Low Obesity-Related Indices Are Associated with a Low Baseline Calcaneus Ultrasound T-Score, and a Rapid Decline in T-Score in a Large Taiwanese Population Follow-Up Study
Abstract
1. Introduction
2. Materials and Methods
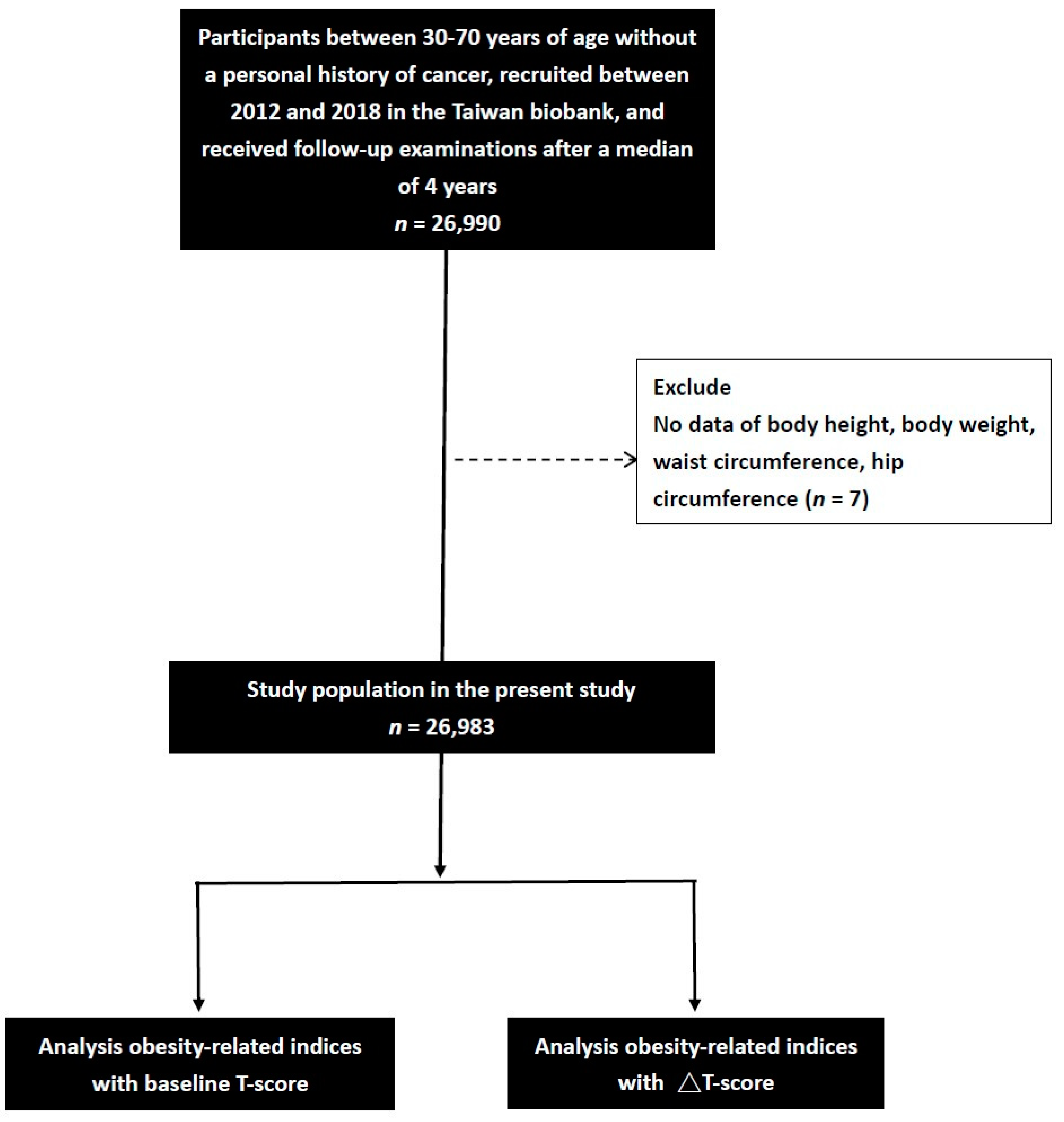
2.1. Taiwan Biobank
2.2. Assessment of Calcaneus Ultrasound T-Score
2.3. Calculation of Obesity-Related Indices
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Study Groups
3.2. Factors Associated with Baseline Calcaneus T-Score Using Univariable Analysis
3.3. Multivariable Analysis to Analyze the Relationships between the Indices Related to Obesity and Baseline Calcaneus Ultrasound T-Score
3.4. Factors Associated with ΔT-Score Using Univariable Analysis
3.5. Relationships among the Indices Related to Obesity with ΔT-Score in Multivariable Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pouresmaeili, F.; Kamalidehghan, B.; Kamarehei, M.; Goh, Y.M. A comprehensive overview on osteoporosis and its risk factors. Ther. Clin. Risk Manag. 2018, 14, 2029–2049. [Google Scholar] [CrossRef]
- Kanis, J.A. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: Synopsis of a WHO report. WHO Study Group. Osteoporos. Int. 1994, 4, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Chavda, S.; Chavda, B.; Dube, R. Osteoporosis Screening and Fracture Risk Assessment Tool: Its Scope and Role in General Clinical Practice. Cureus 2022, 14, e26518. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Ghasemi, H.; Mohammadi, L.; Behzadi, M.H.; Rabieenia, E.; Shohaimi, S.; Mohammadi, M. The global prevalence of osteoporosis in the world: A comprehensive systematic review and meta-analysis. J. Orthop. Surg. Res. 2021, 16, 609. [Google Scholar] [CrossRef]
- Nancy, E.; Lane, E. Etiology, and Diagnosis of Osteoporosis. Am. J. Obstet. Gynecol. 2006, 194, S3–S11. [Google Scholar]
- Sözen, T.; Özışık, L.; Başaran, N.Ç. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef]
- Chandran, M.; Lau, T.C.; Gagnon-Arpin, I.; Dobrescu, A.; Li, W.; Leung, M.Y.M.; Patil, N.; Zhao, Z. The health and economic burden of osteoporotic fractures in Singapore and the potential impact of increasing treatment rates through more pharmacological options. Arch. Osteoporos. 2019, 14, 114. [Google Scholar] [CrossRef]
- Wu, L.; Zhu, W.; Qiao, Q.; Huang, L.; Li, Y.; Chen, L. Novel and traditional anthropometric indices for identifying metabolic syndrome in non-overweight/obese adults. Nutr. Metab. 2021, 18, 3. [Google Scholar] [CrossRef]
