condition | 15 (3.83) | 212 (4.48) | 0.569 |
| Cigarette smoking | 60 (15.3) | 867 (18.3) | 0.004 |
| Model 1 OR (95% CI) | p-Value | Model 2 OR (95% CI) | p-Value | Model 3 OR (95% CI) | p-Value | Model 4 OR (95% CI) | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Age-related Macular Degeneration | ||||||||
| Calcium | 0.629 (0.458–0.853) | 0.004 | 0.687 (0.488–0.967) | 0.031 | 0.672 (0.476–0.949) | 0.024 | 0.680 (0.482–0.960) | 0.029 |
| Magnesium | 0.160 (0.051–0.502) | 0.002 | 0.308 (0.089–1.065) | 0.063 | 0.291 (0.083–1.027) | 0.055 | 0.343 (0.097–1.215) | 0.097 |
| Iron | 0.000 (0.000–8.454) | 0.082 | 0.000 (0.000–9.542) | 0.175 | 0.000 (0.000–4.356) | 0.191 | 0.000 (0.000–7.307) | 0.259 |
| Zinc | 0.000 (0.000–0.258) | 0.038 | 0.000 (0.000–7.040) | 0.244 | 0.000 (0.000–6.722) | 0.244 | 0.000 (0.000–4.544) | 0.249 |
| Copper | 0.000 (0.000–2.595) | 0.623 | 0.000 (0.000–3.330) | 0.828 | 0.000 (0.000–5.315) | 0.778 | 0.000 (0.000–7.310) | 0.796 |
| Sodium | 0.858 (0.780–0.944) | 0.002 | 0.950 (0.858–1.053) | 0.330 | 0.946 (0.853–1.048) | 0.287 | 0.954 (0.861–1.057) | 0.365 |
| Selenium | 0.018 (0.001–0.312) | 0.006 | 0.208 (0.010–4.484) | 0.316 | 0.174 (0.008–3.856) | 0.269 | 0.222 (0.010–4.866) | 0.339 |
| Model 1 OR (95% CI) | p-Value | Model 2 OR (95% CI) | p-Value | Model 3 OR (95% CI) | p Value | Model 4 OR (95% CI) | p Value | |
|---|---|---|---|---|---|---|---|---|
| Age-related Macular Degeneration | ||||||||
| Dietary calcium | 0.629 (0.458–0.853) | 0.004 | 0.687 (0.488–0.967) | 0.031 | 0.671 (0.475–0.948) | 0.024 | 0.680 (0.482–0.960) | 0.029 |
| Serum calcium | 0.768 (0.523–1.129) | 0.179 | 0.784 (0.525–1.169) | 0.233 | 0.836 (0.554–1.261) | 0.392 | 0.839 (0.555–1.269) | 0.405 |
| Dietary Calcium | Model 1 OR (95% CI) | p Value | Model 2 OR (95% CI) | p Value | Model 3 OR (95% CI) | p Value | Model 4 OR (95% CI) | p Value |
|---|---|---|---|---|---|---|---|---|
| Age-related Macular Degeneration | ||||||||
| T1 vs T3 | 0.886 (0.635–1.237) | 0.478 | 0.891 (0.631–1.256) | 0.509 | 0.891 (0.630–1.261) | 0.514 | 0.910 (0.643–1.289) | 0.597 |
| T2 vs T3 | 0.647 (0.452–0.928) | 0.018 | 0.696 (0.479–1.012) | 0.058 | 0.680 (0.466–0.992) | 0.045 | 0.684 (0.468–1.000) | 0.050 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-Y.; Chen, Y.-J. The Relationship between Dietary Calcium and Age-Related Macular Degeneration. Nutrients 2023, 15, 671. https://doi.org/10.3390/nu15030671
Chen Y-Y, Chen Y-J. The Relationship between Dietary Calcium and Age-Related Macular Degeneration. Nutrients. 2023; 15(3):671. https://doi.org/10.3390/nu15030671
Chicago/Turabian StyleChen, Yuan-Yuei, and Ying-Jen Chen. 2023. "The Relationship between Dietary Calcium and Age-Related Macular Degeneration" Nutrients 15, no. 3: 671. https://doi.org/10.3390/nu15030671
APA StyleChen, Y.-Y., & Chen, Y.-J. (2023). The Relationship between Dietary Calcium and Age-Related Macular Degeneration. Nutrients, 15(3), 671. https://doi.org/10.3390/nu15030671






