Malnutrition, Cancer Stage and Gastrostomy Timing as Markers of Poor Outcomes in Gastrostomy-Fed Head and Neck Cancer Patients
Abstract
1. Introduction
- According to guidelines, is the patient a suitable candidate for gastrostomy, with a life expectancy longer than 3 weeks?
- How to use nutritional and laboratory data to identify HNC patients with severely impaired nutritional status, and unfavorable outcomes months after the gastrostomy, requiring more powerful nutritional support with larger protein energy intake?
- Evaluate the clinical and nutritional status of HNC patients when referred to endoscopic gastrostomy for long-term enteral nutrition, using anthropometry, laboratory data and accessible tools, even with patients who cannot speak.
- Evaluate the clinical outcome of PEG-fed HNC patients.
- Evaluate the relations between survival, severity, and nutritional status:
- Compare the nutritional status with the different TNM-defined stages.
- Compare the nutritional status with the different grades of stage IV, grouped as having metastases and no distant metastases (IVa and IVb, against IVc).
- Evaluate the impact of clinical and nutritional status on the survival of PEG-fed HNC patients, using TNM-defined stages, anthropometry, and laboratory data.
2. Materials and Methods
2.1. Patients
2.2. The Clinic, Anthropometric and Laboratory Data
2.3. Head and Neck Cancer TNM Classification of Malignant Tumors
2.4. Clinical Outcome
2.5. Anthropometric Evaluation
- Body mass index (BMI) was obtained in most patients using the equation Weight (Kg)/Height (m)2. If patients were bedridden and could not stand up for weight and height evaluation, BMI was estimated using the mid-upper arm circumference (MUAC) and regression equations described by Powell-Tuck and Hennessy [27]; this method has been previously used and proved to provide a reliable BMI estimation in PEG patients [28,29]. Each patient was classified by the WHO classification according to their age as having low BMI if was <18.5 kg/m2 or < 22 kg/m2, normal BMI if 18.5–25 kg/m2 or 22–27 kg/m2, and high BMI if >25 kg/m2 or >27 kg/m2, for patients under 65 years or 65 years old or older, respectively [30] (Table 1).
| Low | Normal | High | |
|---|---|---|---|
| <65 Years | <18.5 kg/m2 | ≥18.5–<25 kg/m2 | ≥25 kg/m2 |
| ≥65 Years | <22 kg/m2 | ≥22–<27 kg/m2 | ≥27 kg/m2 |
- 2.
- Mid-upper arm circumference (MUAC) was evaluated using an inextensible measuring tape, with a 1 mm resolution. MUAC results were obtained from evaluating several tissues representing fat and lean mass.
- 3.
- Tricipital skinfold (TSF), was measured using a Lange skinfold caliper with a 1 mm resolution. TSF evaluates the subcutaneous adipose tissue and estimates adipose reserves.
- 4.
- The mid-arm muscle circumference (MAMC) was calculated according to the equation: MAMC = MUAC (cm) − 0.314 × TSF (mm). The MAMC allows us to estimate lean and muscle mass.
