Association of Healthy Lifestyles with Non-Alcoholic Fatty Liver Disease: A Prospective Cohort Study in Chinese Government Employees
Abstract
1. Introduction
2. Methods
2.1. Research Design and Study Participants
2.2. Assessment of Healthy Lifestyle Factors
2.3. Healthy Lifestyle Definition
2.4. Diagnosis of NAFLD
2.5. Assessment of Covariates
2.6. Statistical Analyses
3. Results
3.1. Basic Characteristics of Study Participants
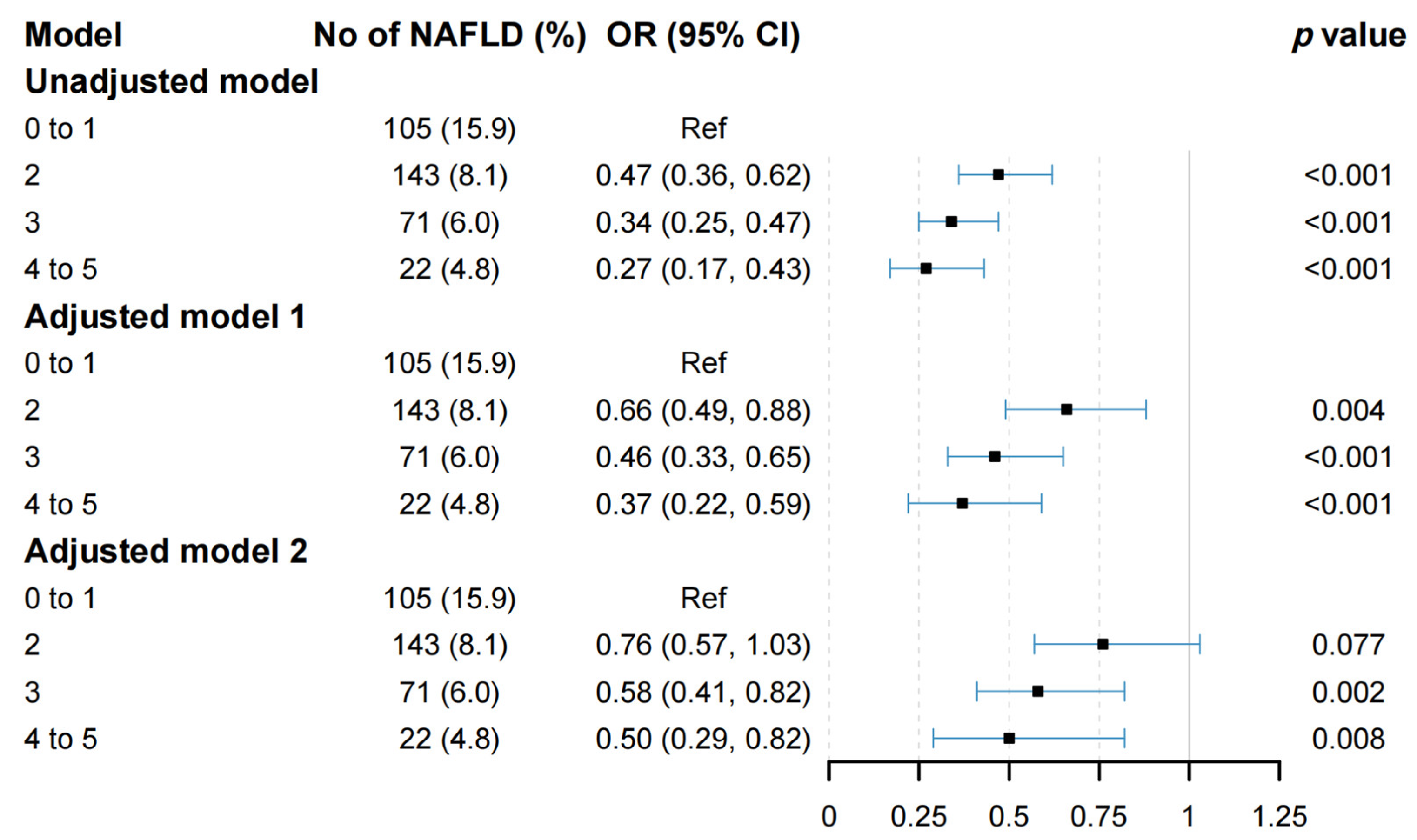
3.2. Associations between Combined Lifestyle Factors and NAFLD
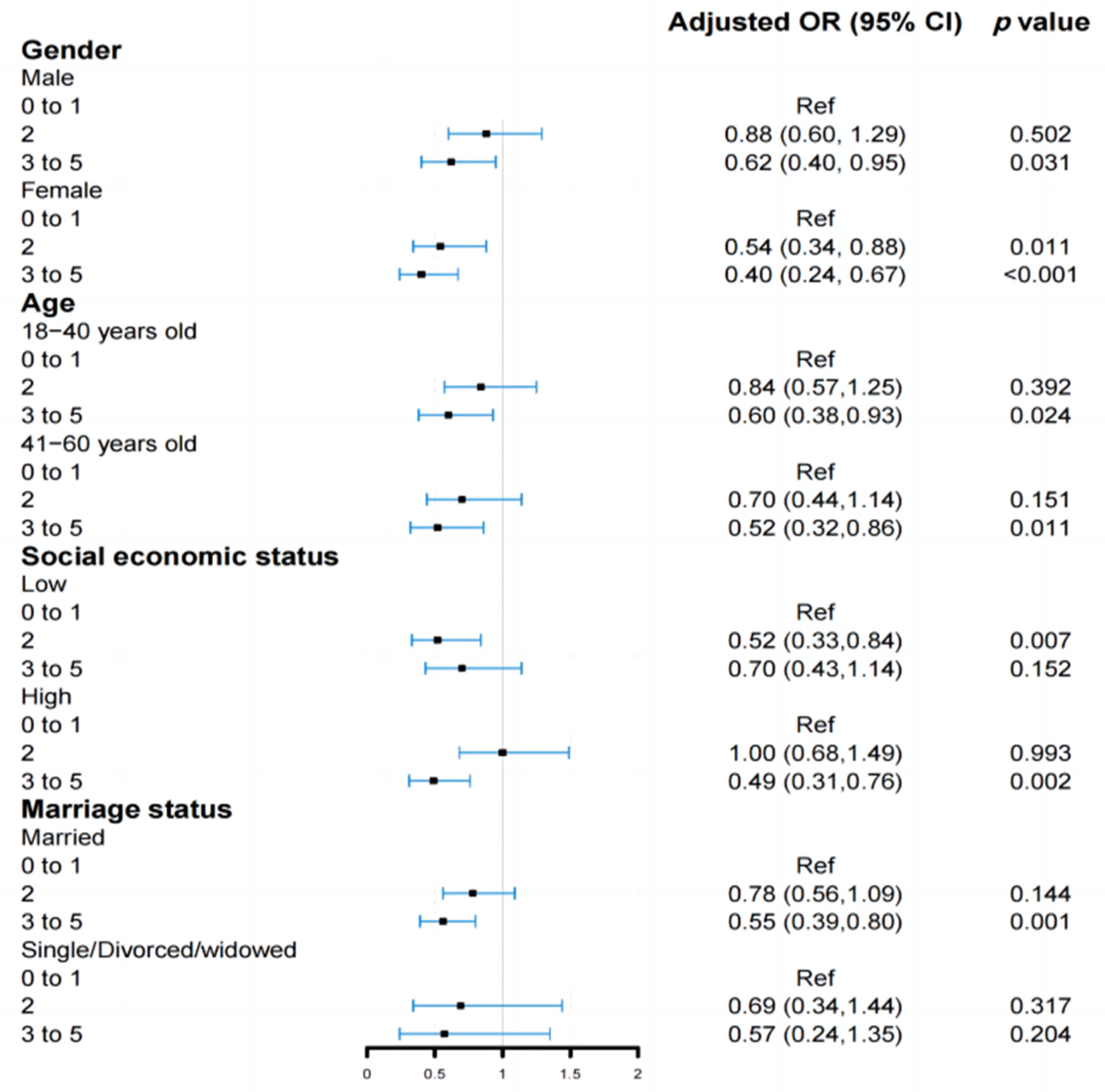
3.3. Analyses of Subgroups and Sensitivity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Powell, E.E.; Wong, V.W.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef]
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef] [PubMed]
- Sarin, S.K.; Kumar, M.; Eslam, M.; George, J.; Al Mahtab, M.; Akbar, S.M.F.; Jia, J.; Tian, Q.; Aggarwal, R.; Muljono, D.H.; et al. Liver diseases in the Asia-Pacific region: A Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol. Hepatol. 2020, 5, 167–228. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wei, Y.; Hu, H.; Wang, J.; Li, Z.; Wang, F.; Long, T.; Yuan, J.; Yao, P.; Wei, S.; et al. Genetic Risk, a Healthy Lifestyle, and Type 2 Diabetes: The Dongfeng-Tongji Cohort Study. J. Clin. Endocrinol. Metab. 2020, 105, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wang, T.; Huang, Y.; Zhao, X.; Ding, Y.; Zhu, M.; Ji, M.; Wang, C.; Dai, J.; Yin, R.; et al. Genetic Risk for Overall Cancer and the Benefit of Adherence to a Healthy Lifestyle. Cancer Res. 2021, 81, 4618–4627. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Chen, C.; Pan, X.F.; Guo, J.; Li, Y.; Franco, O.H.; Liu, G.; Pan, A. Associations of healthy lifestyle and socioeconomic status with mortality and incident cardiovascular disease: Two prospective cohort studies. BMJ 2021, 373, n604. [Google Scholar] [CrossRef]
- Sun, Q.; Yu, D.; Fan, J.; Yu, C.; Guo, Y.; Pei, P.; Yang, L.; Chen, Y.; Du, H.; Yang, X.; et al. Healthy lifestyle and life expectancy at age 30 years in the Chinese population: An observational study. Lancet Public Health 2022, 7, E994–E1004. [Google Scholar] [CrossRef] [PubMed]
- Akhavan Rezayat, A.; Dadgar Moghadam, M.; Ghasemi Nour, M.; Shirazinia, M.; Ghodsi, H.; Rouhbakhsh Zahmatkesh, M.R.; Tavakolizadeh Noghabi, M.; Hoseini, B.; Akhavan Rezayat, K. Association between smoking and non-alcoholic fatty liver disease: A systematic review and meta-analysis. SAGE Open Med. 2018, 6, 2050312117745223. [Google Scholar] [CrossRef]