- Jao, H.F.; Wung, C.H.; Yu, H.C.; Lee, M.Y.; Chen, P.C.; Chen, S.C.; Chang, J.M. Sex Difference in the Associations among Obesity-Related Indices with Metabolic Syndrome in Patients with Type 2 Diabetes Mellitus. Int. J. Med. Sci. 2021, 18, 3470–3477. [Google Scholar] [CrossRef]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef]
- Chiu, T.H.; Huang, Y.C.; Chiu, H.; Wu, P.Y.; Chiou, H.C.; Huang, J.C.; Chen, S.C. Comparison of Various Obesity-Related Indices for Identification of Metabolic Syndrome: A Population-Based Study from Taiwan Biobank. Diagnostics 2020, 10, 1081. [Google Scholar] [CrossRef] [PubMed]
- Janghorbani, M.; Aminorroaya, A.; Amini, M. Comparison of Different Obesity Indices for Predicting Incident Hypertension. High Blood Press. Cardiovasc. Prev. 2017, 24, 157–166. [Google Scholar] [CrossRef]
- Lin, Y.A.; Chen, Y.J.; Tsao, Y.C.; Yeh, W.C.; Li, W.C.; Tzeng, I.S.; Chen, J.Y. Relationship between obesity indices and hypertension among middle-aged and elderly populations in Taiwan: A community-based, cross-sectional study. BMJ Open 2019, 9, e031660. [Google Scholar] [CrossRef]
- Lin, I.T.; Lee, M.Y.; Wang, C.W.; Wu, D.W.; Chen, S.C. Gender Differences in the Relationships among Metabolic Syndrome and Various Obesity-Related Indices with Nonalcoholic Fatty Liver Disease in a Taiwanese Population. Int. J. Environ. Res. Public Health 2021, 18, 857. [Google Scholar] [CrossRef]
- Ou, Y.L.; Lee, M.Y.; Lin, I.T.; Wen, W.L.; Hsu, W.H.; Chen, S.C. Obesity-related indices are associated with albuminuria and advanced kidney disease in type 2 diabetes mellitus. Ren. Fail. 2021, 43, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.E.; Chen, S.C.; Geng, J.H.; Wu, D.W.; Wu, P.Y.; Huang, J.C. Obesity-Related Indices Are Associated with Longitudinal Changes in Lung Function: A Large Taiwanese Population Follow-Up Study. Nutrients 2021, 13, 4055. [Google Scholar] [CrossRef] [PubMed]
- Wung, C.H.; Chung, C.Y.; Wu, P.Y.; Huang, J.C.; Tsai, Y.C.; Chen, S.C.; Chiu, Y.W.; Chang, J.M. Associations between Metabolic Syndrome and Obesity-Related Indices and Bone Mineral Density T-Score in Hemodialysis Patients. J. Pers. Med. 2021, 11, 775. [Google Scholar] [CrossRef]