2.6. Laboratory Evaluation
2.7. Statistics
3. Results
3.1. Subjects
3.2. Head or Neck Cancers
Cancer Location
3.3. Anthropometry
3.3.1. Body Mass Index (BMI)
3.3.2. Mid-Upper Arm Circumference (MUAC)
3.3.3. Tricipital Skinfold (TSF)
3.3.4. Mid-Arm Muscle Circumference (MAMC)
3.4. Laboratory Assessment
3.4.1. Serum albumin
3.4.2. Serum Transferrin
3.4.3. Total Serum Cholesterol
3.5. Clinical Outcome
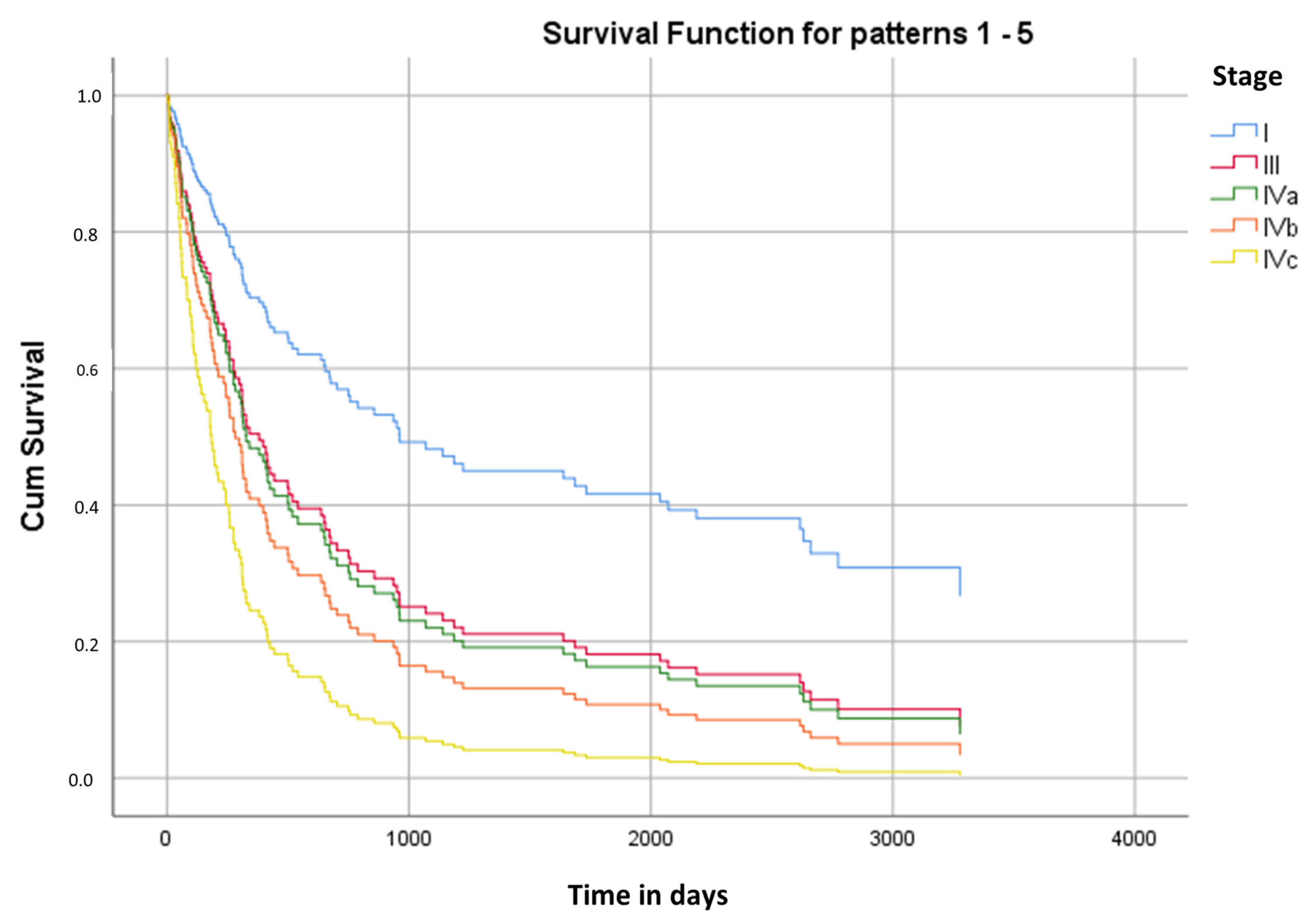
3.6. Kaplan–Meier Survival Analysis
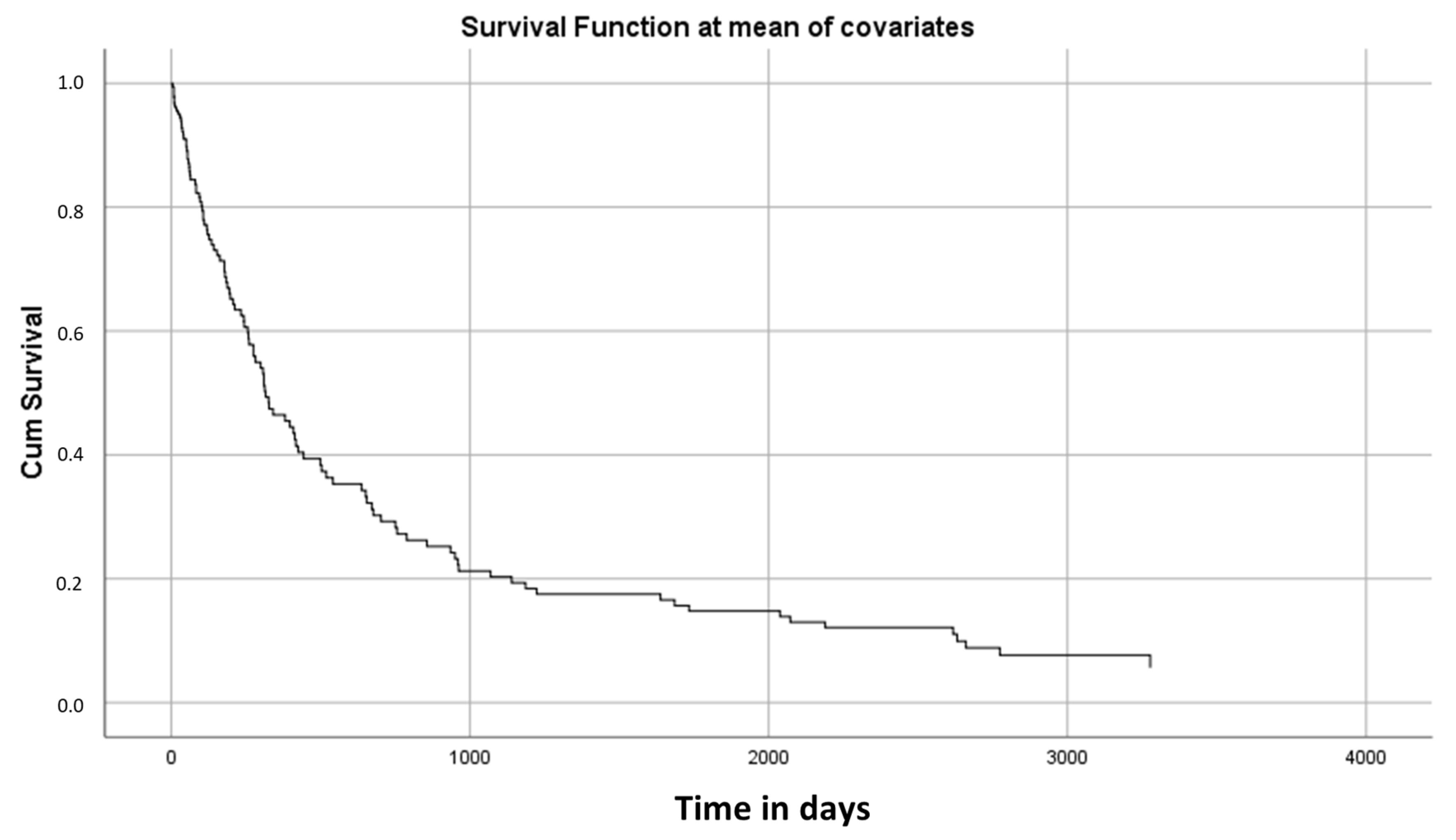
3.7. Cox Regression Analysis
3.8. Regression Analysis of Cancer Stage Impact on Nutrition Markers
3.8.1. TNM-Defined Stages (I vs. II vs. III vs. IVa vs. IVb vs. IVc)
3.8.2. TNM-Defined Stages IVa and IVb vs. IVc
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ross, L.J.; Wilson, M.; Banks, M.; Rezannah, F.; Daglish, M. Prevalence of Malnutrition and Nutritional Risk Factors in Patients Undergoing Alcohol and Drug Treatment. Nutrition 2012, 28, 738–743. [Google Scholar] [CrossRef] [PubMed]
- White, J.V.; Guenter, P.; Jensen, G.; Malone, A.; Schofield, M. Consensus Statement: Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition. J. Parenter. Enter. Nutr. 2012, 36, 275–283. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J.P.; Shaha, A.R. Nutrition Management of Patients with Malignancies of the Head and Neck. Surg. Clin. N. Am. 2011, 91, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Silander, E.; Nyman, J.; Hammerlid, E. An Exploration of Factors Predicting Malnutrition in Patients with Advanced Head and Neck Cancer. Laryngoscope 2013, 123, 2428–2434. [Google Scholar] [CrossRef]
- Alshadwi, A.; Nadershah, M.; Carlson, E.; Young, L.; Burke, P.; Daley, B. Nutritional Considerations for Head and Neck Cancer Patients: A Review of the Literature. J. Oral Maxillofac. Surg. 2013, 71, 1853–1860. [Google Scholar] [CrossRef]
- Kubrak, C.; Olson, K.; Jha, N.; Jensen, L.; McCargar, L.; Seikaly, H.; Harris, J.; Scrimger, R.; Parliament, M.; Baracos, V.E. Nutrition Impact Symptoms: Key Determinants of Reduced Dietary Intake, Weight Loss, and Reduced Functional Capacity of Patients with Head and Neck Cancer before Treatment. Head Neck 2009, 32, 290–300. [Google Scholar] [CrossRef]