- Qiu, S.; Cai, X.; Sun, Z.; Li, L.; Zugel, M.; Steinacker, J.M.; Schumann, U. Association between physical activity and risk of nonalcoholic fatty liver disease: A meta-analysis. Therap. Adv. Gastroenterol. 2017, 10, 701–713. [Google Scholar] [CrossRef]
- Ryu, S.; Chang, Y.; Jung, H.S.; Yun, K.E.; Kwon, M.J.; Choi, Y.; Kim, C.W.; Cho, J.; Suh, B.S.; Cho, Y.K.; et al. Relationship of sitting time and physical activity with non-alcoholic fatty liver disease. J. Hepatol. 2015, 63, 1229–1237. [Google Scholar] [CrossRef]
- Wei, H.; Qu, H.; Wang, H.; Deng, H. Associations between sitting time and non-alcoholic fatty liver diseases in Chinese male workers: A cross-sectional study. BMJ Open 2016, 6, e011939. [Google Scholar] [CrossRef]
- Zelber-Sagi, S.; Lotan, R.; Shlomai, A.; Webb, M.; Harrari, G.; Buch, A.; Nitzan Kaluski, D.; Halpern, Z.; Oren, R. Predictors for incidence and remission of NAFLD in the general population during a seven-year prospective follow-up. J. Hepatol. 2012, 56, 1145–1151. [Google Scholar] [CrossRef]
- Romero-Gomez, M.; Zelber-Sagi, S.; Trenell, M. Treatment of NAFLD with diet, physical activity and exercise. J. Hepatol. 2017, 67, 829–846. [Google Scholar] [CrossRef]
- Bjermo, H.; Iggman, D.; Kullberg, J.; Dahlman, I.; Johansson, L.; Persson, L.; Berglund, J.; Pulkki, K.; Basu, S.; Uusitupa, M.; et al. Effects of n-6 PUFAs compared with SFAs on liver fat, lipoproteins, and inflammation in abdominal obesity: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 95, 1003–1012. [Google Scholar] [CrossRef]
- Qiu, D.; Li, R.; Li, Y.; He, J.; Ouyang, F.; Luo, D.; Xiao, S. Job Dissatisfaction Mediated the Associations Between Work Stress and Mental Health Problems. Front. Psychiatry 2021, 12, 711263. [Google Scholar] [CrossRef]
- Li, L.; Ouyang, F.; He, J.; Qiu, D.; Luo, D.; Xiao, S. Associations of Socioeconomic Status and Healthy Lifestyle with Incidence of Dyslipidemia: A Prospective Chinese Governmental Employee Cohort Study. Front. Public Health 2022, 10, 878126. [Google Scholar] [CrossRef]
- Qiu, D.; He, J.; Li, Y.; Li, R.; Ouyang, F.; Li, L.; Luo, D.; Xiao, S. Stressful Life Events and Chronic Fatigue Among Chinese Government Employees: A Population-Based Cohort Study. Front. Public Health 2022, 10, 890604. [Google Scholar] [CrossRef]