- Lee, W.C.; Wu, P.Y.; Huang, J.C.; Tsai, Y.C.; Chiu, Y.W.; Chen, S.C.; Chang, J.M.; Chen, H.C. Sex Difference in the Associations among Obesity-Related Indices with Incident Hypertension in a Large Taiwanese Population Follow-Up Study. J. Pers. Med. 2022, 12, 972. [Google Scholar] [CrossRef] [PubMed]
- Wung, C.H.; Lee, M.Y.; Wu, P.Y.; Huang, J.C.; Chen, S.C. Obesity-Related Indices Are Associated with Peripheral Artery Occlusive Disease in Patients with Type 2 Diabetes Mellitus. J. Pers. Med. 2021, 11, 533. [Google Scholar] [CrossRef]
- Huang, S.H.; Chen, S.C.; Geng, J.H.; Wu, D.W.; Li, C.H. Metabolic Syndrome and High-Obesity-Related Indices Are Associated with Poor Cognitive Function in a Large Taiwanese Population Study Older than 60 Years. Nutrients 2022, 14, 1535. [Google Scholar] [CrossRef]
- Nielson, C.M.; Srikanth, P.; Orwoll, E.S. Obesity and fracture in men and women: An epidemiologic perspective. J. Bone Miner. Res. 2012, 27, 1–10. [Google Scholar] [CrossRef]
- Compston, J. Obesity and bone. Curr. Osteoporos. Rep. 2013, 11, 30–35. [Google Scholar] [CrossRef]
- De Laet, C.; Kanis, J.A.; Oden, A.; Johanson, H.; Johnell, O.; Delmas, P.; Eisman, J.A.; Kroger, H.; Fujiwara, S.; Garnero, P.; et al. Body mass index as a predictor of fracture risk: A meta-analysis. Osteoporos. Int. 2005, 16, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Qiao, D.; Li, Y.; Liu, X.; Zhang, X.; Qian, X.; Zhang, H.; Zhang, G.; Wang, C. Association of obesity with bone mineral density and osteoporosis in adults: A systematic review and meta-analysis. Public Health 2020, 180, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Espallargues, M.; Sampietro-Colom, L.; Estrada, M.D.; Sola, M.; del Rio, L.; Setoain, J.; Granados, A. Identifying bone-mass-related risk factors for fracture to guide bone densitometry measurements: A systematic review of the literature. Osteoporos. Int. 2001, 12, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Yang, J.H.; Chiang, C.W.K.; Hsiung, C.N.; Wu, P.E.; Chang, L.C.; Chu, H.W.; Chang, J.; Song, I.W.; Yang, S.L.; et al. Population structure of Han Chinese in the modern Taiwanese population based on 10,000 participants in the Taiwan Biobank project. Hum. Mol. Genet. 2016, 25, 5321–5331. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.T.; Hung, T.H.; Yeh, C.K. Taiwan Regulation of Biobanks. J. Law Med. Ethics 2015, 43, 816–826. [Google Scholar] [CrossRef]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef]
- Ministry of Education. Physical Fitness 333 Plan; Ministry of Education: Taiwan, China, 1999.