- Paccagnella, A.; Morello, M.; Da Mosto, M.C.; Baruffi, C.; Marcon, M.L.; Gava, A.; Baggio, V.; Lamon, S.; Babare, R.; Rosti, G.; et al. Early Nutritional Intervention Improves Treatment Tolerance and Outcomes in Head and Neck Cancer Patients Undergoing Concurrent Chemoradiotherapy. Support. Cancer Ther. 2009, 18, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Gómez, C.C.; Olivar, R.J.; García, M.; Marín, M.; Madero, R.; Pérez-Portabella, C.; Planás, M.; Mokoroa, A.; Pereyra, F.; Martín Palmero, A. Assessment of a malnutrition screening tool in cancer patients. Nutr. Hosp. 2010, 25, 400–405. [Google Scholar]
- Ravasco, P.; Monteiro-Grillo, I.; Vidal, P.M.; Camilo, M.E. Nutritional Deterioration in Cancer: The Role of Disease and Diet. Clin. Oncol. 2003, 15, 443–450. [Google Scholar] [CrossRef]
- Arends, J.; Bodoky, G.; Bozzetti, F.; Fearon, K.; Muscaritoli, M.; Selga, G.; Von Meyenfeldt, M.; Zürcher, G.; Fietkau, R.; Aulbert, E.; et al. ESPEN Guidelines on Enteral Nutrition: Non-Surgical Oncology. Clin. Nutr. 2006, 25, 245–259. [Google Scholar] [CrossRef]
- Sandmæl, J.A.; Sand, K.; Bye, A.; Solheim, T.S.; Oldervoll, L.; Helvik, A. Nutritional Experiences in Head and Neck Cancer Patients. Eur. J. Cancer Care 2019, 28, e13168. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, M.; Liu, C.; Ye, Y.; Huang, G. Percutaneous Endoscopic Gastrostomy versus Nasogastric Tube Feeding for Patients with Head and Neck Cancer: A Systematic Review. J. Radiat. Res. 2014, 55, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Yagishita, A.; Kakushima, N.; Tanaka, M.; Takizawa, K.; Yamaguchi, Y.; Matsubayashi, H.; Ono, H. Percutaneous Endoscopic Gastrostomy Using the Direct Method for Aerodigestive Cancer Patients. Eur. J. Gastroenterol. Hepatol. 2012, 24, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Nicholl, M.B.; Lyons, D.A.; Wheeler, A.A.; Jorgensen, J.B. Repeat PEG Placement Is Safe for Head and Neck Cancer Patients. Am. J. Otolaryngol. 2014, 35, 89–92. [Google Scholar] [CrossRef]
- Rutter, C.E.; Yovino, S.; Taylor, R.; Wolf, J.; Cullen, K.J.; Ord, R.; Athas, M.; Zimrin, A.; Strome, S.; Suntharalingam, M. Impact of Early Percutaneous Endoscopic Gastrostomy Tube Placement on Nutritional Status and Hospitalization in Patients with Head and Neck Cancer Receiving Definitive Chemoradiation Therapy. Head Neck 2010, 33, 1441–1447. [Google Scholar] [CrossRef]