- Tremblay, M.S.; Aubert, S.; Barnes, J.D.; Saunders, T.J.; Carson, V.; Latimer-Cheung, A.E.; Chastin, S.F.M.; Altenburg, T.M.; Chinapaw, M.J.M.; Participants, S.T.C.P. Sedentary Behavior Research Network (SBRN)—Terminology Consensus Project process and outcome. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 75. [Google Scholar] [CrossRef]
- Healy, G.N.; Clark, B.K.; Winkler, E.A.; Gardiner, P.A.; Brown, W.J.; Matthews, C.E. Measurement of adults’ sedentary time in population-based studies. Am. J. Prev. Med. 2011, 41, 216–227. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R., Jr.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 Compendium of Physical Activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef]
- Harrison, S.A.; Day, C.P. Benefits of lifestyle modification in NAFLD. Gut 2007, 56, 1760–1769. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Gong, W.; Ding, C.; Li, H.; Feng, G.; Ma, Y.; Fan, J.; Song, C.; Liu, A. Association of Physical Activity and Sitting Time with Overweight/Obesity in Chinese Occupational Populations. Obes. Facts 2021, 14, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Lu, F.C.; Department of Disease Control Ministry of Health, PR China. The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed. Environ. Sci. 2004, 17, 1–36. [Google Scholar]
- Zeng, M.D.; Fan, J.G.; Lu, L.G.; Li, Y.M.; Chen, C.W.; Wang, B.Y.; Mao, Y.M.; Chinese National Consensus Workshop on Nonalcoholic Fatty Liver Disease. Guidelines for the diagnosis and treatment of nonalcoholic fatty liver diseases. J. Dig. Dis. 2008, 9, 108–112. [Google Scholar] [CrossRef]
- Deng, Y.Y.; Zhong, Q.W.; Zhong, H.L.; Xiong, F.; Ke, Y.B.; Chen, Y.M. Higher Healthy Lifestyle Score is associated with lower presence of non-alcoholic fatty liver disease in middle-aged and older Chinese adults: A community-based cross-sectional study. Public Health Nutr. 2021, 24, 5081–5089. [Google Scholar] [CrossRef] [PubMed]
- Van Kleef, L.A.; Hofman, A.; Voortman, T.; de Knegt, R.J. Objectively Measured Physical Activity Is Inversely Associated with Nonalcoholic Fatty Liver Disease: The Rotterdam Study. Am. J. Gastroenterol. 2022, 117, 311–318. [Google Scholar] [CrossRef]