- Thomas, D.M.; Bredlau, C.; Bosy-Westphal, A.; Mueller, M.; Shen, W.; Gallagher, D.; Maeda, Y.; McDougall, A.; Peterson, C.M.; Ravussin, E.; et al. Relationships between body roundness with body fat and visceral adipose tissue emerging from a new geometrical model. Obesity (Silver Spring) 2013, 21, 2264–2271. [Google Scholar] [CrossRef] [PubMed]
- Bergman, R.N.; Stefanovski, D.; Buchanan, T.A.; Sumner, A.E.; Reynolds, J.C.; Sebring, N.G.; Xiang, A.H.; Watanabe, R.M. A better index of body adiposity. Obesity (Silver Spring) 2011, 19, 1083–1089. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Rodriguez-Moran, M. Abdominal volume index. An anthropometry-based index for estimation of obesity is strongly related to impaired glucose tolerance and type 2 diabetes mellitus. Arch. Med. Res. 2003, 34, 428–432. [Google Scholar] [CrossRef]
- Kahn, H.S. The “lipid accumulation product” performs better than the body mass index for recognizing cardiovascular risk: A population-based comparison. BMC Cardiovasc. Disord. 2005, 5, 26. [Google Scholar] [CrossRef]
- Amato, M.C.; Giordano, C.; Galia, M.; Criscimanna, A.; Vitabile, S.; Midiri, M.; Galluzzo, A. Visceral Adiposity Index: A reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care 2010, 33, 920–922. [Google Scholar] [CrossRef]
- Cummings, S.R.; Nevitt, M.C.; Browner, W.S.; Stone, K.; Fox, K.M.; Ensrud, K.E.; Cauley, J.; Black, D.; Vogt, T.M. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N. Engl. J. Med. 1995, 332, 767–773. [Google Scholar] [CrossRef]
- Osteoporosis: Review of the evidence for prevention, diagnosis and treatment and cost-effectiveness analysis. Osteoporos. Int. 1998, 8 (Suppl. 4), S7–S80. [CrossRef]
- Honkanen, R.J.; Honkanen, K.; Kröger, H.; Alhava, E.; Tuppurainen, M.; Saarikoski, S. Risk factors for perimenopausal distal forearm fracture. Osteoporos. Int. 2000, 11, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Galvard, H.; Elmståhl, S.; Elmståhl, B.; Samuelsson, S.M.; Robertsson, E. Differences in body composition between female geriatric hip fracture patients and healthy controls: Body fat is more important as explanatory factor for the fracture than body weight and lean body mass. Aging 1996, 8, 282–286. [Google Scholar] [CrossRef]
- Joakimsen, R.M.; Fønnebø, V.; Magnus, J.H.; Tollan, A.; Søgaard, A.J. The Tromsø Study: Body height, body mass index and fractures. Osteoporos. Int. 1998, 8, 436–442. [Google Scholar] [CrossRef]
- Van der Voort, D.J.; Geusens, P.P.; Dinant, G.J. Risk factors for osteoporosis related to their outcome: Fractures. Osteoporos. Int. 2001, 12, 630–638. [Google Scholar] [CrossRef]
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef]
- Ha, J.; Baek, K.H. Body mass index at the crossroads of osteoporosis and type 2 diabetes. Korean J. Intern. Med. 2020, 35, 1333–1335. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Alhambra, D.; Premaor, M.O.; Fina Aviles, F.; Hermosilla, E.; Martinez-Laguna, D.; Carbonell-Abella, C.; Nogues, X.; Compston, J.E.; Diez-Perez, A. The association between fracture and obesity is site-dependent: A population-based study in postmenopausal women. J. Bone Miner. Res. 2012, 27, 294–300. [Google Scholar] [CrossRef]