- Müller-Richter, U.; Betz, C.; Hartmann, S.; Brands, R.C. Nutrition Management for Head and Neck Cancer Patients Improves Clinical Outcome and Survival. Nutr. Res. 2017, 48, 1–8. [Google Scholar] [CrossRef]
- Reis, T.G.; da Silva, R.A.W.P.; Nascimento, E.D.S.; de Bessa, J.; Oliveira, M.C.; Fava, A.S.; Lehn, C.N. Early Postoperative Serum Albumin Levels as Predictors of Surgical Outcomes in Head and Neck Squamous Cell Carcinoma. Braz. J. Otorhinolaryngol. 2022, 88 (Suppl. S1), S48–S56. [Google Scholar] [CrossRef]
- Fonseca, J.; Adriana Santos, C.; Brito, J. Predicting Survival of Endoscopic Gastrostomy Candidates Using the Underlying Disease, Serum Cholesterol, Albumin and Transferrin Levels. Nutr. Hosp. 2013, 28, 1280–1285. [Google Scholar] [CrossRef]
- Santos, C.A.; Fonseca, J.; Brito, J.; Fernandes, T.; Gonçalves, L.; Sousa Guerreiro, A. Serum Zn Levels in Dysphagic Patients Who Underwent Endoscopic Gastrostomy for Long Term Enteral Nutrition. Nutr. Hosp. 2014, 29, 359–364. [Google Scholar] [CrossRef]
- Fuhrman, M.P.; Charney, P.; Mueller, C.M. Hepatic Proteins and Nutrition Assessment. J. Am. Diet. Assoc. 2004, 104, 1258–1264. [Google Scholar] [CrossRef]
- Duguet, A.; Bachmann, P.; Lallemand, Y.; Blanc-Vincent, M.P. Summary Report of the Standards, Options and Recommendations for Malnutrition and Nutritional Assessment in Patients with Cancer (1999). Br. J. Cancer 2003, 89 (Suppl. S1), S92–S97. [Google Scholar] [CrossRef]
- Guerra, L.T.; Rosa, A.R.; Romani, R.F.; Gurski, R.R.; Schirmer, C.C.; Kruel, C.D.P. Serum Transferrin and Serum Prealbumin as Markers of Response to Nutritional Support in Patients with Esophageal Cancer. Nutr. Hosp. 2009, 24, 241–242. [Google Scholar]
- Ellegård, L.H.; Bosaeus, I.G. Biochemical Indices to Evaluate Nutritional Support for Malignant Disease. Clin. Chim. Acta 2008, 390, 23–27. [Google Scholar] [CrossRef]
- Valenzuela-Landaeta, K.; Rojas, P.; Basfi-fer, K. Nutritional assessment for cancer patient. Nutr. Hosp. 2012, 27, 516–523. [Google Scholar] [CrossRef]
- Löser, C.; Aschl, G.; Hébuterne, X.; Mathus-Vliegen, E.M.H.; Muscaritoli, M.; Niv, Y.; Rollins, H.; Singer, P.; Skelly, R.H. ESPEN Guidelines on Artificial Enteral Nutrition--Percutaneous Endoscopic Gastrostomy (PEG). Clin. Nutr. 2005, 24, 848–861. [Google Scholar] [CrossRef]