- Du, S.; Wang, C.; Jiang, W.; Li, C.; Li, Y.; Feng, R.; Sun, C. The impact of body weight gain on nonalcoholic fatty liver disease and metabolic syndrome during earlier and later adulthood. Diabetes Res. Clin. Pract. 2016, 116, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.M.C.; Martins, E.S.; Pedrosa, D.; Evangelista, M. Relationship between climatic factors and air quality with tuberculosis in the Federal District, Brazil, 2003–2012. Braz. J. Infect. Dis. 2017, 21, 369–375. [Google Scholar] [CrossRef]
- Yu, C.; Gao, J.; Ge, X.; Wang, X.; Ding, Y.; Tian, T.; Xu, X.; Guo, W.; Wang, Q.; Ge, Z.; et al. Healthy Lifestyle Is Associated with Reduced Mortality in Patients with Non-Alcoholic Fatty Liver Disease. Nutrients 2022, 14, 3785. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, H.; Liang, S.; Zhang, H.; Mo, Y.; Rao, S.; Zhang, Y.; Zhang, Z.; Wang, W.; Yang, W. Higher Adherence to Healthy Lifestyle Score Is Associated with Lower Odds of Non-Alcoholic Fatty Liver Disease. Nutrients 2022, 14, 4462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; He, J.; Pan, L.L.; Ma, Z.M.; Han, C.K.; Chen, C.S.; Chen, Z.; Han, H.W.; Chen, S.; Sun, Q.; et al. Effects of Moderate and Vigorous Exercise on Nonalcoholic Fatty Liver Disease: A Randomized Clinical Trial. JAMA Intern. Med. 2016, 176, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Promrat, K.; Kleiner, D.E.; Niemeier, H.M.; Jackvony, E.; Kearns, M.; Wands, J.R.; Fava, J.L.; Wing, R.R. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 2010, 51, 121–129. [Google Scholar] [CrossRef]
- Wong, V.W.; Wong, G.L.; Chan, R.S.; Shu, S.S.; Cheung, B.H.; Li, L.S.; Chim, A.M.; Chan, C.K.; Leung, J.K.; Chu, W.C.; et al. Beneficial effects of lifestyle intervention in non-obese patients with non-alcoholic fatty liver disease. J. Hepatol. 2018, 69, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, M.; Zimny, S.; Feiner, S.; Sauter, J.; Sydor, S.; Denk, G.; Nagel, J.M.; Bischoff, G.; Rust, C.; Hohenester, S. Multidisciplinary lifestyle intervention is associated with improvements in liver damage and in surrogate scores of NAFLD and liver fibrosis in morbidly obese patients. Eur. J. Nutr. 2022, 61, 2725–2735. [Google Scholar] [CrossRef]
- World Health Statistics 2019: Monitoring Health for the SDGs, Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2019.
- Lonardo, A.; Nascimbeni, F.; Ballestri, S.; Fairweather, D.; Win, S.; Than, T.A.; Abdelmalek, M.F.; Suzuki, A. Sex Differences in Nonalcoholic Fatty Liver Disease: State of the Art and Identification of Research Gaps. Hepatology 2019, 70, 1457–1469. [Google Scholar] [CrossRef]