- Premaor, M.O.; Compston, J.E.; Fina Avilés, F.; Pagès-Castellà, A.; Nogués, X.; Díez-Pérez, A.; Prieto-Alhambra, D. The association between fracture site and obesity in men: A population-Based cohort study. J. Bone Miner. Res. 2013, 28, 1771–1777. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Klimentidis, Y.C.; Bea, J.W.; Ernst, K.C.; Hu, C.; Jackson, R.; Thomson, C.A. Body Mass Index, Waist Circumference, and Mortality in a Large Multiethnic Postmenopausal Cohort-Results from the Women’s Health Initiative. J. Am. Geriatr. Soc. 2017, 65, 1907–1915. [Google Scholar] [CrossRef] [PubMed]
- Padwal, R.; Leslie, W.D.; Lix, L.M.; Majumdar, S.R. Relationship Among Body Fat Percentage, Body Mass Index, and All-Cause Mortality: A Cohort Study. Ann. Intern. Med. 2016, 164, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Nishida, C.; Ko, G.T.; Kumanyika, S. Body fat distribution and noncommunicable diseases in populations: Overview of the 2008 WHO Expert Consultation on Waist Circumference and Waist-Hip Ratio. Eur. J. Clin. Nutr. 2010, 64, 2–5. [Google Scholar] [CrossRef]
- Hassan, N.E.; El-Masr, S.A.; El Bann, R.A.; Al-Tohamy, M.; El-Lebedy, D.; Abdel, D.A.; Amin, D.; Megahed, S.; Khalil, A. Bone Health and its Relation to Energy Intake, Fat Mass and its Distribution. Pak. J. Biol. Sci. 2020, 23, 1075–1085. [Google Scholar] [CrossRef]
- Qin, K.; He, M.; Cao, X.T.; Yang, H.W.; Yang, Y.; Wang, Y.J.; Yu, C.; An, Z.M.; Li, S.Y. Obesity and Osteoporosis in Men Aged Above 50. Sichuan Da Xue Xue Bao Yi Xue Ban 2017, 48, 17–22. [Google Scholar]
- Bland, V.L.; Klimentidis, Y.C.; Bea, J.W.; Roe, D.J.; Funk, J.L.; Going, S.B. Cross-sectional associations between adipose tissue depots and areal bone mineral density in the UK Biobank imaging study. Osteoporos. Int. 2022, 33, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; He, C.; He, W.; Yang, M.; Luo, X.; Li, C. Obesity and Bone Health: A Complex Link. Front. Cell Dev. Biol. 2020, 8, 600181. [Google Scholar] [CrossRef]
- Karunananthan, S.; Bergman, H.; Vedel, I.; Retornaz, F. Frailty: Searching for a relevant clinical and research paradigm. Rev. Med. Interne 2009, 30, 105–109. [Google Scholar] [CrossRef]
- Gaffney-Stomberg, E.; Insogna, K.L.; Rodriguez, N.R.; Kerstetter, J.E. Increasing dietary protein requirements in elderly people for optimal muscle and bone health. J. Am. Geriatr. Soc. 2009, 57, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Corti, M.C.; Guralnik, J.M.; Salive, M.E.; Sorkin, J.D. Serum albumin level and physical disability as predictors of mortality in older persons. Jama 1994, 272, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Tengstrand, B.; Cederholm, T.; Söderqvist, A.; Tidermark, J. Effects of protein-rich supplementation and nandrolone on bone tissue after a hip fracture. Clin. Nutr. 2007, 26, 460–465. [Google Scholar] [CrossRef]
- Coin, A.; Sergi, G.; Beninca, P.; Lupoli, L.; Cinti, G.; Ferrara, L.; Benedetti, G.; Tomasi, G.; Pisent, C.; Enzi, G. Bone mineral density and body composition in underweight and normal elderly subjects. Osteoporos. Int. 2000, 11, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Montalcini, T.; Romeo, S.; Ferro, Y.; Migliaccio, V.; Gazzaruso, C.; Pujia, A. Osteoporosis in chronic inflammatory disease: The role of malnutrition. Endocrine 2013, 43, 59–64. [Google Scholar] [CrossRef]