- Fonseca, J.; Santos, C.A.; Brito, J. Malnutrition and Clinical Outcome of 234 Head and Neck Cancer Patients Who Underwent Percutaneous Endoscopic Gastrostomy. Nutr. Cancer 2016, 68, 589–597. [Google Scholar] [CrossRef]
- Powell-Tuck, J.; Hennessy, E.M. A Comparison of Mid Upper Arm Circumference, Body Mass Index and Weight Loss as Indices of Undernutrition in Acutely Hospitalized Patients. Clin. Nutr. 2003, 22, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.; Santos, C.; Fonseca, J. Body mass index estimation on gastrostomy patients using the mid-upper arm circumference. J. Aging Res. Clin. Pract. 2012, 1, 252–255. [Google Scholar]
- Barosa, R.; Roque Ramos, L.; Santos, C.A.; Pereira, M.; Fonseca, J. Mid Upper Arm Circumference and Powell-Tuck and Hennessy’s Equation Correlate with Body Mass Index and Can Be Used Sequentially in Gastrostomy Fed Patients. Clin. Nutr. 2018, 37, 1584–1588. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization—Europe. Nutrition—Body Mass Index—BMI. Available online: https://www.euro.who.int/en/healthtopics/disease-prevention/nutrition/a-healthylifestyle/body-mass-index-bmi~ (accessed on 6 January 2020).
- Frisancho, A.R. New Standards of Weight and Body Composition by Frame Size and Height for Assessment of Nutritional Status of Adults and the Elderly. Am. J. Clin. Nutr. 1984, 40, 808–819. [Google Scholar] [CrossRef]
- Fonseca, J.; Santos, C.A. Clinical anatomy: Anthropometry for nutritional assessment of 367 adults who underwent endoscopic gastrostomy. Acta Med. Port. 2013, 26, 212–218. [Google Scholar] [PubMed]
- McDowell, M.A.; Fryar, C.D.; Ogden, C.L.; Flegal, K.M. Anthropometric Reference Data for Children and Adults: United States, 2003–2006. Natl. Health Stat. Rep. 2008, 10, 1–48. [Google Scholar]
- Sultan, S.; Nasir, K.; Qureshi, R.; Dhrolia, M.; Ahmad, A. Assessment of the Nutritional Status of the Hemodialysis Patients by Anthropometric Measurements. Cureus 2021, 13, e18605. [Google Scholar] [CrossRef] [PubMed]