- Lonardo, A.; Suzuki, A. Sexual Dimorphism of NAFLD in Adults. Focus on Clinical Aspects and Implications for Practice and Translational Research. J. Clin. Med. 2020, 9, 1278. [Google Scholar] [CrossRef]
- Shima, T.; Seki, K.; Umemura, A.; Ogawa, R.; Horimoto, R.; Oya, H.; Sendo, R.; Mizuno, M.; Okanoue, T. Influence of lifestyle-related diseases and age on the development and progression of non-alcoholic fatty liver disease. Hepatol. Res. 2015, 45, 548–559. [Google Scholar] [CrossRef]
- Yatsuji, S.; Hashimoto, E.; Tobari, M.; Tokushige, K.; Shiratori, K. Influence of age and gender in Japanese patients with non-alcoholic steatohepatitis. Hepatol. Res. 2007, 37, 1034–1043. [Google Scholar] [CrossRef]
- Long, M.T.; Pedley, A.; Massaro, J.M.; Hoffmann, U.; Ma, J.; Loomba, R.; Chung, R.T.; Benjamin, E.J. A simple clinical model predicts incident hepatic steatosis in a community-based cohort: The Framingham Heart Study. Liver Int. 2018, 38, 1495–1503. [Google Scholar] [CrossRef]
- Li, L.; Liu, D.W.; Yan, H.Y.; Wang, Z.Y.; Zhao, S.H.; Wang, B. Obesity is an independent risk factor for non-alcoholic fatty liver disease: Evidence from a meta-analysis of 21 cohort studies. Obes. Rev. 2016, 17, 510–519. [Google Scholar] [CrossRef]
- Fan, J.G.; Kim, S.U.; Wong, V.W. New trends on obesity and NAFLD in Asia. J. Hepatol. 2017, 67, 862–873. [Google Scholar] [CrossRef]
- Lu, F.B.; Hu, E.D.; Xu, L.M.; Chen, L.; Wu, J.L.; Li, H.; Chen, D.Z.; Chen, Y.P. The relationship between obesity and the severity of non-alcoholic fatty liver disease: Systematic review and meta-analysis. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 491–502. [Google Scholar] [CrossRef]
- Camhi, S.M.; Bray, G.A.; Bouchard, C.; Greenway, F.L.; Johnson, W.D.; Newton, R.L.; Ravussin, E.; Ryan, D.H.; Smith, S.R.; Katzmarzyk, P.T. The relationship of waist circumference and BMI to visceral, subcutaneous, and total body fat: Sex and race differences. Obesity 2011, 19, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Mongraw-Chaffin, M.; Golden, S.H.; Allison, M.A.; Ding, J.; Ouyang, P.; Schreiner, P.J.; Szklo, M.; Woodward, M.; Young, J.H.; Anderson, C.A. The Sex and Race Specific Relationship between Anthropometry and Body Fat Composition Determined from Computed Tomography: Evidence from the Multi-Ethnic Study of Atherosclerosis. PLoS ONE 2015, 10, e0139559. [Google Scholar] [CrossRef]