- Bischoff, H.A.; Stahelin, H.B.; Dick, W.; Akos, R.; Knecht, M.; Salis, C.; Nebiker, M.; Theiler, R.; Pfeifer, M.; Begerow, B.; et al. Effects of vitamin D and calcium supplementation on falls: A randomized controlled trial. J. Bone Miner. Res. 2003, 18, 343–351. [Google Scholar] [CrossRef]
- Wootton, R.; Brereton, P.J.; Clark, M.B.; Hesp, R.; Hodkinson, H.M.; Klenerman, L.; Reeve, J.; Slavin, G.; Tellez-Yudilevich, M. Fractured neck of femur in the elderly: An attempt to identify patients at risk. Clin. Sci. 1979, 57, 93–101. [Google Scholar] [CrossRef]
- Nilsson, B.E. Spinal osteoporosis and femoral neck fracture. Clin. Orthop. Relat. Res. 1970, 68, 93–95. [Google Scholar] [CrossRef]
- Rizzoli, R.; Bonjour, J.P. Malnutrition and osteoporosis. Z. Gerontol. Geriatr. 1999, 32 (Suppl. 1), I31–I37. [Google Scholar] [CrossRef]
- Rolland, Y.; Vellas, B. Sarcopenia. Rev. Med. Interne 2009, 30, 150–160. [Google Scholar] [CrossRef]
- Lang, T.; Streeper, T.; Cawthon, P.; Baldwin, K.; Taaffe, D.R.; Harris, T.B. Sarcopenia: Etiology, clinical consequences, intervention, and assessment. Osteoporos. Int. 2010, 21, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Baumgartner, R.N.; Roubenoff, R.; Mayer, J.; Nair, K.S. Sarcopenia. J. Lab. Clin. Med. 2001, 137, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, P.; Body, J.J.; Boonen, S.; Boutsen, Y.; Devogelaer, J.P.; Goemaere, S.; Kaufman, J.; Reginster, J.Y.; Rozenberg, S. Loading and skeletal development and maintenance. J. Osteoporos. 2010, 2011, 786752. [Google Scholar] [CrossRef]
- Klein-Nulend, J.; van der Plas, A.; Semeins, C.M.; Ajubi, N.E.; Frangos, J.A.; Nijweide, P.J.; Burger, E.H. Sensitivity of osteocytes to biomechanical stress in vitro. FASEB J. 1995, 9, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, J.I.; Plotkin, L.I.; Stewart, S.A.; Weinstein, R.S.; Parfitt, A.M.; Manolagas, S.C.; Bellido, T. Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J. Bone Miner. Res. 2006, 21, 605–615. [Google Scholar] [CrossRef]
- Gu, G.; Mulari, M.; Peng, Z.; Hentunen, T.A.; Vaananen, H.K. Death of osteocytes turns off the inhibition of osteoclasts and triggers local bone resorption. Biochem. Biophys. Res. Commun. 2005, 335, 1095–1101. [Google Scholar] [CrossRef]
- Jin, N.; Lin, S.; Zhang, Y.; Chen, F. Assess the discrimination of Achilles InSight calcaneus quantitative ultrasound device for osteoporosis in Chinese women: Compared with dual energy X-ray absorptiometry measurements. Eur. J. Radiol. 2010, 76, 265–268. [Google Scholar] [CrossRef]
| Item | Calculation Formula |
|---|---|
| BMI | BW (kg)/BH2 (m) |
| WHR | WC (cm)/HC (cm) |
| WHtR | WC (cm)/BH (cm) |
| BRI | [30] |
| BAI | [31] |
| AVI | [32] |
| LAP | in males, and in females [33] |
| VAI | in males, and in females [34] |
| Characteristics | T-Score ≥ −2.5 (n = 24,932) | T-Score < −2.5 (n = 2051) | p |
|---|---|---|---|
| Age (year) | 50.7 ± 10.4 | 57.2 ± 8.4 | <0.001 |
| Male gender (%) | 34.8 | 41.8 | <0.001 |
| DM (%) | 5.2 | 6.0 | 0.119 |