- Friedenberg, F.; Jensen, G.; Gujral, N.; Braitman, L.E.; Levine, G.M. Serum Albumin Is Predictive of 30-Day Survival after Percutaneous Endoscopic Gastrostomy. JPEN J. Parenter. Enter. Nutr. 1997, 21, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Kuzuya, M.; Izawa, S.; Enoki, H.; Okada, K.; Iguchi, A. Is Serum Albumin a Good Marker for Malnutrition in the Physically Impaired Elderly? Clin. Nutr. 2007, 26, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Badia-Tahull, M.B.; Llop-Talaveron, J.; Fort-Casamartina, E.; Farran-Teixidor, L.; Ramon-Torrel, J.M.; Jódar-Masanés, R. Preoperative Albumin as a Predictor of Outcome in Gastrointestinal Surgery. e-SPEN Eur. J. Clin. Nutr. Metab. 2009, 4, e248–e251. [Google Scholar] [CrossRef]
- Vyroubal, P.; Chiarla, C.; Giovannini, I.; Hyspler, R.; Ticha, A.; Hrnciarikova, D.; Zadak, Z. Hypocholesterolemia in Clinically Serious Conditions—Review. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2008, 152, 181–189. [Google Scholar] [CrossRef]
- Casas-Rodera, P.; Gómez-Candela, C.; Benítez, S.; Mateo, R.; Armero, M.; Castillo, R.; Culebras, J.M. Immunoenhanced Enteral Nutrition Formulas in Head and Neck Cancer Surgery: A Prospective, Randomized Clinical Trial. Nutr. Hosp. 2008, 23, 105–110. [Google Scholar]
- van Wayenburg, C.A.M.; Rasmussen-Conrad, E.L.; van den Berg, M.G.A.; Merkx, M.A.W.; van Staveren, W.A.; van Weel, C.; van Binsbergen, J.J. Weight Loss in Head and Neck Cancer Patients Little Noticed in General Practice. J. Prim. Health Care 2010, 2, 16–21. [Google Scholar] [CrossRef]
- Platek, M.E. The Role of Dietary Counseling and Nutrition Support in Head and Neck Cancer Patients. Curr. Opin. Support. Palliat. Care 2012, 6, 438–445. [Google Scholar] [CrossRef]
- Cao, J.; Xu, H.; Li, W.; Guo, Z.; Lin, Y.; Shi, Y.; Hu, W.; Ba, Y.; Li, S.; Li, Z.; et al. Nutritional Assessment and Risk Factors Associated to Malnutrition in Patients with Esophageal Cancer. Curr. Probl. Cancer 2021, 45, 100638. [Google Scholar] [CrossRef] [PubMed]
- Bossi, P.; Delrio, P.; Mascheroni, A.; Zanetti, M. The Spectrum of Malnutrition/Cachexia/Sarcopenia in Oncology According to Different Cancer Types and Settings: A Narrative Review. Nutrients 2021, 13, 1980. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, D.; Laszlo, M.; Provisor, A.; Yu, A. Nutrition Management for the Head and Neck Cancer Patient. Cancer Treat. Res. 2018, 174, 187–208. [Google Scholar] [CrossRef] [PubMed]