- Vilar-Gomez, E.; Martinez-Perez, Y.; Calzadilla-Bertot, L.; Torres-Gonzalez, A.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Friedman, S.L.; Diago, M.; Romero-Gomez, M. Weight Loss through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology 2015, 149, 367–378.e5, quiz e314–365. [Google Scholar] [CrossRef] [PubMed]
- Koutoukidis, D.A.; Koshiaris, C.; Henry, J.A.; Noreik, M.; Morris, E.; Manoharan, I.; Tudor, K.; Bodenham, E.; Dunnigan, A.; Jebb, S.A.; et al. The effect of the magnitude of weight loss on non-alcoholic fatty liver disease: A systematic review and meta-analysis. Metabolism 2021, 115, 154455. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Total | Healthy Lifestyle Score | p Value | |||
|---|---|---|---|---|---|---|
| 0–1 | 2 | 3 | 4–5 | |||
| N | 5411 | 1299 | 2201 | 1390 | 521 | |
| Age (years), mean ± SD | 38.19 ± 9.32 | 38.48 ± 9.66 | 37.03 ± 9.03 | 38.86 ± 9.21 | 40.60 ± 9.28 | <0.001 |
| Gender, n (%) | <0.001 | |||||
| Female | 3472 (64.2) | 500 (38.5) | 1527 (69.4) | 1034 (74.4) | 411 (78.9) | |
| Male | 1939 (35.8) | 799 (61.5) | 674 (30.6) | 356 (25.6) | 110 (21.1) | |
| Education level, n (%) | 0.057 | |||||
| High school or below | 252 (4.7) | 77 (5.9) | 89 (4.0) | 69 (5.0) | 17 (3.3) | |
| University | 2704 (50.0) | 648 (49.9) | 1129 (51.3) | 673 (48.4) | 254 (48.8) | |
| Postgraduate or above | 2455 (45.4) | 574 (44.2) | 983 (44.7) | 648 (46.6) | 250 (48.0) | |
| Marriage status, n (%) | <0.001 | |||||
| Divorced/widowed | 137 (2.5) | 26 (2.0) | 50 (2.3) | 46 (3.3) | 15 (2.9) | |
| Single | 854 (15.8) | 222 (17.1) | 405 (18.4) | 181 (13.0) | 46 (8.8) | |
| Married | 4420 (81.7) | 1051 (80.9) | 1746 (79.3) | 1163 (83.7) | 460 (88.3) | |
| Grades of employment | <0.001 | |||||
| Primary | 2174 (40.2) | 550 (42.3) | 953 (43.3) | 518 (37.3) | 153 (29.4) | |
| Intermediate | 2117 (39.1) | 456 (35.1) | 852 (38.7) | 590 (42.4) | 219 (42.0) | |
| Senior/deputy senior | 1120 (20.7) | 293 (22.6) | 396 (18.0) | 282 (20.3) | 149 (28.6) | |
| Annual household income (CNY), n (%) | 0.127 | |||||
| <100,000 | 1850 (34.2) | 470 (36.2) | 755 (34.3) | 457 (32.9) | 168 (32.2) | |
| 100,000–300,000 | 3040 (56.2) | 703 (54.1) | 1253 (56.9) | 795 (57.2) | 289 (55.5) | |
| >300,000 | 521 (9.6) | 126 (9.7) | 193 (8.8) | 138 (9.9) | 64 (12.3) | |
| Alcohol | <0.001 | |||||
| Current | 303 (5.6) | 146 (11.2) | 96 (4.4) | 54 (3.9) | 7 (1.3) | |
| Never/former | 5108 (94.4) | 1153 (88.8) | 2105 (95.6) | 1336 (96.1) | 514 (98.7) | |
| History of using statins, n (%) | 39 (0.7) | 17 (1.3) | 11 (0.5) | 7 (0.5) | 4 (0.8) | 0.033 |