| Hypertension (%) | 12.8 | 16.7 | <0.001 |
| Systolic BP (mmHg) | 117.2 ± 17.6 | 121.5 ± 18.3 | <0.001 |
| Diastolic BP (mmHg) | 72.4 ± 10.8 | 73.2 ± 10.9 | 0.002 |
| Smoking history (%) | 25.3 | 29.5 | <0.001 |
| Alcohol history (%) | 2.9 | 3.2 | 0.427 |
| Regular exercise habit (%) | 47.8 | 54.3 | <0.001 |
| Menstruation in female (%) | 47.3 | 10.9 | <0.001 |
| Body height (cm) | 161.1 ± 8.0 | 161.0 ± 8.7 | 0.416 |
| Body weight (Kg) | 62.9 ± 11.8 | 61.2 ± 12.1 | <0.001 |
| Waist circumference (cm) | 83.2 ± 9.8 | 83.0 ± 9.9 | 0.415 |
| Hip circumference (cm) | 95.9 ± 6.8 | 94.7 ± 6.7 | <0.001 |
| Baseline T-score | −0.265 ± 1.472 | −3.073 ± 0.499 | <0.001 |
| Follow-up T-score | −0.571 ± 1.482 | −2.832 ± 1.067 | <0.001 |
| Laboratory parameters | |||
| Fasting glucose (mg/dL) | 96.1 ± 20.0 | 98.0 ± 23.5 | <0.001 |
| Hemoglobin (g/dL) | 13.7 ± 1.6 | 13.8 ± 1.4 | 0.045 |
| Triglyceride (mg/dL) | 114.0 ± 83.9 | 113.5 ± 72.3 | 0.770 |
| Total cholesterol (mg/dL) | 195.3 ± 35.4 | 196.8 ± 35.8 | 0.062 |
| HDL-C (mg/dL) | 54.2 ± 13.2 | 54.5 ± 13.6 | 0.464 |
| LDL-C (mg/dL) | 121.6 ± 31.7 | 121.7 ± 31.0 | 0.942 |
| eGFR (mL/min/1.73 m2) | 109.3 ± 25.3 | 107.5 ± 26.5 | <0.001 |
| Uric acid (mg/dL) | 5.5 ± 1.4 | 5.5 ± 1.4 | 0.961 |
| Obesity-related indices | |||
| BMI (kg/m2) | 24.1 ± 3.6 | 23.5 ± 3.6 | <0.001 |
| WHR (%) | 86.7 ± 6.8 | 87.5 ± 6.8 | <0.001 |
| WHtR (%) | 51.7 ± 6.0 | 51.6 ± 6.0 | 0.685 |
| BRI | 6.8 ± 1.9 | 6.7 ± 1.9 | 0.559 |
| BAI | 29.0 ± 4.1 | 28.5 ± 4.1 | <0.001 |
| AVI | 14.2 ± 3.3 | 14.1 ± 3.3 | 0.332 |
| LAP | 31.5 ± 30.8 | 30.3 ± 27.5 | 0.097 |
| VAI | 1.69 ± 1.67 | 1.66 ± 1.45 | 0.353 |
| Parameters | Baseline T-Score | ||
|---|---|---|---|
| Univariable | |||
| Unstandardized Coefficient β | 95% CI | p | |
| Age (per 1 year) | −0.053 | −0.055, −0.051 | <0.001 |
| Female (vs. male) | 0.432 | 0.392, 0.472 | <0.001 |
| DM | −0.267 | −0.353, −0.182 | <0.001 |
| Hypertension | −0.416 | −0.472, −0.359 | <0.001 |
| Systolic BP (per 1 mmHg) | −0.014 | −0.015, −0.012 | <0.001 |
| Diastolic BP (per 1 mmHg) | −0.012 | −0.014, −0.010 | <0.001 |
| Smoking history | −0.272 | −0.315, −0.228 | <0.001 |
| Alcohol history | −0.162 | −0.276, −0.047 | 0.006 |
| Regular exercise habits | −0.218 | −0.256, −0.179 | <0.001 |
| Menstruation in female | 1.371 | 1.325, 1.416 | <0.001 |
| Laboratory parameters | |||
| Fasting glucose (per 1 mg/dL) | −0.006 | −0.007, −0.005 | <0.001 |
| Hemoglobin (per 1 g/dL) | −0.086 | −0.098. −0.074 | <0.001 |
| Triglyceride (per 1 mg/dL) | −0.001 | −0.001, −0.001 | <0.001 |
| Total cholesterol (per 1 mg/dL) | −0.003 | −0.004, −0.003 | <0.001 |
| HDL-cholesterol (per 1 mg/dL) | 0.005 | 0.003, 0.006 | <0.001 |
| LDL-cholesterol (per 1 mg/dL) | −0.003 | −0.003, −0.002 | <0.001 |
| eGFR (per 1 mL/min/1.73 m2) | 0.005 | 0.005, 0.006 | <0.001 |
| Uric acid (per 1 mg/dL) | −0.072 | −0.086, −0.059 | <0.001 |
| Obesity-Related Indices | Baseline T-Score | ||
|---|---|---|---|
| Multivariable | |||
| Unstandardized Coefficient β | 95% CI | p | |
| BMI (per 1 kg/m2) a | 0.065 | 0.058, 0.072 | <0.001 |
| WHR (per 1%) a | 0.012 | 0.008, 0.016 | <0.001 |
| WHtR (per 1%) a | 0.024 | 0.020, 0.029 | <0.001 |