- Meerkerk, C.D.A.; Chargi, N.; de Jong, P.A.; van den Bos, F.; de Bree, R. Low Skeletal Muscle Mass Predicts Frailty in Elderly Head and Neck Cancer Patients. Eur. Arch. Otorhinolaryngol. 2022, 279, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Lapornik, N.; Avramovič Brumen, B.; Plavc, G.; Strojan, P.; Rotovnik Kozjek, N. Influence of Fat-Free Mass Index on the Survival of Patients with Head and Neck Cancer. Eur. Arch. Otorhinolaryngol. 2022, 1–9. [Google Scholar] [CrossRef]
| Subject Characteristics | n (%) |
|---|---|
| Age (years) | |
| 42–94 (mean 61.3) | |
| Gender | |
| Female | 9 (6.5%) |
| Male | 129 (93.5%) |
| Cancer site | |
| Mouth | 39 (28.3%) |
| Pharyngeal | 42 (30.4%) |
| Laryngeal | 55 (39.9%) |
| Others | 2 (1.4%) |
| Total (n = 138) | Total Mean | |
|---|---|---|
| Primary Tumor located | ||
| Mouth | 39 | |
| Pharynx | 42 | |
| Larynx | 55 | |
| Other HNC location | 2 | |
| Classification of Malignant Tumors (TNM) Classification | ||
| Stage I | 6 | |
| Stage II | 1 | |
| Stage III | 22 | |
| Stage Iva | 81 | |
| Stage IVb | 12 | |
| Stage IVc | 16 | |
| Anthropometry Results | ||
| BMI | 76 Low BMI | |
| 46 Normal BMI | ||
| 16 High BMI | ||
| MUAC | 114 Low | |
| 24 Normal | ||
| TSF | 58 Low | |
| 80 Normal | ||
| MAMC | 81 Low | |
| 57 Normal | ||
| Laboratory serum data | ||
| Albumin | 47 Low | 3.7 g/dL |
| 91 Normal | ||
| Transferrin | 93 Low | 182.0 mg/dL |
| 45 Normal | ||
| Total Cholesterol | 53 Low | 173.2 mg/dL |
| 85 Normal | ||
| Stage (N) | Mean | Median | ||||
|---|---|---|---|---|---|---|
| Survival Time | Std. Error | 95% Confidence Interval | Survival Time | Std. Error | ||
| Lower Bound | Upper Bound | |||||
| I (6) | 1135 | 496 | 164 | 2106 | 178 | 515 |
| III (22) | 1074 | 291 | 503 | 1645 | 275 | 268 |
| IVa (81) | 1054 | 149 | 762 | 1347 | 397 | 93 |
| IVb (12) | 1082 | 431 | 237 | 1927 | 233 | 202 |
| IVc (16) | 219 | 55 | 111 | 327 | 135 | 26 |
| Overall (137) | 996 | 117 | 767 | 1226 | 316 | 56 |
| Time in Days | ||||
|---|---|---|---|---|
| Local | Mouth | Stage | I (n = 1) | 555 |
| III (n = 6) | 75 | |||
| IVa (n = 27) | 450 | |||
| IVb (n = 2) | 400 | |||
| IVc (n = 3) | 175 | |||
| Pharynx | Stage | I (n = 1) | 2700 | |
| III (n = 4) | 2000 | |||
| IVa (n = 25) | 305 | |||