| TC (mmol/L), mean ± SD | 4.77 ± 0.91 | 4.92 ± 0.96 | 4.73 ± 0.90 | 4.74 ± 0.88 | 4.68 ± 0.90 | <0.001 |
| TG (mmol/L), mean ± SD | 1.35 ± 1.19 | 1.82 ± 1.62 | 1.26 ± 1.03 | 1.14 ± 0.90 | 1.08 ± 0.81 | <0.001 |
| FPG (mmol/L), mean ± SD | 5.32 ± 0.88 | 5.49 ± 1.09 | 5.26 ± 0.81 | 5.28 ± 0.83 | 5.22 ± 0.54 | <0.001 |
| TBIL (μmol/L), mean ± SD | 13.34 ± 4.91 | 12.90 ± 4.42 | 13.31 ± 4.82 | 13.65 ± 5.26 | 13.72 ± 5.38 | <0.001 |
| HDL-C (mmol/L), mean ± SD | 1.44 ± 0.33 | 1.30 ± 0.31 | 1.46 ± 0.33 | 1.51 ± 0.32 | 1.53 ± 0.32 | <0.001 |
| LDL-C (mmol/L), mean ± SD | 2.72 ± 0.76 | 2.79 ± 0.81 | 2.69 ± 0.75 | 2.72 ± 0.73 | 2.66 ± 0.73 | 0.001 |
| PLT (109/L), mean ± SD | 224.95 ± 51.39 | 227.11 ± 50.78 | 225.93 ± 51.74 | 222.37 ± 50.34 | 222.32 ± 53.88 | 0.047 |
| ALB (g/L), mean ± SD | 45.44 ± 3.03 | 45.84 ± 3.17 | 45.36 ± 3.06 | 45.36 ± 2.88 | 45.00 ± 2.88 | <0.001 |
| ALT (U/L), mean ± SD | 21.68 ± 18.85 | 28.99 ± 27.76 | 20.11 ± 15.19 | 18.67 ± 13.36 | 18.18 ± 11.82 | <0.001 |
| Ultrasound-based NAFLD, n (%) | 1280 (23.7) | 618 (47.6) | 414 (18.8) | 189 (13.6) | 59 (11.3) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ling, Z.; Zhang, C.; He, J.; Ouyang, F.; Qiu, D.; Li, L.; Li, Y.; Li, X.; Duan, Y.; Luo, D.; et al. Association of Healthy Lifestyles with Non-Alcoholic Fatty Liver Disease: A Prospective Cohort Study in Chinese Government Employees. Nutrients 2023, 15, 604. https://doi.org/10.3390/nu15030604
Ling Z, Zhang C, He J, Ouyang F, Qiu D, Li L, Li Y, Li X, Duan Y, Luo D, et al. Association of Healthy Lifestyles with Non-Alcoholic Fatty Liver Disease: A Prospective Cohort Study in Chinese Government Employees. Nutrients. 2023; 15(3):604. https://doi.org/10.3390/nu15030604
Chicago/Turabian StyleLing, Zhen, Chengcheng Zhang, Jun He, Feiyun Ouyang, Dan Qiu, Ling Li, Yilu Li, Xuping Li, Yanying Duan, Dan Luo, and et al. 2023. "Association of Healthy Lifestyles with Non-Alcoholic Fatty Liver Disease: A Prospective Cohort Study in Chinese Government Employees" Nutrients 15, no. 3: 604. https://doi.org/10.3390/nu15030604
APA StyleLing, Z., Zhang, C., He, J., Ouyang, F., Qiu, D., Li, L., Li, Y., Li, X., Duan, Y., Luo, D., Xiao, S., & Shen, M. (2023). Association of Healthy Lifestyles with Non-Alcoholic Fatty Liver Disease: A Prospective Cohort Study in Chinese Government Employees. Nutrients, 15(3), 604. https://doi.org/10.3390/nu15030604