| BRI (per 1) a | 0.079 | 0.066, 0.093 | <0.001 |
| BAI (per 1) a | 0.032 | 0.025, 0.038 | <0.001 |
| AVI (per 1) a | 0.049 | 0.041, 0.057 | <0.001 |
| LAP (per 1) b | 0.005 | 0.004, 0.006 | <0.001 |
| VAI (per 1) c | 0.003 | −0.012, 0.019 | 0.667 |
| Parameters | ΔT-Score | ||
|---|---|---|---|
| Univariable | |||
| Unstandardized Coefficient β | 95% CI | p | |
| Age (per 1 year) | −0.005 | −0.006, −0.004 | <0.001 |
| Female (vs. male) | −0.160 | −0.185, −0.135 | <0.001 |
| DM | 0.035 | −0.018, 0.089 | 0.194 |
| Hypertension | 0.024 | −0.012, 0.059 | 0.186 |
| Systolic BP (per 1 mmHg) | −7.13 × 10−5 | −0.001, 0.001 | 0.836 |
| Diastolic BP (per 1 mmHg) | 0.002 | 0.001, 0.003 | <0.001 |
| Smoking history | 0.111 | 0.084, 0.138 | <0.001 |
| Alcohol history | 0.110 | 0.038, 0.181 | 0.003 |
| Regular exercise habits | −0.036 | −0.059, −0.012 | 0.004 |
| Menstruation in female | 0.122 | 0.090, 0.153 | <0.001 |
| Laboratory parameters | |||
| Fasting glucose (per 1 mg/dL) | 0.001 | 0, 0.002 | 0.001 |
| Hemoglobin (per 1 g/dL) | 0.019 | 0.012, 0.027 | <0.001 |
| Triglyceride (per 1 mg/dL) | 0 | 0, 0 | 0.003 |
| Total cholesterol (per 1 mg/dL) | −0.001 | −0.001, −0.001 | <0.001 |
| HDL-cholesterol (per 1 mg/dL) | −0.005 | −0.006, −0.004 | <0.001 |
| LDL-cholesterol (per 1 mg/dL) | 0 | −0.001, 0 | 0.105 |
| eGFR (per 1 mL/min/1.73 m2) | −1.142 × 10−5 | 0, 0 | 0.962 |
| Uric acid (per 1 mg/dL) | 0.031 | 0.023, 0.040 | <0.001 |
| Obesity-Related Indices | ΔT-Score | ||
|---|---|---|---|
| Multivariable | |||
| Unstandardized Coefficient β | 95% CI | p | |
| BMI (per 1 kg/m2) a | 0.005 | 0, 0.011 | 0.036 |
| WHR (per 1%) a | −0.002 | −0.005, 0.001 | 0.153 |
| WHtR (per 1%) a | 0.003 | 0, 0.006 | 0.076 |
| BRI (per 1) a | 0.007 | −0.002, 0.017 | 0.122 |
| BAI (per 1) a | 0.010 | 0.005, 0.014 | <0.001 |
| AVI (per 1) a | 0.003 | −0.002, 0.009 | 0.233 |
| LAP (per 1) b | 0 | −0.001, 0.001 | 0.728 |
| VAI (per 1) c | 0.017 | 0.006, 0.027 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.-H.; Liu, Y.-H.; Chen, S.-C.; Su, H.-M. Low Obesity-Related Indices Are Associated with a Low Baseline Calcaneus Ultrasound T-Score, and a Rapid Decline in T-Score in a Large Taiwanese Population Follow-Up Study. Nutrients 2023, 15, 605. https://doi.org/10.3390/nu15030605
Chen L-H, Liu Y-H, Chen S-C, Su H-M. Low Obesity-Related Indices Are Associated with a Low Baseline Calcaneus Ultrasound T-Score, and a Rapid Decline in T-Score in a Large Taiwanese Population Follow-Up Study. Nutrients. 2023; 15(3):605. https://doi.org/10.3390/nu15030605
Chicago/Turabian StyleChen, Li-Han, Yi-Hsueh Liu, Szu-Chia Chen, and Ho-Ming Su. 2023. "Low Obesity-Related Indices Are Associated with a Low Baseline Calcaneus Ultrasound T-Score, and a Rapid Decline in T-Score in a Large Taiwanese Population Follow-Up Study" Nutrients 15, no. 3: 605. https://doi.org/10.3390/nu15030605
APA StyleChen, L.-H., Liu, Y.-H., Chen, S.-C., & Su, H.-M. (2023). Low Obesity-Related Indices Are Associated with a Low Baseline Calcaneus Ultrasound T-Score, and a Rapid Decline in T-Score in a Large Taiwanese Population Follow-Up Study. Nutrients, 15(3), 605. https://doi.org/10.3390/nu15030605