| IVb (n = 6) | 1700 | |||
| IVc (n = 5) | 120 | |||
| Larynx | Stage | I (n = 3) | 950 | |
| III (n = 11) | 631 | |||
| IVa (n = 29) | 452 | |||
| IVb (n = 4) | 250 | |||
| IVc (n = 8) | 200 | |||
| Other locations | Stage | I (n = 1) | 150 | |
| III (n = 1) | 150 | |||
| Coef | SE | p-Value | OR | 95.0% CI for OR | |||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| TNM Stage | I | 0.117 | |||||
| III | 0.668 | 0.583 | 0.252 | 1.949 | 0.622 | 3.276 | |
| IVa | 0.728 | 0.529 | 0.169 | 2.071 | 0.734 | 3.408 | |
| IVb | 0.934 | 0.616 | 0.130 | 2.546 | 0.761 | 4.331 | |
| IVc | 1.386 | 0.592 | 0.019 | 3.998 | 1.252 | 6.744 | |
| MAMC | −0.177 | 0.040 | 0.000 | 0.838 | 0.775 | 0.901 | |
| Albumin | 0.251 | 0.162 | 0.122 | 1.285 | 0.935 | 1.635 | |
| Time with PEG | −0.001 | 0.000 | 0.000 | 0.999 | 0.999 | 0.999 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa-Catita, D.; Ferreira-Santos, C.; Mascarenhas, P.; Oliveira, C.; Madeira, R.; Santos, C.A.; André, C.; Godinho, C.; Antunes, L.; Fonseca, J. Malnutrition, Cancer Stage and Gastrostomy Timing as Markers of Poor Outcomes in Gastrostomy-Fed Head and Neck Cancer Patients. Nutrients 2023, 15, 662. https://doi.org/10.3390/nu15030662
Sousa-Catita D, Ferreira-Santos C, Mascarenhas P, Oliveira C, Madeira R, Santos CA, André C, Godinho C, Antunes L, Fonseca J. Malnutrition, Cancer Stage and Gastrostomy Timing as Markers of Poor Outcomes in Gastrostomy-Fed Head and Neck Cancer Patients. Nutrients. 2023; 15(3):662. https://doi.org/10.3390/nu15030662
Chicago/Turabian StyleSousa-Catita, Diogo, Cláudia Ferreira-Santos, Paulo Mascarenhas, Cátia Oliveira, Raquel Madeira, Carla Adriana Santos, Carla André, Catarina Godinho, Luís Antunes, and Jorge Fonseca. 2023. "Malnutrition, Cancer Stage and Gastrostomy Timing as Markers of Poor Outcomes in Gastrostomy-Fed Head and Neck Cancer Patients" Nutrients 15, no. 3: 662. https://doi.org/10.3390/nu15030662
APA StyleSousa-Catita, D., Ferreira-Santos, C., Mascarenhas, P., Oliveira, C., Madeira, R., Santos, C. A., André, C., Godinho, C., Antunes, L., & Fonseca, J. (2023). Malnutrition, Cancer Stage and Gastrostomy Timing as Markers of Poor Outcomes in Gastrostomy-Fed Head and Neck Cancer Patients. Nutrients, 15(3), 662. https://doi.org/10.3390/nu15030662









